Diagnosis and Management of Malignant Pleural Effusion: A Decade in Review
Abstract
:1. Background
2. Diagnosis
2.1. Clinical Presentation
2.2. Imaging
2.2.1. Chest Radiography
2.2.2. Thoracic Ultrasonography
2.2.3. Chest Computed Tomography (CT)
2.2.4. PET Scan
2.3. Diagnostic Pleural Procedures
2.3.1. Thoracentesis and Pleural Fluid Analysis
2.3.2. Pleural Biopsy: Blind and Image-Guided
2.3.3. Medical Thoracoscopy and Video-Assisted Thoracoscopic Surgery
3. Prognosis
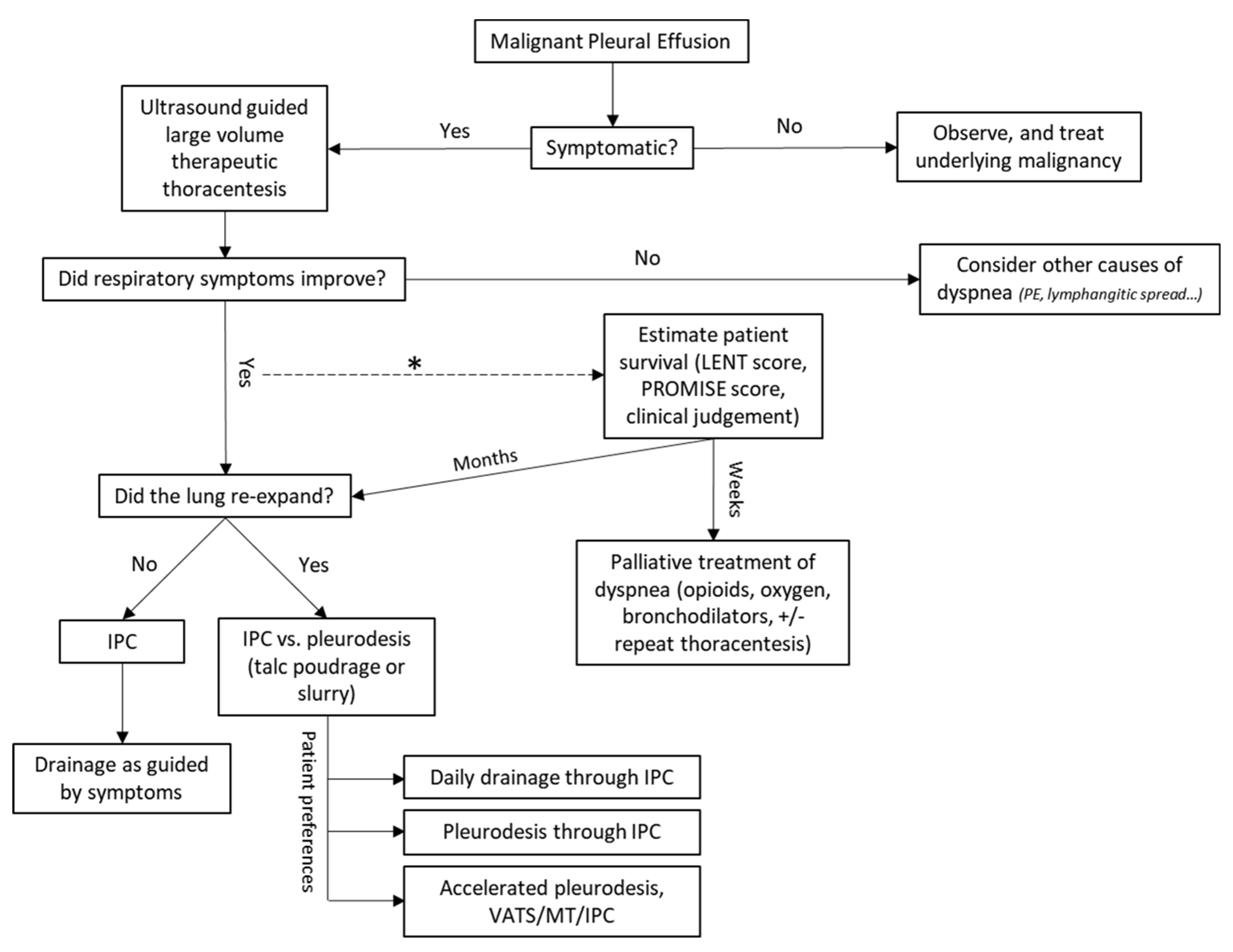
4. Management of MPE
4.1. Asymptomatic Malignant Pleural Effusion
4.2. Therapeutic Thoracentesis
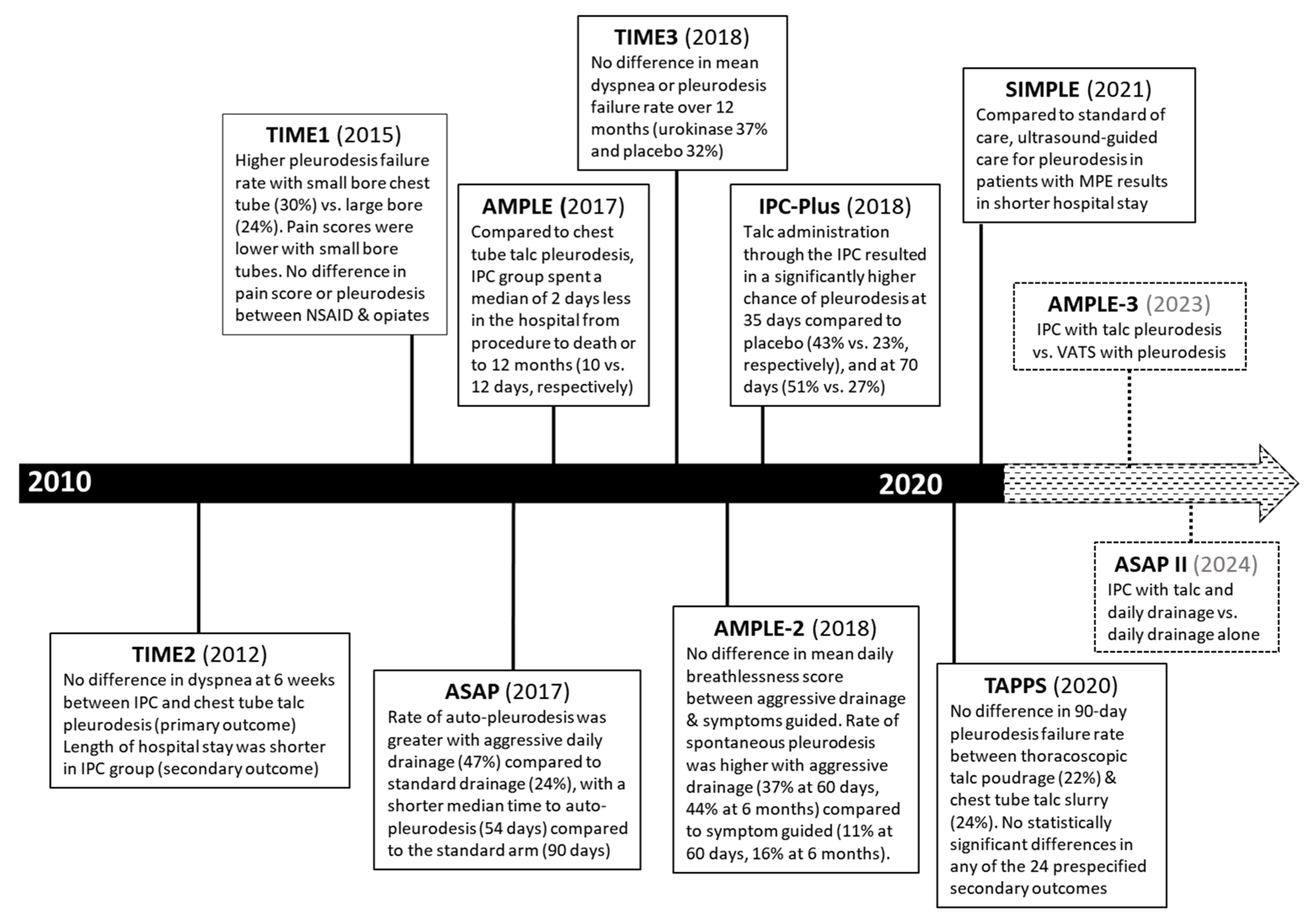
4.3. Pleurodesis
4.4. Indwelling Pleural Catheter (IPC)
4.5. IPC-Related Complications
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antony, V.B.; Loddenkemper, R.; Astoul, P.; Boutin, C.; Goldstraw, P.; Hott, J.; Panadero, F.R.; Sahn, S.A. Management of malignant pleural effusions. Am. J. Respir. Crit. Care Med. 2000, 162, 1987–2001. [Google Scholar] [CrossRef] [PubMed]
- Feller-Kopman, D.; Light, R. Pleural Disease. N. Engl. J. Med. 2018, 378, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Ma, X.; Taghizadeh, N.; Kharrazi, H.; Feller-Kopman, D.; Tremblay, A.; Yarmus, L.B. Healthcare Costs and Utilization among Patients Hospitalized for Malignant Pleural Effusion. Respiration 2020, 99, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, N.; Fortin, M.; Tremblay, A. US Hospitalizations for Malignant Pleural Effusions: Data From the 2012 National Inpatient Sample. Chest 2017, 151, 845–854. [Google Scholar] [CrossRef]
- Psallidas, I.; Kalomenidis, I.; Porcel, J.M.; Robinson, B.W.; Stathopoulos, G. Malignant pleural effusion: From bench to bedside. Eur. Respir. Rev. 2016, 25, 189–198. [Google Scholar] [CrossRef]
- Bibby, A.C.; Dorn, P.; Psallidas, I.; Porcel, J.M.; Janssen, J.; Froudarakis, M.; Subotic, D.; Astoul, P.; Licht, P.; Schmid, R.A.; et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur. Respir. J. 2018, 52, 1800349. [Google Scholar] [CrossRef] [Green Version]
- Feller-Kopman, D.J.; Reddy, C.B.; DeCamp, M.M.; Diekemper, R.L.; Gould, M.K.; Henry, T.; Iyer, N.P.; Lee, Y.G.; Lewis, S.Z.; Maskell, N.A.; et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, 839–849. [Google Scholar] [CrossRef]
- Chernow, B.; Sahn, S.A. Carcinomatous involvement of the pleura: An analysis of 96 patients. Am. J. Med. 1977, 63, 695–702. [Google Scholar] [CrossRef]
- Roberts, M.E.; Neville, E.; Berrisford, R.G.; Antunes, G.; Ali, N.J.; on behalf of the BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65, ii32–ii40. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.; Ahn, H.Y.; Kim, I.; Son, J.H.; Seol, H.Y.; Kim, M.H.; Lee, M.K.; Eom, J.S. Clinical course of asymptomatic malignant pleural effusion in non-small cell lung cancer patients: A multicenter retrospective study. Medicine 2021, 100, e25748. [Google Scholar] [CrossRef]
- Estenne, M.; Yernault, J.C.; De Troyer, A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am. J. Med. 1983, 74, 813–819. [Google Scholar] [CrossRef]
- Thomas, R.; Jenkins, S.; Eastwood, P.; Lee, Y.G.; Singh, B. Physiology of breathlessness associated with pleural effusions. Curr. Opin. Pulm. Med. 2015, 21, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muruganandan, S.; Azzopardi, M.; Thomas, R.; Fitzgerald, D.B.; Kuok, Y.J.; Cheah, H.M.; Read, C.A.; Budgeon, C.A.; Eastwood, P.R.; Jenkins, S.; et al. The Pleural Effusion and Symptom Evaluation (PLEASE) study of breathlessness in patients with a symptomatic pleural effusion. Eur. Respir. J. 2020, 55, 1900980. [Google Scholar] [CrossRef] [PubMed]
- Tammilehto, L.; Maasilta, P.; Kostiainen, S.; Appelqvist, P.; Holsti, L.; Mattson, K. Diagnosis and Prognostic Factors in Malignant Pleural Mesothelioma: A Retrospective Analysis of Sixty-Five Patients. Respiration 1992, 59, 129–135. [Google Scholar] [CrossRef]
- Maher, G.G.; Berger, H.W. Massive pleural effusion: Malignant and nonmalignant causes in 46 patients. Am. Rev. Respir. Dis. 1972, 105, 458–460. [Google Scholar] [CrossRef]
- Yousefifard, M.; Baikpour, M.; Ghelichkhani, P.; Asady, H.; Nia, K.S.; Jafari, A.M.; Hosseini, M.; Safari, S. Screening Performance Characteristic of Ultrasonography and Radiography in Detection of Pleural Effusion; a Meta-Analysis. Emergency 2016, 4, 1–10. [Google Scholar]
- Qureshi, N.R.; Rahman, N.M.; Gleeson, F.V. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009, 64, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Shiroshita, A.; Nozaki, S.; Tanaka, Y.; Luo, Y.; Kataoka, Y. Thoracic ultrasound for malignant pleural effusion: A systematic review and meta-analysis. ERJ Open Res. 2020, 6, 00464-2020. [Google Scholar] [CrossRef]
- Desai, N.R.; Lee, H.J. Diagnosis and management of malignant pleural effusions: State of the art in 2017. J. Thorac. Dis. 2017, 9, S1111–S1122. [Google Scholar] [CrossRef] [Green Version]
- Hooper, C.; Lee, Y.C.G.; Maskell, N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2020. Thorax 2010, 65, ii4–ii17. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.J.; Reddy, G.P.; Gotway, M.B.; Higgins, C.B.; Jablons, D.M.; Ramaswamy, M.; Hawkins, R.A.; Webb, W.R. Malignant Pleural Mesothelioma: Evaluation with CT, MR Imaging, and PET. RadioGraphics 2004, 24, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M.; Pardina, M.; Bielsa, S.; González, A.; Light, R.W. Derivation and validation of a CT scan scoring system for discriminating malignant from benign pleural effusions. Chest 2015, 147, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M.; Hernández, P.; Martínez-Alonso, M.; Bielsa, S.; Salud, A. Accuracy of fluorodeoxyglucose-PET imaging for differentiating benign from malignant pleural effusions: A meta-analysis. Chest 2015, 147, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-F.; Tong, Z.-H.; Wang, Z.; Zhang, Y.-Y.; Xu, L.-L.; Wang, X.-J.; Li, W.; Wu, X.-Z.; Wang, W.; Zhang, Y.-H.; et al. Development and validation of the PET-CT score for diagnosis of malignant pleural effusion. Eur. J. Pediatr. 2019, 46, 1457–1467. [Google Scholar] [CrossRef] [Green Version]
- Ferreiro, L.; Gude, F.; Toubes, M.E.; Lama, A.; Suárez-Antelo, J.; San-José, E.; González-Barcala, F.J.; Golpe, A.; Álvarez-Dobaño, J.M.; Rábade, C.; et al. Predictive models of malignant transudative pleural effusions. J. Thorac. Dis. 2017, 9, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Sahn, S.A.; Good, J.T. Pleural Fluid pH in Malignant Effusions. Ann. Intern. Med. 1988, 108, 345–349. [Google Scholar] [CrossRef]
- Arnold, D.T.; De Fonseka, D.; Perry, S.; Morley, A.; Harvey, J.E.; Medford, A.; Brett, M.; Maskell, N.A. Investigating unilateral pleural effusions: The role of cytology. Eur. Respir. J. 2018, 52, 1801254. [Google Scholar] [CrossRef]
- Heffner, J.E.; Nietert, P.; Barbieri, C. Pleural Fluid pH as a Predictor of Survival for Patients with Malignant Pleural Effusions. Chest 2000, 117, 79–86. [Google Scholar] [CrossRef]
- Heffner, J.E.; Nietert, P.J.; Barbieri, C. Pleural fluid pH as a predictor of pleurodesis failure: Analysis of primary data. Chest 2000, 117, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.W.; Ducatman, B.S.; Wang, H.H. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod. Pathol. 1994, 7, 665–668. [Google Scholar]
- Rakha, E.A.; Patil, S.; Abdulla, K.; Abdulkader, M.; Chaudry, Z.; Soomro, I.N. The sensitivity of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Diagn. Cytopathol. 2010, 38, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Grosu, H.B.; Kazzaz, F.; Vakil, E.; Molina, S.; Ost, D. Sensitivity of Initial Thoracentesis for Malignant Pleural Effusion Stratified by Tumor Type in Patients with Strong Evidence of Metastatic Disease. Respiration 2018, 96, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.; Ali, S.Z.; Olson, M.T. A minimum fluid volume of 75 mL is needed to ensure adequacy in a pleural effusion: A retrospective analysis of 2540 cases. Cancer Cytopathol. 2014, 122, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M.; Quirós, M.; Gatius, S.; Bielsa, S. Examination of cytological smears and cell blocks of pleural fluid: Complementary diagnostic value for malignant effusions. Rev. Clin. Esp. 2017, 217, 144–148. [Google Scholar] [CrossRef]
- Swiderek, J.; Morcos, S.; Donthireddy, V.; Surapaneni, R.; Jackson-Thompson, V.; Schultz, L.; Kini, S.; Kvale, P. Prospective Study to Determine the Volume of Pleural Fluid Required to Diagnose Malignancy. Chest 2010, 137, 68–73. [Google Scholar] [CrossRef]
- Pereyra, M.F.; San-José, E.; Ferreiro, L.; Golpe, A.; Antúnez, J.; Gonzalez-Barcala, F.-J.; Abdulkader, I.; Álvarez-Dobaño, J.M.; Rodríguez-Núñez, N.; Valdés, L. Role of Blind Closed Pleural Biopsy in the Management of Pleural Exudates. Can. Respir. J. 2013, 20, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Prakash, U.B.; Reiman, H.M. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: Analysis of 414 cases. Mayo Clin. Proc. 1985, 60, 158–164. [Google Scholar] [CrossRef]
- Maskell, N.A.; Gleeson, F.V.; Davies, R.J. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: A randomised controlled trial. Lancet 2003, 361, 1326–1330. [Google Scholar] [CrossRef]
- Chang, D.-B.; Yang, P.-C.; Luh, K.-T.; Kuo, S.-H.; Yu, C.-J. Ultrasound-Guided Pleural Biopsy with Tru-Cut Needle. Chest 1991, 100, 1328–1333. [Google Scholar] [CrossRef]
- Hallifax, R.J.; Corcoran, J.P.; Ahmed, A.; Nagendran, M.; Rostom, H.; Hassan, N.; Maruthappu, M.; Psallidas, I.; Manuel, A.; Gleeson, F.; et al. Physician-Based Ultrasound-Guided Biopsy for Diagnosing Pleural Disease. Chest 2014, 146, 1001–1006. [Google Scholar] [CrossRef]
- Rahman, N.M.; Ali, N.J.; Brown, G.; Chapman, S.J.; O Davies, R.J.; Downer, N.J.; Gleeson, F.V.; Howes, T.Q.; Treasure, T.; Singh, S.; et al. Local anaesthetic thoracoscopy: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65, ii54–ii60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shojaee, S.; Lee, H.J. Thoracoscopy: Medical versus surgical—in the management of pleural diseases. J. Thorac. Dis. 2015, 7, S339–S351. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Opitz, I.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 2020, 55, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Rey, F.; Viallat, J.R. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995, 108, 754–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clive, A.O.; Taylor, H.; Dobson, L.; Wilson, P.; de Winton, E.; Panakis, N.; Pepperell, J.; Howell, T.; Stewart, S.; Penz, E.; et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): A multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 1094–1104. [Google Scholar] [CrossRef] [Green Version]
- Bayman, N.; Appel, W.; Ashcroft, L.; Baldwin, D.R.; Bates, A.; Darlison, L.; Edwards, J.G.; Ezhil, V.; Gilligan, D.; Hatton, M.; et al. Prophylactic Irradiation of Tracts in Patients with Malignant Pleural Mesothelioma: An Open-Label, Multicenter, Phase III Randomized Trial. J. Clin. Oncol. 2019, 37, 1200–1208. [Google Scholar] [CrossRef]
- Clive, A.O.; Kahan, B.C.; Hooper, C.E.; Bhatnagar, R.; Morley, A.J.; Zahan-Evans, N.; Bintcliffe, O.J.; Boshuizen, R.C.; Fysh, E.; Tobin, C.L.; et al. Predicting survival in malignant pleural effusion: Development and validation of the LENT prognostic score. Thorax 2014, 69, 1098–1104. [Google Scholar] [CrossRef] [Green Version]
- Brims, F.J.; Meniawy, T.M.; Duffus, I.; de Fonseka, D.; Segal, A.; Creaney, J.; Maskell, N.; Lake, R.A.; de Klerk, N.; Nowak, A.K. A Novel Clinical Prediction Model for Prognosis in Malignant Pleural Mesothelioma Using Decision Tree Analysis. J. Thorac. Oncol. 2016, 11, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Burrows, C.M.; Mathews, W.C.; Colt, H.G. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: An assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest 2000, 117, 73–78. [Google Scholar] [CrossRef]
- Anevlavis, S.; Kouliatsis, G.; Sotiriou, I.; Koukourakis, M.I.; Archontogeorgis, K.; Karpathiou, G.; Giatromanolaki, A.; Froudarakis, M.E. Prognostic Factors in Patients Presenting with Pleural Effusion Revealing Malignancy. Respiration 2014, 87, 311–316. [Google Scholar] [CrossRef]
- Pilling, J.E.; Dusmet, M.E.; Ladas, G.; Goldstraw, P. Prognostic Factors for Survival after Surgical Palliation of Malignant Pleural Effusion. J. Thorac. Oncol. 2010, 5, 1544–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, E.K.; Muruganandan, S.; Clark, A.; Bhatnagar, R.; Maskell, N.; Lee, Y.G.; Rahman, N.M. Breathlessness Predicts Survival in Patients with Malignant Pleural Effusions: Meta-analysis of Individual Patient Data from Five Randomized Controlled Trials. Chest 2021, 160, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Mauri, F.A.; Ramakrishnan, R.; Wahab, L.; Lloyd, T.; Sharma, R. Inflammation-Based Prognostic Indices in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2012, 7, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielsa, S.; Salud, A.; Martínez, M.; Esquerda, A.; Martín, A.; Rodríguez-Panadero, F.; Porcel, J.M. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur. J. Intern. Med. 2008, 19, 334–339. [Google Scholar] [CrossRef]
- Psallidas, I.; Kanellakis, N.I.; Gerry, S.; Thézénas, M.L.; Charles, P.D.; Samsonova, A.; Schiller, H.B.; Fischer, R.; Asciak, R.; Hallifax, R.J.; et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): A multicohort analysis. Lancet Oncol. 2018, 19, 930–939. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, A.; Robbins, S.; Berthiaume, L.; Michaud, G. Natural History of Asymptomatic Pleural Effusions in Lung Cancer Patients. J. Bronchol. Interv. Pulmonol. 2007, 14, 98–100. [Google Scholar] [CrossRef]
- Porcel, J.M.; Gasol, A.; Bielsa, S.; Civit, C.; Light, R.W.; Salud, A. Clinical features and survival of lung cancer patients with pleural effusions. Respirology 2015, 20, 654–659. [Google Scholar] [CrossRef]
- Grosu, H.B.; Molina, S.; Casal, R.; Song, J.; Li, L.; Diaz-Mendoza, J.; Reddy, C.; Yarmus, L.; Schiavo, D.; Simoff, M.; et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology 2018, 24, 76–82. [Google Scholar] [CrossRef]
- Gordon, C.E.; Feller-Kopman, D.; Balk, E.M.; Smetana, G.W. Pneumothorax following thoracentesis: A systematic review and meta-analysis. Arch. Intern. Med. 2010, 170, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Mercaldi, C.J.; Lanes, S.F. Ultrasound Guidance Decreases Complications and Improves the Cost of Care Among Patients Undergoing Thoracentesis and Paracentesis. Chest 2013, 143, 532–538. [Google Scholar] [CrossRef]
- Lentz, R.J.; Lerner, A.D.; Pannu, J.K.; Merrick, C.M.; Roller, L.; Walston, C.; Valenti, S.; Goddard, T.; Chen, H.; Huggins, J.T.; et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: A multicentre, single-blind randomised controlled trial. Lancet Respir. Med. 2019, 7, 447–455. [Google Scholar] [CrossRef]
- Martin, G.A.; Tsim, S.; Kidd, A.C.; Foster, J.E.; McLoone, P.; Chalmers, A.; Blyth, K.G. Pre-EDIT: A Randomized Feasibility Trial of Elastance-Directed Intrapleural Catheter or Talc Pleurodesis in Malignant Pleural Effusion. Chest 2019, 156, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Judson, M.A.; Doelken, P.; Maldonado, F.; Rahman, N.M.; Huggins, J.T. The Relationship of Pleural Manometry with Postthoracentesis Chest Radiographic Findings in Malignant Pleural Effusion. Chest 2019, 157, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Dipper, A.; Jones, H.; Bhatnagar, R.; Preston, N.; Maskell, N.; Clive, A.O. Interventions for the management of malignant pleural effusions: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 4, CD010529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dresler, C.M.; Olak, J.; Herndon, J.E.; Richards, W.G.; Scalzetti, E.; Fleishman, S.B.; Kernstine, K.H.; Demmy, T.; Jablons, D.M.; Kohman, L.; et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005, 127, 909–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, J.; Montes, J.F.; Villarino, M.A.; Light, R.W.; Garcìa-Valero, J. Influence of Particle Size on Extrapleural Talc Dissemination After Talc Slurry Pleurodesis. Chest 2002, 122, 1018–1027. [Google Scholar] [CrossRef] [Green Version]
- Janssen, J.P.; Collier, G.; Astoul, P.; Tassi, G.F.; Noppen, M.; Rodriguez-Panadero, F.; Loddenkemper, R.; Herth, F.J.; Gasparini, S.; Marquette, C.H.; et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: A prospective cohort study. Lancet 2007, 369, 1535–1539. [Google Scholar] [CrossRef]
- Leemans, J.; Dooms, C.; Ninane, V.; Yserbyt, J. Success rate of medical thoracoscopy and talc pleurodesis in malignant pleurisy: A single-centre experience. Respirology 2018, 23, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.M.; Pepperell, J.; Rehal, S.; Saba, T.; Tang, A.; Ali, N.; West, A.; Hettiarachchi, G.; Mukherjee, D.; Samuel, J.; et al. Effect of Opioids vs NSAIDs and Larger vs Smaller Chest Tube Size on Pain Control and Pleurodesis Efficacy among Patients with Malignant Pleural Effusion: The TIME1 Randomized Clinical Trial. JAMA 2015, 314, 2641–2653. [Google Scholar] [CrossRef]
- Thethi, I.; Ramirez, S.; Shen, W.; Zhang, D.; Mohamad, M.; Kaphle, U.; Kheir, F. Effect of chest tube size on pleurodesis efficacy in malignant pleural effusion: A meta-analysis of randomized controlled trials. J. Thorac. Dis. 2018, 10, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, R.; Piotrowska, H.E.; Laskawiec-Szkonter, M.; Kahan, B.C.; Luengo-Fernandez, R.; Pepperell, J.C.; Evison, M.D.; Holme, J.; Al-Aloul, M.; Psallidas, I.; et al. Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients with Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2020, 323, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Psallidas, I.; Hassan, M.; Yousuf, A.; Duncan, T.; Khan, S.L.; Blyth, K.G.; Evison, M.; Corcoran, J.P.; Barnes, S.; Reddy, R.; et al. Role of thoracic ultrasonography in pleurodesis pathways for malignant pleural effusions (SIMPLE): An open-label, randomised controlled trial. Lancet Respir. Med. 2022, 10, 139–148. [Google Scholar] [CrossRef]
- Okur, E.; Baysungur, V.; Tezel, C.; Ergene, G.; Okur, H.K.; Halezeroglu, S. Streptokinase for malignant pleural effusions: A randomized controlled study. Asian Cardiovasc. Thorac. Ann. 2011, 19, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Saydam, O.; Karapinar, K.; Gokce, M.; Kilic, L.; Metin, M.; Oz, I.I.; Tanriverdi, O. The palliative treatment with intrapleural streptokinase in patients with multiloculated malignant pleural effusion: A double-blind, placebo-controlled, randomized study. Med. Oncol. 2015, 32, 612. [Google Scholar] [CrossRef] [PubMed]
- Lansley, S.M.; Cheah, H.M.; Varano della Vergiliana, J.F.; Chakera, A.; Lee, Y.G. Tissue plasminogen activator potently stimulates pleural effusion via a monocyte chemo-tactic protein-1-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 2015, 53, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Mishra, E.K.; Clive, A.O.; Wills, G.H.; Davies, H.E.; Stanton, A.E.; Al-Aloul, M.; Hart-Thomas, A.; Pepperell, J.; Evison, M.; Saba, T.; et al. Randomized Controlled Trial of Urokinase versus Placebo for Nondraining Malignant Pleural Effusion. Am. J. Respir. Crit. Care Med. 2018, 197, 502–508. [Google Scholar] [CrossRef]
- Van Meter, M.E.M.; McKee, K.Y.; Kohlwes, R.J. Efficacy and Safety of Tunneled Pleural Catheters in Adults with Malignant Pleural Effusions: A Systematic Review. J. Gen. Intern. Med. 2010, 26, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Wilshire, C.L.; Chang, S.C.; Gilbert, C.R.; Akulian, J.A.; AlSarraj, M.K.; Asciak, R.; Bevill, B.T.; Davidson, K.R.; Delgado, A.; Grosu, H.B.; et al. Temporal Trends in Tunneled Pleural Catheter Utilization in Patients with Malignancy: A Multicenter Review. Chest 2021, 159, 2483–2487. [Google Scholar] [CrossRef]
- Putnam, J.B.; Light, R.W.; Rodriguez, R.M.; Ponn, R.; Olak, J.; Pollak, J.S.; Lee, R.B.; Payne, D.K.; Graeber, G.; Kovitz, K.L. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999, 86, 1992–1999. [Google Scholar] [CrossRef]
- Davies, H.E.; Mishra, E.K.; Kahan, B.C.; Wrightson, J.M.; Stanton, A.E.; Guhan, A.; Davies, C.W.; Grayez, J.; Harrison, R.; Prasad, A.; et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: The TIME2 randomized controlled trial. JAMA 2012, 307, 2383–2389. [Google Scholar] [CrossRef]
- Thomas, R.; Fysh, E.T.; Smith, N.A.; Lee, P.; Kwan, B.C.; Yap, E.; Horwood, F.C.; Piccolo, F.; Lam, D.C.; Garske, L.A.; et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients with Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA 2017, 318, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Wahidi, M.M.; Reddy, C.; Yarmus, L.; Feller-Kopman, D.; Musani, A.; Shepherd, R.W.; Lee, H.; Bechara, R.; Lamb, C.; Shofer, S.; et al. Randomized Trial of Pleural Fluid Drainage Frequency in Patients with Malignant Pleural Effusions. The ASAP Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 1050–1057. [Google Scholar] [CrossRef]
- Muruganandan, S.; Azzopardi, M.; Fitzgerald, D.B.; Shrestha, R.; Kwan, B.C.; Lam, D.C.; De Chaneet, C.C.; Ali, M.R.; Yap, E.; Tobin, C.L.; et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via in-dwelling pleural catheters (AMPLE-2): An open-label randomised trial. Lancet Respir. Med. 2018, 6, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, L.; Ip, H.; Rao, D.; Patel, N.; Noorzad, F. Talc pleurodesis through indwelling pleural catheters for malignant pleural effusions: Retrospective case series of a novel clinical pathway. Chest 2014, 146, e190–e194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, R.; Keenan, E.K.; Morley, A.J.; Kahan, B.C.; Stanton, A.E.; Haris, M.; Harrison, R.N.; Mustafa, R.A.; Bishop, L.J.; Ahmed, L.; et al. Outpatient Talc Administration by Indwelling Pleural Catheter for Malignant Effusion. N. Engl. J. Med. 2018, 378, 1313–1322. [Google Scholar] [CrossRef]
- Fitzgerald, D.B.; Muruganandan, S.; Stanley, C.; Badiei, A.; Murray, K.; Read, C.A.; Lee, Y.C.G. EPIToME (Early Pleurodesis via IPC with Talc for Malignant Effusion): Evaluation of a new management algorithm. Eur. Respir. Soc. 2019, 54, OA493. [Google Scholar] [CrossRef]
- Duke University. Randomized Controlled Trial of Talc Instillation in Addition to Daily Drainage through a Tunneled Pleural Catheter to Improve Rates of Outpatient Pleurodesis in Patients with Malignant Pleural Effusion. 2021. Available online: https://ClinicalTrials.gov/show/NCT04792970 (accessed on 21 February 2022).
- Bhatnagar, R.; Zahan-Evans, N.; Kearney, C.; Edey, A.J.; Stadon, L.J.; Tremblay, A.; Maskell, N.A. A Novel Drug-Eluting Indwelling Pleural Catheter for the Management of Malignant Effusions. Am. J. Respir. Crit. Care Med. 2018, 197, 136–138. [Google Scholar] [CrossRef] [Green Version]
- CareFusion, Safety and Effectiveness of a New Pleural Catheter for Symptomatic, Recurrent, MPEs versus Approved Pleural Catheter. 2016. Available online: https://ClinicalTrials.gov/show/NCT02649894 (accessed on 24 February 2022).
- Krochmal, R.; Reddy, C.; Yarmus, L.; Desai, N.R.; Feller-Kopman, D.; Lee, H.J. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J. Thorac. Dis. 2016, 8, 2538–2543. [Google Scholar] [CrossRef] [Green Version]
- Foo, C.T.; Pulimood, T.; Knolle, M.; Marciniak, S.J.; Herre, J. Ambulatory Thoracoscopic Pleurodesis Combined with Indwelling Pleural Catheter in Malignant Pleural Effusion. Front. Surg. 2021, 8, 738719. [Google Scholar] [CrossRef]
- Walker, S.; Mercer, R.; Maskell, N.; Rahman, N.M. Malignant pleural effusion management: Keeping the flood gates shut. Lancet Respir. Med. 2019, 8, 609–618. [Google Scholar] [CrossRef]
- Penz, E.D.; Mishra, E.K.; Davies, H.E.; Manns, B.J.; Miller, R.F.; Rahman, N.M. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest 2014, 146, 991–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiq, M.; Simkovich, S.; Hossen, S.; Feller-Kopman, D. Indwelling Pleural Catheter Drainage Strategy for Malignant Effusion: A Cost-Effectiveness Analysis. Ann. Am. Thorac. Soc. 2020, 17, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Frick, K.D.; Lee, H.; Yarmus, L.; Feller-Kopman, D.J. Management of Malignant Pleural Effusion: A Cost-Utility Analysis. J. Bronchol. Interv. Pulmonol. 2015, 22, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Chrissian, A.A.; Lee, Y.C.G.; Rahman, N.M.; Wahidi, M.M.; Tremblay, A.; Hsia, D.W.; Almeida, F.A.; Shojaee, S.; Mudambi, L.; et al. AABIP Evidence-informed Guidelines and Expert Panel Report for the Management of In-dwelling Pleural Catheters. J. Bronchol. Interv. Pulmonol. 2020, 27, 229–245. [Google Scholar] [CrossRef]
- Sundaralingam, A.; Bedawi, E.O.; Harriss, E.K.; Munnavar, M.; Rahman, N.M. The Frequency, Risk Factors, and Management of Complications from Pleural Procedures. Chest 2021. [Google Scholar] [CrossRef]
- Fysh, E.T.; Tremblay, A.; Feller-Kopman, D.; Mishra, E.K.; Slade, M.; Garske, L.; Clive, A.O.; Lamb, C.; Boshuizen, R.; Ng, B.J.; et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: An international multicenter study. Chest 2013, 144, 1597–1602. [Google Scholar] [CrossRef]
- Gilbert, C.R.; Wahidi, M.M.; Light, R.W.; Rivera, M.P.; Sterman, D.H.; Thomas, R.; Shojaee, S.; Shoham, S.; Psallidas, I.; Ost, D.E.; et al. Management of Indwelling Tunneled Pleural Catheters: A Modified Delphi Consensus Statement. Chest 2020, 158, 2221–2228. [Google Scholar] [CrossRef]
- Faiz, S.A.; Pathania, P.; Song, J.; Li, L.; Balachandran, D.D.; Ost, D.E.; Morice, R.C.; Shannon, V.R.; Bashoura, L.; Eapen, G.A.; et al. Indwelling Pleural Catheters for Patients with Hematologic Malignancies. A 14-Year, Single-Center Experience. Ann. Am. Thorac. Soc. 2017, 14, 976–985. [Google Scholar] [CrossRef]
- Thomas, R.; Budgeon, C.A.; Kuok, Y.J.; Read, C.; Fysh, E.; Bydder, S.; Lee, Y.C.G. Catheter Tract Metastasis Associated with Indwelling Pleural Catheters. Chest 2014, 146, 557–562. [Google Scholar] [CrossRef]
- Frost, N.; Brünger, M.; Ruwwe-Glösenkamp, C.; Raspe, M.; Tessmer, A.; Temmesfeld-Wollbrück, B.; Schürmann, D.; Suttorp, N.; Witzenrath, M. Indwelling pleural catheters for malignancy-associated pleural effusion: Report on a single centre’s ten years of experience. BMC Pulm. Med. 2019, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lui, M.M.S.; Thomas, R.; Lee, Y.C.G. Complications of indwelling pleural catheter use and their management. BMJ Open Respir. Res. 2016, 3, e000123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.; Piccolo, F.; Miller, D.; MacEachern, P.R.; Chee, A.C.; Huseini, T.; Yarmus, L.; Bhatnagar, R.; Lee, H.J.; Feller-Kopman, D.; et al. Intrapleural Fibrinolysis for the Treatment of Indwelling Pleural Catheter-Related Symptomatic Loculations: A Multicenter Observational Study. Chest 2015, 148, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.R.; Wilshire, C.L.; Chang, S.-C.; Gorden, J.A. The Use of Intrapleural Thrombolytic or Fibrinolytic Therapy, or Both, via Indwelling Tunneled Pleural Catheters with or without Concurrent Anticoagulation Use. Chest 2021, 160, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Fysh, E.; Wrightson, J.; Lee, Y.G.; Rahman, N.M. Fractured Indwelling Pleural Catheters. Chest 2012, 141, 1090–1094. [Google Scholar] [CrossRef]
- Matus, I.; Colt, H. Pleural Catheter Fracture During IPC Removal: An Under-reported Complication. J. Bronchol. Interv. Pulmonol. 2021, 28, e1–e3. [Google Scholar] [CrossRef]
- Chalhoub, M.; Saqib, A.; Castellano, M. Indwelling pleural catheters: Complications and management strategies. J. Thorac. Dis. 2018, 10, 4659–4666. [Google Scholar] [CrossRef]
- AMPLE-3: IPC Plus Talc vs VATS in Management of Malignant Pleural Effusion. Available online: https://ClinicalTrials.gov/show/NCT04322136 (accessed on 24 February 2022).
- M.D. Anderson Cancer Center; National Cancer Institute (NCI). Indwelling Pleural Catheters with or without Doxycycline in Treating Patients with Malignant Pleural Effusions. Available online: https://ClinicalTrials.gov/show/NCT03465774 (accessed on 26 February 2022).
- Han, H.-S.; Eom, D.-W.; Kim, J.H.; Kim, K.-H.; Shin, H.-M.; An, J.Y.; Lee, K.M.; Choe, K.H.; Kim, S.T.; Koo, J.H.; et al. EGFR Mutation Status in Primary Lung Adenocarcinomas and Corresponding Metastatic Lesions: Discordance in Pleural Metastases. Clin. Lung Cancer 2011, 12, 380–386. [Google Scholar] [CrossRef]
- Stathopoulos, G.T.; Kalomenidis, I. Malignant pleural effusion: Tumor-host interactions unleashed. Am. J. Respir. Crit. Care Med. 2012, 186, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Murthy, P.; Ekeke, C.N.; Russell, K.L.; Butler, S.C.; Wang, Y.; Luketich, J.D.; Soloff, A.C.; Dhupar, R.; Lotze, M.T. Making cold malignant pleural effusions hot: Driving novel immunotherapies. OncoImmunology 2019, 8, e1554969. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.R.; Taylor, M.D.; Petroni, G.R.; Shu, J.; Burks, S.G.; Daniel, T.M.; Gillenwater, H.H. Phase I Trial of Intrapleural Docetaxel Administered through an Implantable Catheter in Subjects with a Malignant Pleural Effusion. J. Thorac. Oncol. 2010, 5, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, C.; Haas, A.R.; Metzger, S.; Aguilar, L.K.; Aguilar-Cordova, E.; Manzanera, A.G.; Gómez-Hernández, G.; Katz, S.I.; Alley, E.W.; Evans, T.L.; et al. Phase I Study of Intrapleural Gene-Mediated Cytotoxic Immunotherapy in Patients with Malignant Pleural Ef-fusion. Mol. Ther. 2018, 26, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Points | ||
|---|---|---|---|
| Clinical PROMISE Score | Biological PROMISE Score | ||
| Previous chemotherapy | No | 0 | 0 |
| Yes | 4 | 3 | |
| Previous radiotherapy | No | 0 | 0 |
| Yes | 2 | 2 | |
| Hemoglobin (g/dL) | ≥16 | 0 | 0 |
| 14 to <16 | 1 | 1 | |
| 12 to <14 | 2 | 2 | |
| 10 to <12 | 3 | 3 | |
| <10 | 4 | 4 | |
| Serum white blood cell count (10⁹ cells/L) | <4 | 0 | 0 |
| 4 to <6.3 | 2 | 2 | |
| 6.3 to <10 | 4 | 4 | |
| 10 to <15.8 | 7 | 7 | |
| ≥15.8 | 10 | 9 | |
| C-reactive protein (IU/L) | <3 | 0 | 0 |
| 3 to <10 | 3 | 3 | |
| 10 to <32 | 5 | 5 | |
| 32 to <100 | 8 | 8 | |
| ≥100 | 11 | 10 | |
| ECOG performance status | 0–1 | 0 | 0 |
| 2–4 | 7 | 7 | |
| Cancer type | Mesothelioma | 0 | 0 |
| All other types of cancer | 4 | 5 | |
| Lung | 5 | 6 | |
| TIMP1 (ng/mg protein) | <40 | n/a | 0 |
| 40 to <160 | n/a | 1 | |
| ≥160 | n/a | 2 | |
| Total score and corresponding 3-month mortality | |||
| Group (3-month mortality) | Clinical score | Biological score | |
| A: <25% | 0–20 | 0–20 | |
| B: 25% to <50% | 21–27 | 21–28 | |
| C: 50% to <75% | 28–35 | 29–37 | |
| D: ≥75% | >35 | >37 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, B.; Sheikh, G.; Youness, H.A.; Keddissi, J.I.; Abdo, T. Diagnosis and Management of Malignant Pleural Effusion: A Decade in Review. Diagnostics 2022, 12, 1016. https://doi.org/10.3390/diagnostics12041016
Jacobs B, Sheikh G, Youness HA, Keddissi JI, Abdo T. Diagnosis and Management of Malignant Pleural Effusion: A Decade in Review. Diagnostics. 2022; 12(4):1016. https://doi.org/10.3390/diagnostics12041016
Chicago/Turabian StyleJacobs, Blake, Ghias Sheikh, Houssein A. Youness, Jean I. Keddissi, and Tony Abdo. 2022. "Diagnosis and Management of Malignant Pleural Effusion: A Decade in Review" Diagnostics 12, no. 4: 1016. https://doi.org/10.3390/diagnostics12041016
APA StyleJacobs, B., Sheikh, G., Youness, H. A., Keddissi, J. I., & Abdo, T. (2022). Diagnosis and Management of Malignant Pleural Effusion: A Decade in Review. Diagnostics, 12(4), 1016. https://doi.org/10.3390/diagnostics12041016






