An Experimental Apparatus for E-Nose Breath Analysis in Respiratory Failure Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setup for Exhaled Breath Collection
2.2. Feasibility Study
2.2.1. Study Population
2.2.2. Exhaled Breath Sampling
- Washout phase: The patient breathed for 3 min through the setup with the exhaled line of the non-rebreathing valve open, which allowed the inhalation of a mixture of medical gasses from the hospital pipeline system and exhalation into the ambient (Figure 1c). The subject wore a nose clip to avoid nasal respiration and breathed through a mouthpiece.
- Exhaled breath sampling: At the end of the washout phase, patients held their breath while the operator connected a NalophanTM bag to the expiratory line of the non-rebreathing valve to collect the exhaled breath (Figure 1d).
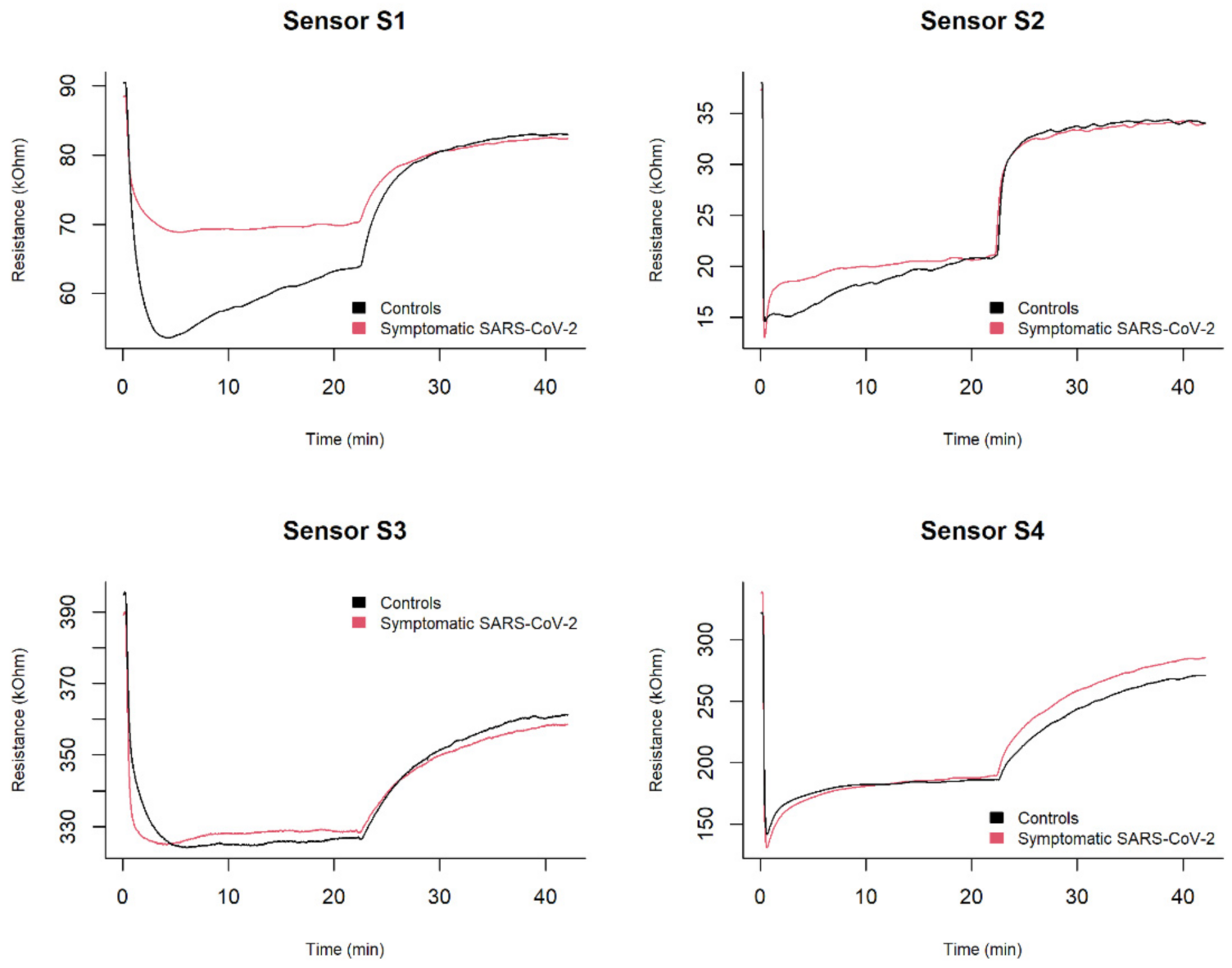
2.2.3. Exhaled Breath Analysis
- Adsorption: the exhaled breath samples were drawn at a constant flow rate of 50 mL/min for 20 min into the sensor chamber using a vacuum pump. In this phase, a decrease in the MOX sensor resistance was recorded.
- Desorption: room reference air was drawn into the sensor chamber at a constant flow rate of 50 mL/min for 20 min to restore the sensor’s baseline. Each sample was analysed once with this instrumentation.
2.2.4. Data Processing
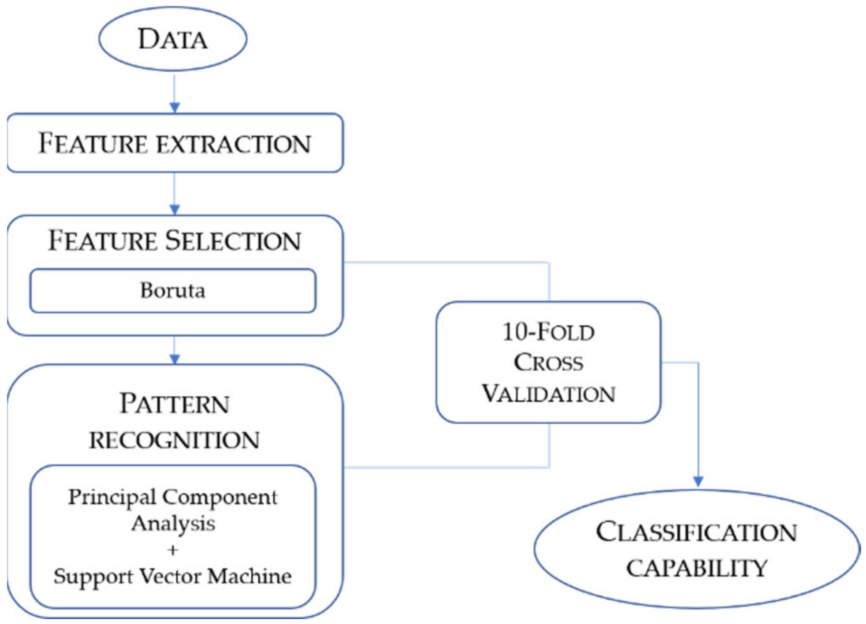
2.3. Feature Extraction
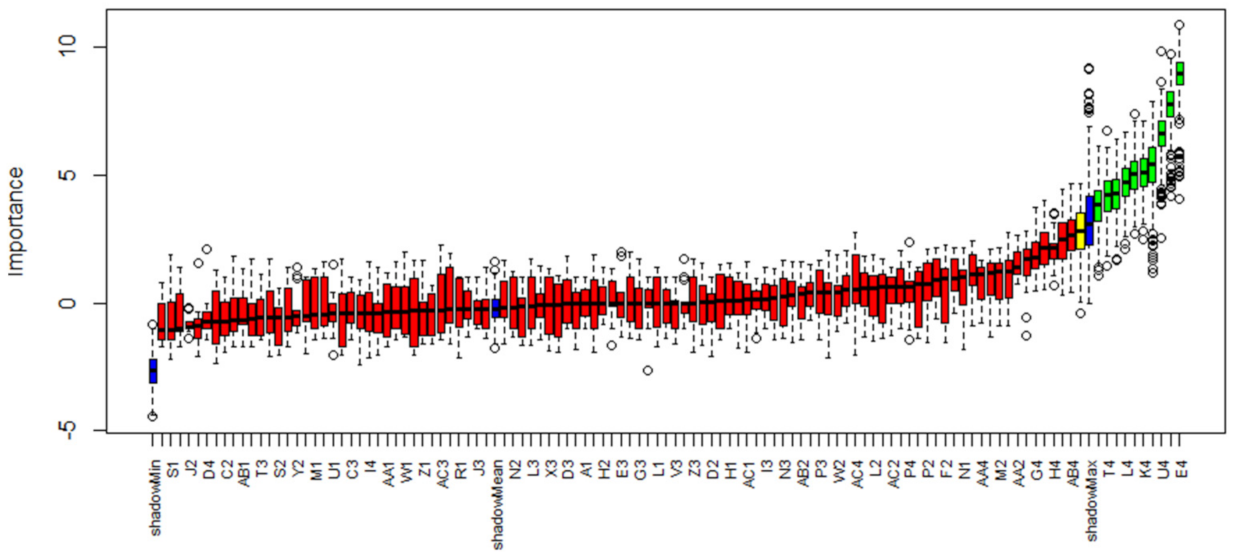
2.4. Feature Selection
2.5. Pattern Recognition
2.6. Ten-Fold Cross-Validation
2.7. Evaluation of Classification Capability
3. Results
3.1. Characteristics of Study Participants
3.2. Features of Interest
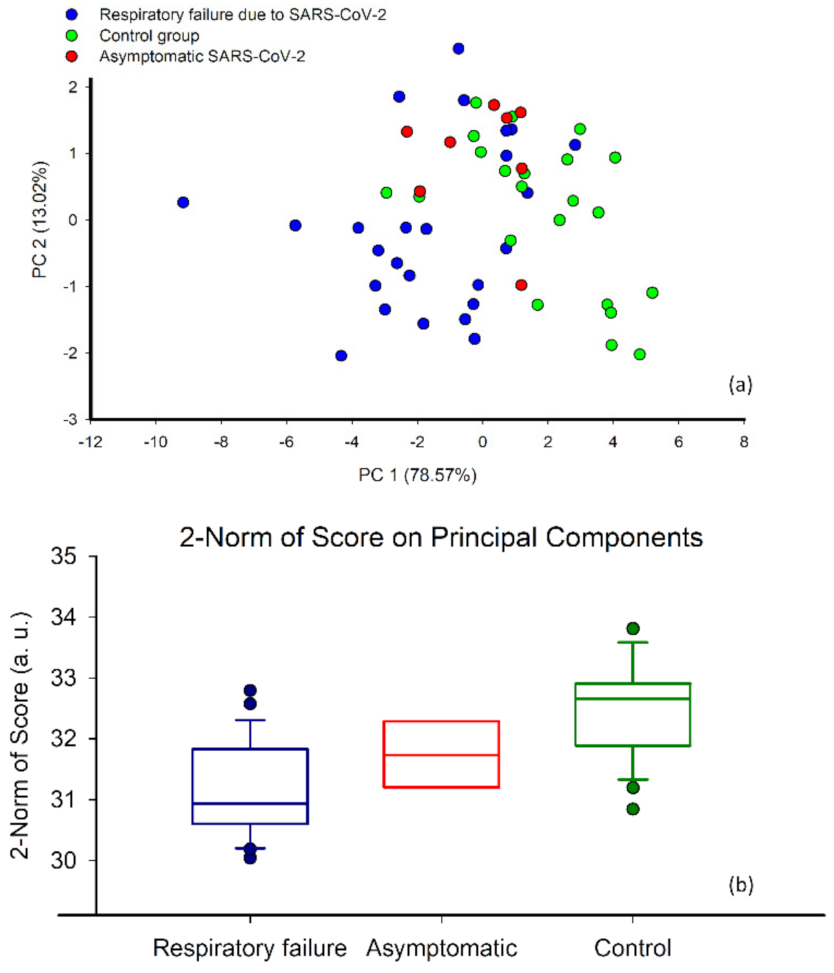
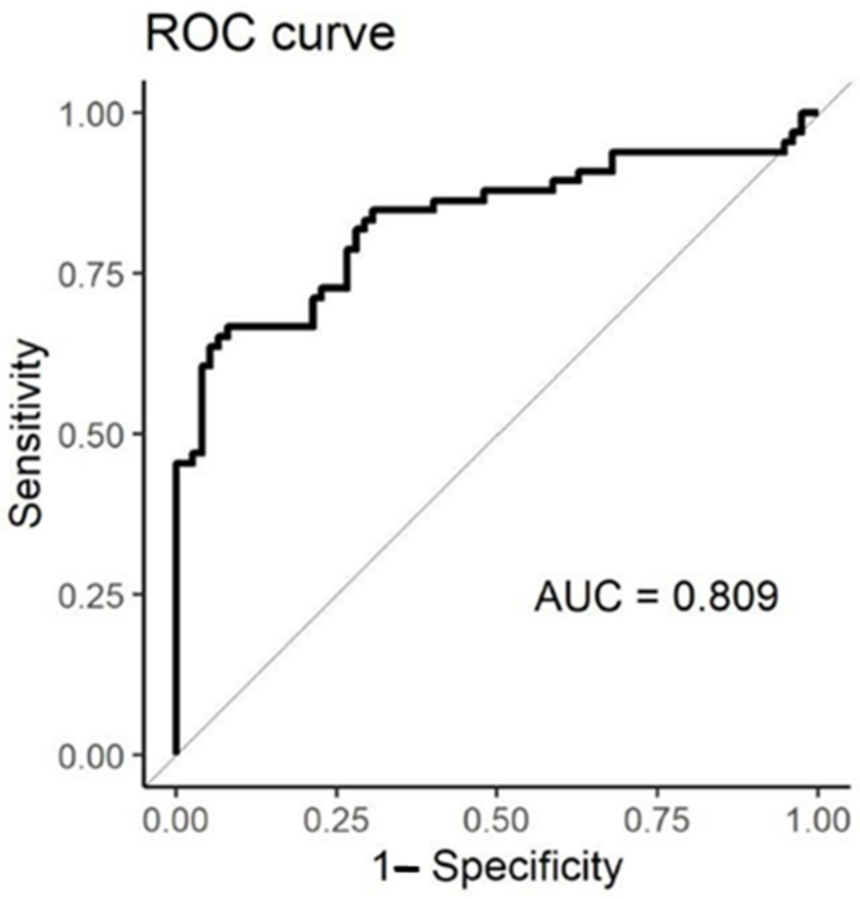
3.3. Classification and Correlation of EN Responses with Respiratory Failure
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papazian, L.; Calfee, C.S.; Chiumello, D.; Luyt, C.E.; Meyer, N.J.; Sekiguchi, H.; Matthay, M.A.; Meduri, G.U. Diagnostic workup for ARDS patients. Intensive Care Med. 2016, 42, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease, 2nd ed.; European Respiratory Society: Sheffield, UK, 2017; ISBN 9781849840873. [Google Scholar]
- Dragonieri, S.; Pennazza, G.; Carratu, P.; Resta, O. Electronic Nose Technology in Respiratory Diseases. Lung 2017, 195, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rocco, G.; Pennazza, G.; Santonico, M.; Longo, F.; Rocco, R.; Crucitti, P.; Incalzi, R.A. Breathprinting and Early Diagnosis of Lung Cancer. J. Thorac. Oncol. 2018, 13, 883–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behera, B.; Joshi, R.; Anil Vishnu, G.K.; Bhalerao, S.; Pandya, H.J. Electronic nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019, 13, 24001. [Google Scholar] [CrossRef]
- Buszewski, B.; Grzywinski, D.; Ligor, T.; Stacewicz, T.; Bielecki, Z.; Wojtas, J. Detection of volatile organic compounds as biomarkers in breath analysis by different analytical techniques. Bioanalysis 2013, 5, 2287–2306. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Podmore, I. Current Challenges in Volatile Organic Compounds Analysis as Potential Biomarkers of Cancer. J. Biomark. 2015, 2015, 981458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, M.; Cataneo, R.N.; Cruz-Ramos, J.A.; Huston, J.; Ornelas, O.; Pappas, N.; Pathak, S. Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res. Treat. 2018, 170, 343–350. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Wang, D.; Yu, K.; Wang, L.; Zou, Y.; Zhao, C.; Zhang, X.; Wang, P.; Ying, K. The analysis of volatile organic compounds biomarkers for lung cancer in exhaled breath, tissues and cell lines. Cancer Biomark. 2012, 11, 129–137. [Google Scholar] [CrossRef]
- Song, G.; Qin, T.; Liu, H.; Xu, G.B.; Pan, Y.Y.; Xiong, F.X.; Gu, K.S.; Sun, G.P.; Chen, Z.D. Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer 2010, 67, 227–231. [Google Scholar] [CrossRef]
- Phillips, M.; Basa-Dalay, V.; Bothamley, G.; Cataneo, R.N.; Lam, P.K.; Natividad, M.P.R.; Schmitt, P.; Wai, J. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis 2010, 90, 145–151. [Google Scholar] [CrossRef]
- Fernández del Río, R.; O’Hara, M.E.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Volatile Biomarkers in Breath Associated With Liver Cirrhosis—Comparisons of Pre- and Post-liver Transplant Breath Samples. EBioMedicine 2015, 2, 1243–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadamio, J.; Van den Velde, S.; Laleman, W.; Van Hee, P.; Coucke, W.; Nevens, F.; Quirynen, M. Breath biomarkers of liver cirrhosis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 905, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pijls, K.E.; Smolinska, A.; Jonkers, D.M.A.E.; Dallinga, J.W.; Masclee, A.A.M.; Koek, G.H.; Van Schooten, F.J. A profile of volatile organic compounds in exhaled air as a potential non-invasive biomarker for liver cirrhosis. Sci. Rep. 2016, 6, 19903. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.; Preti, G. Volatile Disease Biomarkers in Breath: A Critique. Curr. Pharm. Biotechnol. 2011, 12, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Fens, N.; van der Schee, M.P.; Brinkman, P.; Sterk, P.J. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin. Exp. Allergy 2013, 43, 705–715. [Google Scholar] [CrossRef]
- Turner, A.P.F.; Magan, N. Electronic noses and disease diagnostics. Nat. Rev. Microbiol. 2004, 2, 161–166. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Wasilewski, T.; Migoń, D.; Gębicki, J.; Kamysz, W. Critical review of electronic nose and tongue instruments prospects in pharmaceutical analysis. Anal. Chim. Acta 2019, 1077, 14–29. [Google Scholar] [CrossRef]
- Bax, C.; Prudenza, S.; Gaspari, G.; Capelli, L.; Grizzi, F.; Taverna, G. Drift compensation on electronic nose data for non-invasive diagnosis of prostate cancer by urine analysis. iScience 2022, 25, 103622. [Google Scholar] [CrossRef]
- Laref, R.; Losson, E.; Sava, A.; Siadat, M. Support Vector Machine Regression for Calibration Transfer between Electronic Noses Dedicated to Air Pollution Monitoring. Sensors 2018, 18, 3716. [Google Scholar] [CrossRef] [Green Version]
- Rüffer, D.; Hoehne, F.; Bühler, J. New Digital Metal-Oxide (MOx) Sensor Platform. Sensors 2018, 18, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fens, N.; De Nijs, S.B.; Peters, S.; Dekker, T.; Knobel, H.H.; Vink, T.J.; Willard, N.P.; Zwinderman, A.H.; Krouwelse, F.H.; Janssen, H.G.; et al. Exhaled air molecular profiling in relation to inflammatory subtype and activity in COPD. Eur. Respir. J. 2011, 38, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Van Geffen, W.H.; Bruins, M.; Kerstjens, H.A.M. Diagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronic nose: A pilot study. J. Breath Res. 2016, 10, 36001. [Google Scholar] [CrossRef]
- Shafiek, H.; Fiorentino, F.; Merino, J.L.; López, C.; Oliver, A.; Segura, J.; De Paul, I.; Sibila, O.; Agustí, A.; Cosío, B.G. Using the electronic nose to identify airway infection during COPD exacerbations. PLoS ONE 2015, 10, e0135199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, N.; Zakaria, A.; Omar, M.I.; Shakaff, A.Y.M.; Masnan, M.J.; Kamarudin, L.M.; Abdul Rahim, N.; Zakaria, N.Z.I.; Abdullah, A.A.; Othman, A.; et al. In-vitro diagnosis of single and poly microbial species targeted for diabetic foot infection using e-nose technology. BMC Bioinform. 2015, 16, 158. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.; Hokkinen, L.; Karjalainen, M.; Kontunen, A.; Vuento, R.; Numminen, J.; Rautiainen, M.; Oksala, N.; Roine, A.; Kivekäs, I. In vitro detection of common rhinosinusitis bacteria by the eNose utilising differential mobility spectrometry. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2273–2279. [Google Scholar] [CrossRef] [Green Version]
- Tiele, A.; Wicaksono, A.; Kansara, J.; Arasaradnam, R.P.; Covington, J.A. Breath analysis using enose and ion mobility technology to diagnose inflammatory bowel disease—A pilot study. Biosensors 2019, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Wintjens, A.G.W.E.; Hintzen, K.F.H.; Engelen, S.M.E.; Lubbers, T.; Savelkoul, P.H.M.; Wesseling, G.; van der Palen, J.A.M.; Bouvy, N.D. Applying the electronic nose for pre-operative SARS-CoV-2 screening. Surg. Endosc. 2021, 35, 6671–6678. [Google Scholar] [CrossRef]
- Kurstjens, S.; García-Tardón, N.; Fokkert, M.; van Geffen, W.H.; Slingerland, R.; Van Der Palen, J.; Kusters, R. Identifying COVID-19-infected healthcare workers using an electronic ‘nose’. Eur. Respir. J. 2021, 58, PA3869. [Google Scholar] [CrossRef]
- Krauss, E.; Zoelitz, J.; Wagner, J.; Barretto, G.; Degen, M.; Seeger, W.; Guenther, A. The use of electronic nose technology for the detection of Lung Cancer (LC): Analysis of exhaled volatile compounds by Aeonose®. Eur. Respir. J. 2018, 52, PA1758. [Google Scholar] [CrossRef]
- de Vries, R.; Dagelet, J.W.F.; Frey, U.; Lutter, R.; Maitland-Van der Zee, A.H.; Sterk, P.J.; Sinha, A. Assessment of repeatability of eNose (SpiroNose) measurements in healthy and asthmatic subjects. Eur. Respir. J. 2018, 52, OA315. [Google Scholar] [CrossRef]
- Giovannini, G.; Haick, H.; Garoli, D. Detecting COVID-19 from Breath: A Game Changer for a Big Challenge. ACS Sens. 2021, 6, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Snitz, K.; Andelman-Gur, M.; Pinchover, L.; Weissgross, R.; Weissbrod, A.; Mishor, E.; Zoller, R.; Linetsky, V.; Medhanie, A.; Shushan, S.; et al. Proof of concept for real-time detection of SARS CoV-2 infection with an electronic nose. PLoS ONE 2021, 16, e0252121. [Google Scholar] [CrossRef] [PubMed]
- Subali, A.D.; Wiyono, L.; Yusuf, M.; Zaky, M.F.A. The potential of volatile organic compounds-based breath analysis for COVID-19 screening: A systematic review & meta-analysis. Diagn. Microbiol. Infect. Dis. 2022, 102, 293. [Google Scholar]
- Adigal, S.S.; Rayaroth, N.V.; John, R.V.; Pai, K.M.; Bhandari, S.; Mohapatra, A.K.; Lukose, J.; Patil, A.; Bankapur, A.; Chidangil, S. A review on human body fluids for the diagnosis of viral infections: Scope for rapid detection of COVID-19. Expert Rev. Mol. Diagn. 2021, 21, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Maurer, F.; Wolf, A.; Fink, T.; Rittershofer, B.; Heim, N.; Volk, T.; Baumbach, J.I.; Kreuer, S. Wash-out of ambient air contaminations for breath measurements. J. Breath Res. 2014, 8, 27107. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.; Buckley, T.; Pellizzari, E.; Gordon, S. Breath measurements as volatile organic compound biomarkers. Environ. Health Perspect. 1996, 104, 861–869. [Google Scholar] [CrossRef]
- Saktiawati, A.M.I.; Stienstra, Y.; Subronto, Y.W.; Rintiswati, N.; Sumardi; Gerritsen, J.W.; Oord, H.; Akkerman, O.W.; Van Der Werf, T.S. Sensitivity and specificity of an electronic nose in diagnosing pulmonary tuberculosis among patients with suspected tuberculosis. PLoS ONE 2019, 14, e0217963. [Google Scholar] [CrossRef] [Green Version]
- Capelli, L.; Bax, C.; Grizzi, F.; Taverna, G. Optimization of training and measurement protocol for eNose analysis of urine headspace aimed at prostate cancer diagnosis. Sci. Rep. 2021, 11, 20898. [Google Scholar] [CrossRef]
- Kursa, M.B.; Jankowski, A.; Rudnicki, W.R. Boruta—A system for feature selection. Fundam. Inform. 2010, 101, 271–285. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature selection with the boruta package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Sun, M. Support Vector Machine Models for Classification. In Encyclopedia of Business Analytics and Optimization; IGI Global: Hershey, PA, USA, 2014; pp. 2395–2409. [Google Scholar]
- Rodríguez, J.D.; Pérez, A.; Lozano, J.A. Sensitivity Analysis of k-Fold Cross Validation in Prediction Error Estimation. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 32, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Mendoza, B.N.; Díaz de León-Martínez, L.; Rodríguez-Aguilar, M.; Mizaikoff, B.; Flores-Ramírez, R. Chemometric analysis of the global pattern of volatile organic compounds in the exhaled breath of patients with COVID-19, post-COVID and healthy subjects. Proof of concept for post-COVID assessment. Talanta 2022, 236, 122832. [Google Scholar] [CrossRef]
- Nurputra, D.; Kusumaatmadja, A.; Hakim, M.; Hidayat, S.; Julian, T.; Sumanto, B.; Mahendradhata, Y.; Saktiawati, A.; Wasisto, H.; Triyana, K. Fast and noninvasive electronic nose for sniffing out COVID-19 based on exhaled breath-print recognition. ResearchSquare 2021. [Google Scholar] [CrossRef]


| Feature | Description | Point of Evaluation |
|---|---|---|
| Resistance ratio | i = End of Adsorption and/or Desorption phase | |
| Resistance difference | i = End of Adsorption and/or Desorption phase | |
| Area under the curve | Area under the curve | Adsorption phase, Desorption phase, Overall analysis |
| Phase integral | Area under the plot x = R, y = dR/dt | Adsorption phase, Desorption phase, Overall analysis |
| Single point | Adsorption and Desorption phase i = Middle and End of Adsorption phase | |
| Difference ratio | 1 = Beginning of Adsorption phase 2 = Middle of Adsorption phase 3 = End of Adsorption phase | |
| Last difference | R3 − R2 | 2 = Middle of Adsorption phase 3 = End of Adsorption phase |
| Exponential moving average | yk = (1 − α)yk−1 + α(xk − xk−1) | Max, Min, Area under peaks α = (0.01, 0.001) |
| SARS-CoV-2 and Respiratory Failure | SARS-CoV-2 and Asymptomatic | Controls | p-Value | |
|---|---|---|---|---|
| N | 25 | 8 | 22 | |
| Male sex, n (%) | 17 (68.0) | 3 (37.5) | 14 (63.6) | 0.295 |
| Age (years), median (IQR) | 50 (18, 46) | 50 (24, 15) | 50 (23, 76) | 0.403 |
| Smokers, n (%) | 2 (8.0) | 2 (25.0) | 1 (4.5) | 0.219 |
| * Ex-smokers, n (%) | 5 (20.0) | 2 (25.0) | 5 (22.7) | 0.948 |
| Subjects with other comorbidities, n (%) | 10 (40.0) | 5 (62.5) | 8 (36.4) | 0.425 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bax, C.; Robbiani, S.; Zannin, E.; Capelli, L.; Ratti, C.; Bonetti, S.; Novelli, L.; Raimondi, F.; Di Marco, F.; Dellacà, R.L. An Experimental Apparatus for E-Nose Breath Analysis in Respiratory Failure Patients. Diagnostics 2022, 12, 776. https://doi.org/10.3390/diagnostics12040776
Bax C, Robbiani S, Zannin E, Capelli L, Ratti C, Bonetti S, Novelli L, Raimondi F, Di Marco F, Dellacà RL. An Experimental Apparatus for E-Nose Breath Analysis in Respiratory Failure Patients. Diagnostics. 2022; 12(4):776. https://doi.org/10.3390/diagnostics12040776
Chicago/Turabian StyleBax, Carmen, Stefano Robbiani, Emanuela Zannin, Laura Capelli, Christian Ratti, Simone Bonetti, Luca Novelli, Federico Raimondi, Fabiano Di Marco, and Raffaele L. Dellacà. 2022. "An Experimental Apparatus for E-Nose Breath Analysis in Respiratory Failure Patients" Diagnostics 12, no. 4: 776. https://doi.org/10.3390/diagnostics12040776
APA StyleBax, C., Robbiani, S., Zannin, E., Capelli, L., Ratti, C., Bonetti, S., Novelli, L., Raimondi, F., Di Marco, F., & Dellacà, R. L. (2022). An Experimental Apparatus for E-Nose Breath Analysis in Respiratory Failure Patients. Diagnostics, 12(4), 776. https://doi.org/10.3390/diagnostics12040776









