Parkinson’s Disease Symptoms Associated with Developing On-State Axial Symptoms Early after Subthalamic Deep Brain Stimulation
Abstract
:1. Introduction
2. Patients and Methods
Statistical Analysis
3. Results
4. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hely, M.A.; Morris, J.G.; Reid, W.G.; Trafficante, R. Sydney Multicenter Study of Parkinson’s disease: Non-L-dopa-responsive problems dominate at 15 years. Mov. Disord. 2005, 20, 190–199. [Google Scholar] [CrossRef]
- Kulisevsky, J.; Luquin, M.R.; Arbelo, J.M.; Burguera, J.A.; Carrillo, F.; Castro, A.; Chacón, J.; García-Ruiz, P.J.; Lezcano, E.; Mir, P.; et al. Advanced Parkinson’s disease: Clinical characteristics and treatment (part 1). Neurología 2013, 28, 503–521. [Google Scholar] [CrossRef]
- Bronstein, J.M.; Tagliati, M.; Alterman, R.L.; Lozano, A.M.; Volkmann, J.; Stefani, A.; Horak, F.B.; Okun, M.S.; Foote, K.D.; Krack, P.; et al. Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Arch. Neurol. 2011, 68, 165. [Google Scholar] [CrossRef]
- Sesar, Á.; Fernández-Pajarín, G.; Ares, B.; Relova, J.L.; Arán, E.; Rivas, M.T.; Gelabert-González, M.; Castro, A. Continuous subcutaneous apomorphine in advanced Parkinson’s disease patients treated with deep brain stimulation. J. Neurol. 2019, 266, 659–666. [Google Scholar] [CrossRef]
- Tard, C.; Delval, A.; Devos, D.; Lopes, R.; Lenfant, P.; Dujardin, K.; Hossein-Foucher, C.; Semah, F.; Duhamel, A.; Defebvre, L.; et al. Brain metabolic abnormalities during gait with freezing in Parkinson’s disease. Neuroscience 2015, 307, 281–301. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Romenets, S.R.; Anang, J.B.; Latreille, V.; Gagnon, J.F.; Postuma, R.B. New clinical subtypes of Parkinson disease and their longitudinal progression. A prospective cohort comparison with other phenotypes. JAMA Neurol. 2015, 72, 863–873. [Google Scholar] [CrossRef]
- De Pablo-Fernández, E.; Lees, A.J.; Holton, J.L.; Warner, T.T. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol. 2019, 76, 470–479. [Google Scholar] [CrossRef]
- Kempster, P.A.; O’Sullivan, S.S.; Holton, J.L.; Revesz, T.; Lees, A.J. Relationships between age and late progression of Parkinson’s disease: A clinico-pathological study. Brain 2010, 133, 1755–1762. [Google Scholar] [CrossRef]
- Kempster, P.A.; Williams, D.R.; Selikhova, M.; Holton, J.; Revesz, T.; Lees, A.J. Patterns of levodopa response in Parkinson’s disease: A clinico-pathological study. Brain 2007, 130, 2123–2128. [Google Scholar] [CrossRef] [Green Version]
- Selikhova, M.; Williams, D.R.; Kempster, P.A.; Holton, J.L.; Revesz, T.; Lees, A.J. A clinico-pathological study of subtypes in Parkinson’s disease. Brain 2009, 132, 2947–2957. [Google Scholar] [CrossRef] [Green Version]
- Halliday, G.; Hely, M.; Reid, W.; Morris, J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol. 2008, 115, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.R.; David, R.V.; Lord, S.; Galna, B.; Burn, D.J. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain 2012, 135, 2779–2788. [Google Scholar]
- Morris, R.; Lord, S.; Lawson, R.A.; Coleman, S.; Galna, B.; Duncan, G.W.; Khoo, T.K.; Yarnall, A.J.; Burn, D.J.; Rochester, L. Gait Rather than Cognition Predicts Decline in Specific Cognitive Domains in Early Parkinson’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1656–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohnen, N.I.; Frey, K.A.; Studenski, S.; Kotagal, V.; Koeppe, R.A.; Scott, P.J.H.; Albin, R.L.; Müller, M.L.T.M. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 2013, 81, 1611–1616. [Google Scholar] [CrossRef] [Green Version]
- Schlenstedt, C.; Shalash, A.; Muthuraman, M.; Falk, D.; Witt, K.; Deuschl, G. Effect of high-frequency subthalamic neurostimulation on gait and freezing of gait in Parkinson’s disease: A systematic review and meta-analysis. Eur. J. Neurol. 2017, 24, 18–26. [Google Scholar] [CrossRef]
- Kim, R.; Kim, H.J.; Shin, C.; Park, H.; Kim, A.; Paek, S.H.; Jeon, B. Long-term effect of subthalamic nucleus deep brain stimulation on freezing of gait in Parkinson’s disease. J. Neurosurg. 2019, 131, 1797–1804. [Google Scholar] [CrossRef]
- Barbe, M.T.; Tonder, L.; Krack, P.; Debû, B.; Schüpbach, M.; Paschen, S.; Dembek, T.A.; Kühn, A.A.; Fraix, V.; Brefel-Courbon, C.; et al. Deep Brain Stimulation for Freezing of Gait in Parkinson’s Disease with Early Motor Complications. Mov. Disord. 2020, 35, 82–90. [Google Scholar] [CrossRef]
- Moreau, C.; Defebvre, L.; Destée, A.; Bleuse, S.; Clement, F.; Blatt, J.L.; Krystkowiak, P.; Devos, D. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 2008, 71, 80–84. [Google Scholar] [CrossRef]
- Brozova, H.; Barnaure, I.; Alterman, R.L.; Tagliati, M. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 2009, 72, 770. [Google Scholar] [CrossRef]
- Hamani, C.; Moro, E.; Lozano, A.M. The pedunculopontine nucleus as a target for deep brain stimulation. J. Neural. Transm. 2011, 118, 1461–1468. [Google Scholar] [CrossRef]
- Thevathasan, W.; Debu, B.; Aziz, T.; Bloem, B.R.; Blahak, C.; Butson, C.; Czernecki, V.; Foltynie, T.; Fraix, V.; Grabli, D.; et al. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: A clinical review. Mov. Disord. 2013, 33, 10–20. [Google Scholar] [CrossRef]
- Weiss, D.; Walach, M.; Meisner, C.; Fritz, M.; Scholten, M.; Breit, S.; Plewnia, C.; Bender, B.; Gharabaghi, A.; Wächter, T.; et al. Nigral stimulation for resistant axial motor impairment in Parkinson’s disease? A randomised controlled trial. Brain 2013, 136, 2098–2108. [Google Scholar] [CrossRef] [Green Version]
- Valldeoriola, F. Simultaneous low-frequency deep brain stimulation of the substantia nigra pars reticulata and high-frequency stimulation of the subthalamic nucleus to treat levodopa unresponsive freezing of gait in Parkinson’s disease: A pilot study. Parkinsonism Relat. Disord. 2019, 63, 231. [Google Scholar] [CrossRef]
- Defer, G.L.; Widner, H.; Marie, R.M.; Remy, P.; Levivier, M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov. Disord. 1999, 14, 572–584. [Google Scholar] [CrossRef]
- Ricchi, V.; Zibetti, M.; Angrisano, S.; Merola, A.; Arduino, N.; Artusi, C.A.; Rizzone, M.; Lopiano, L.; Lanotte, M. Transient effects of 80 Hz stimulation on gait in STN DBS treated PD patients: A 15 months follow-up study. Brain Stimul. 2012, 5, 388–392. [Google Scholar] [CrossRef]
- Sidiropoulos, C.; Walsh, R.; Meaney, C.; Poon, Y.Y.; Fallis, M.; Moro, E. Low-frequency subthalamic nucleus deep brain stimulation for axial symptoms in advanced Parkinson’s disease. J. Neurol. 2013, 260, 2306–2311. [Google Scholar] [CrossRef]
- Zibetti, M.; Moro, E.; Krishna, V.; Sammartino, F.; Picillo, M.; Munhoz, R.P.; Lozano, A.M.; Fasano, A. Low-frequency Subthalamic Stimulation in Parkinson’s Disease: Long-term Outcome and Predictors. Brain Stimul. 2016, 9, 774–779. [Google Scholar] [CrossRef]
- Schrag, A.; Dodel, R.; Spottke, A.; Bornschein, B.; Siebert, U.; Quinn, N.P. Rate of clinical progression in Parkinson’s disease. A prospective study. Mov. Disord. 2007, 22, 938–945. [Google Scholar] [CrossRef]
- Evans, J.R.; Mason, S.L.; Williams-Gray, C.H.; Foltynie, T.; Brayne, C.; Robbins, T.W.; Barker, R.A. The natural history of treated Parkinson’s disease in an incident, community based cohort. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1112–1118. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Tang, B.S.; Guo, J.F. Parkinson’s Disease and Cognitive Impairment. Park. Dis. 2016, 2016, 6734678. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef]
- Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Rochester, L.; Galna, B.; Lord, S.; Yarnall, A.J.; Morris, R.; Duncan, G.; Khoo, T.K.; Mollenhauer, B.; Burn, D.J. Decrease in Alpha Beta 1–42 predicts dopa-resistant gait progression in early Parkinson’s disease. Neurology 2017, 88, 1501–1511. [Google Scholar] [CrossRef] [Green Version]
- Picillo, M.; Lozano, A.M.; Kou, N.; Puppi Munhoz, R.; Fasano, A. Programming Deep Brain Stimulation for Parkinson’s Disease: The Toronto Western Hospital Algorithms. Brain Stimul. 2016, 9, 425–437. [Google Scholar] [CrossRef]
- Ramdhani, R.A.; Patel, A.; Swope, D.; Kopell, B.H. Early Use of 60 Hz Frequency Subthalamic Stimulation in Parkinson’s Disease: A Case Series and Review. Neuromodulation 2015, 18, 664–669. [Google Scholar] [CrossRef]
- Di Giulio, I.; Kalliolia, E.; Georgiev, D.; Peters, A.L.; Voyce, D.C.; Akram, H.; Foltynie, T.; Limousin, P.; Day, B.L. Chronic Subthalamic Nucleus Stimulation in Parkinson’s Disease: Optimal Frequency for Gait Depends on Stimulation Site and Axial Symptoms. Front. Neurol. 2019, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.M.; Kishima, H.; Hosomi, K.; Maruo, T.; Tani, N.; Oshino, S.; Shimokawa, T.; Yokoe, M.; Mochizuki, H.; Saitoh, Y.; et al. Low-frequency subthalamic nucleus stimulation in Parkinson’s disease: A randomised clinical trial. Mov. Disord. 2014, 29, 270–274. [Google Scholar] [CrossRef]
- Kumar, R.; Johnson, L. Managing Parkinson’s disease patients with deep brain stimulation. In Deep Brain Stimulation Management; Marks, W.J., Ed.; Cambridge Universty Press: Cambrdge, UK, 2010; pp. 62–82. [Google Scholar]
- Keitel, A.; Ferrea, S.; Südmeyer, M.; Schnitzler, A.; Wojtecki, L. Expectation modulates the effect of deep brain stimulation on motor and cognitive function in tremor-dominant Parkinson’s disease. PLoS ONE 2013, 8, e81878. [Google Scholar] [CrossRef]
- Pollo, A.; Torre, E.; Lopiano, L.; Rizzone, M.; Lanotte, M.; Cavanna, A.; Bergamasco, B.; Benedetti, F. Expectation modulates the response to subthalamic nucleus stimulation in Parkinsonian patients. Neuroreport 2002, 13, 1383–1386. [Google Scholar] [CrossRef]
- Mercado, R.; Constantoyannis, C.; Mandat, T.; Kumar, A.; Schulzer, M.; Stoessl, A.J.; Honey, C.R. Expectation and the placebo effect in Parkinson’s disease patients with subthalamic nucleus deep brain stimulation. Mov. Disord. 2006, 21, 1457–1461. [Google Scholar] [CrossRef]
- Keitel, A.; Wojtecki, L.; Hirschmann, J.; Hartmann, C.J.; Ferrea, S.; Südmeyer, M.; Schnitzler, A. Motor and cognitive placebo-/nocebo-responses in Parkinson’s disease patients with deep brain stimulation. Behav. Brain Res. 2013, 250, 199–205. [Google Scholar] [CrossRef]
- Castrioto, A.; Lozano, A.M.; Poon, Y.Y.; Lang, A.E.; Fallis, M.; Moro, E. Ten-year outcome of subthalamic stimulation in Parkinson disease: A blinded evaluation. Arch. Neurol. 2011, 68, 1550–1556. [Google Scholar] [CrossRef] [Green Version]
- Hamani, C.; Richter, E.; Schwalb, J.M.; Lozano, A.M. Bilateral subthalamic nucleus stimulation for Parkinson’s disease: A systematic review of the clinical literature. Neurosurgery 2005, 56, 1313–1324. [Google Scholar] [CrossRef]
- Rodriguez-Oroz, M.C.; Moro, E.; Krack, P. Long-term outcomes of surgical therapies for Parkinson’s disease. Mov. Disord. 2012, 27, 1718–1728. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A.; Aquino, C.C.; Krauss, J.K.; Honey, C.R.; Bloem, B.R. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat. Rev. Neurol. 2015, 11, 98–110. [Google Scholar] [CrossRef]
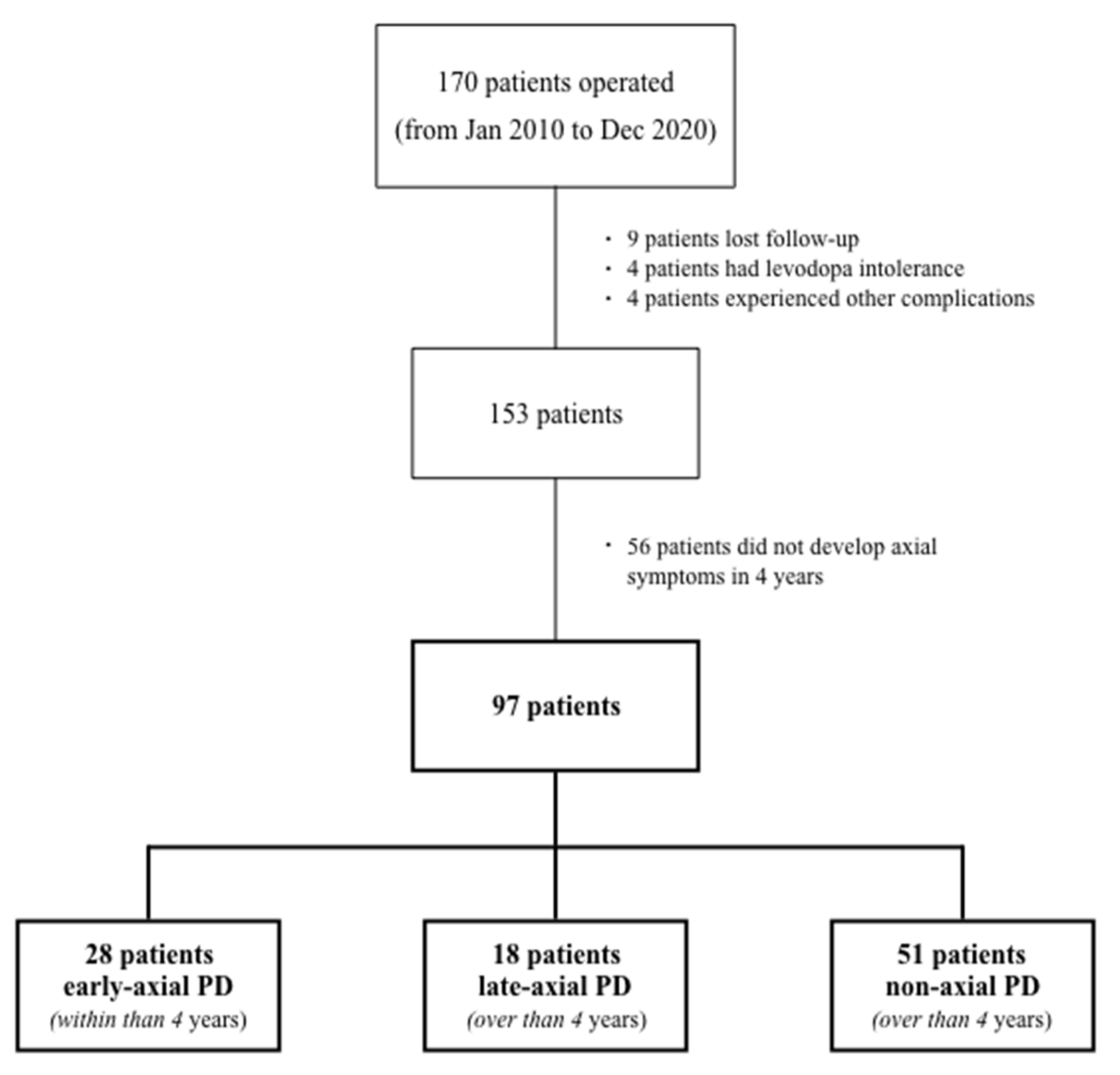
| Non-Axial PD (n = 51) | Early-Axial PD (n = 28) | Late-Axial PD (n = 18) | |
|---|---|---|---|
| Sex (male) | 24 (47.1%) | 16 (57.1%) | 10 (55.6%) |
| Hypertension | 10 (19.6%) | 6 (21.4%) | 5 (27.8%) |
| Age at STN-DBS a | 58.3 ± 8.7 | 62.5 ± 5.6 | 61.1 ± 8.7 |
| Time since PD diagnosis (y) | 10.2 ± 3.6 | 9.9 ± 4.7 | 10.6 ± 3.3 |
| LEDD (mg) | 1348 ± 495 | 1432 ± 337 | 1485 ± 600 |
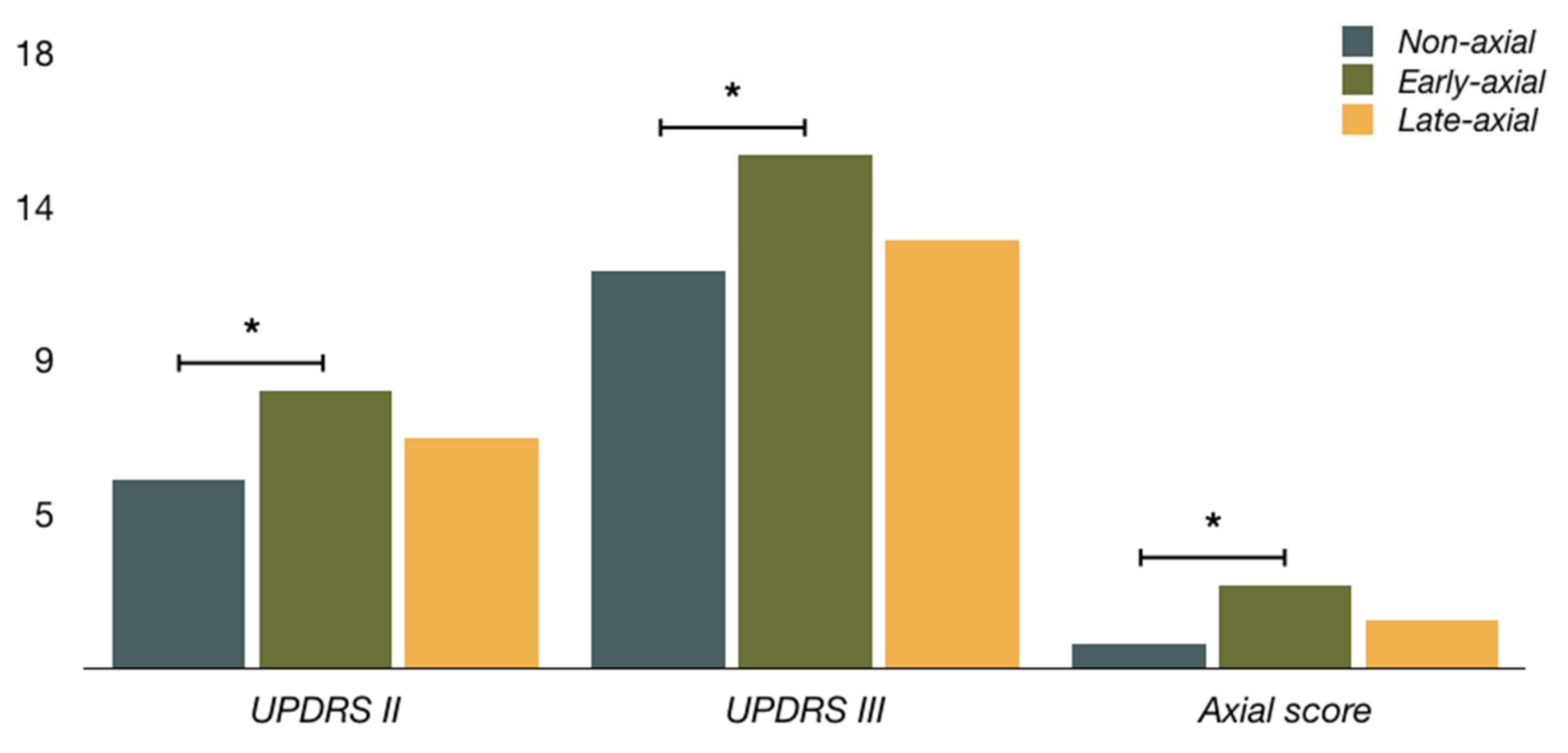
| UPDRS II on meds a | 5.5 ± 4.1 | 8.1 ± 3.9 | 6.7 ± 3.4 |
| Falls | 0.1 ± 0.4 | 0.3 ± 0.6 | 0.2 ± 0.5 |
| Freezing b | 0.2 ± 0.5 | 0.5 ± 0.6 | 0.1 ± 0.3 |
| Gait a,b | 0.4 ± 0.6 | 1.0 ± 0.5 | 0.6 ± 0.5 |
| UPDRS III off meds | 37.7 ± 11.3 | 40.4 ± 11.6 | 40.4 ± 9.9 |
| UPDRS III on meds a | 11.6 ± 6.2 | 15.0 ± 5.6 | 12.5 ± 4.9 |
| UPDRS III (i) a | 0.70 ± 0.11 | 0.62 ± 0.12 | 0.69 ± 0.12 |
| Tremor score c | 0.8 ± 1.3 | 0.8 ± 2.0 | 0.3 ± 0.6 |
| Axial score a | 0.7 ± 1.0 | 2.4 ± 1.8 | 1.4 ± 1.9 |
| Time to axial PD (m) | - | 28.1 ± 12.5 | 75.3 ± 19.2 |
| Follow-up (m) | 75.0 ± 19.7 | 72.9 ± 29.9 | 100.1 ± 17.4 |
| Non-Axial PD (n = 51) | Early-Axial PD (n = 28) | Late-Axial PD (n = 18) | |
|---|---|---|---|
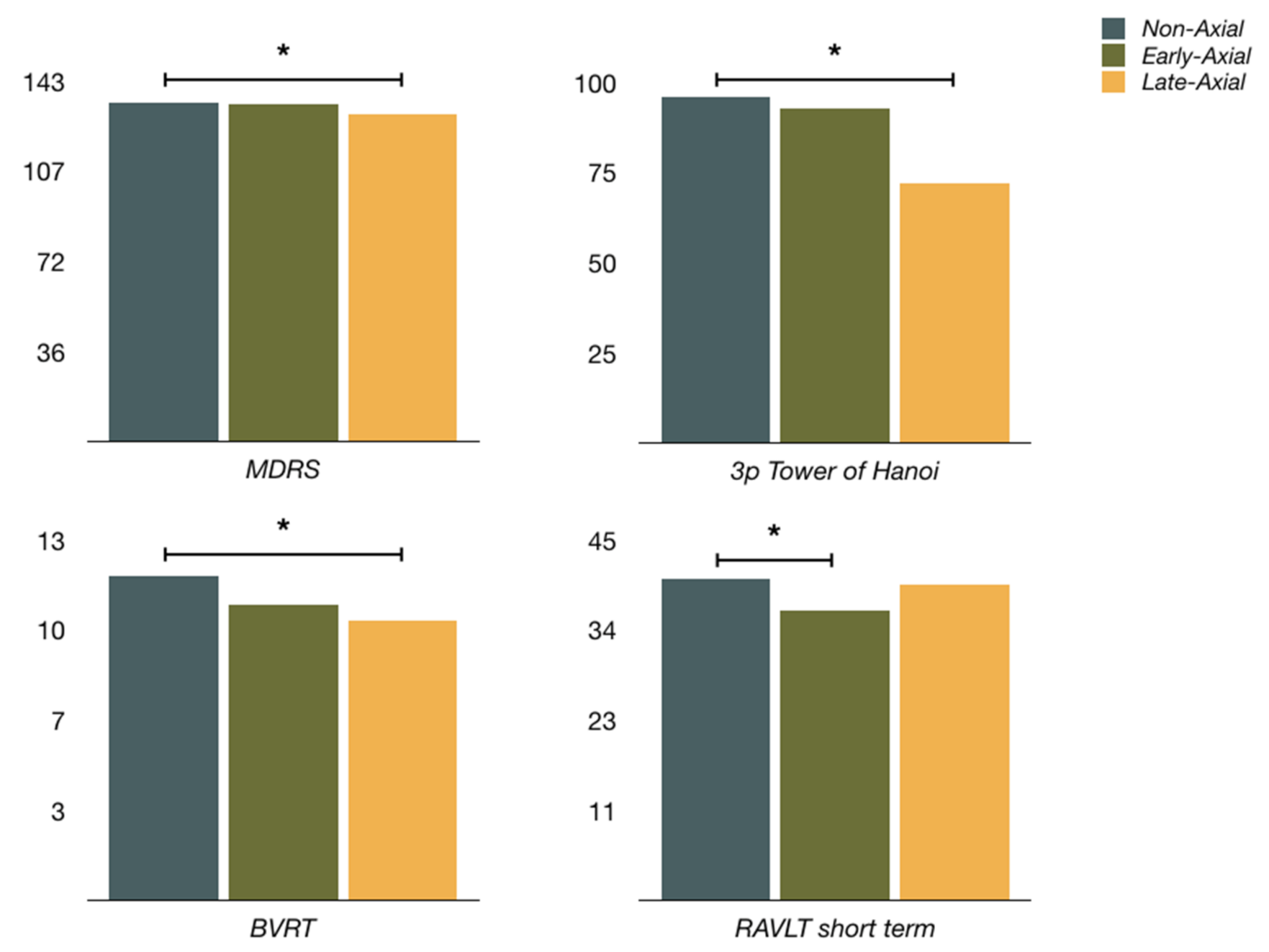
| MDRS c | 134.4 ± 6.2 | 134.1 ± 5.5 | 130.3 ± 7.5 |
| RAVLT (short-term recall) a | 40.3 ± 9.3 | 36.3 ± 7.6 | 39.5 ± 10.6 |
| RAVLT (long-term recall) | 8.3 ± 3.4 | 7.1 ± 2.9 | 7.7 ± 3.4 |
| 3p Tower of Hanoi *,c | 49 (96.1%) | 26 (92.9%) | 13 (72.2%) |
| BVRT **,c | 11.7 ± 2.1 (n = 49) | 10.7 ± 2.7 (n = 27) | 10.1 ± 2.6 (n = 18) |
| BJLO ** | 24.0 ± 4.1 (n = 46) | 22.2 ± 6.1 (n = 26) | 22.7 ± 5.8 (n = 17) |
| WAIS-digits ** | 8.8 ± 2.8 (n = 42) | 9.3 ± 4.3 (n = 25) | 7.1 ± 2.9 (n = 16) |
| Phonetic Fluency | 13.9 ± 6.4 | 12.6 ± 4.7 | 11.9 ± 5.2 |
| Semantic Fluency | 18.9 ± 6.9 | 16.8 ± 4.7 | 16.4 ± 6.0 |
| YDS ** | 10.9 ± 6.1 (n = 33) | 14.5 ± 7.4 (n = 19) | 13.6 ± 8.7 (n = 5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Pajarín, G.; Sesar, Á.; Relova, J.L.; Ares, B.; Jiménez, I.; Gelabert-González, M.; Arán, E.; Castro, A. Parkinson’s Disease Symptoms Associated with Developing On-State Axial Symptoms Early after Subthalamic Deep Brain Stimulation. Diagnostics 2022, 12, 1001. https://doi.org/10.3390/diagnostics12041001
Fernández-Pajarín G, Sesar Á, Relova JL, Ares B, Jiménez I, Gelabert-González M, Arán E, Castro A. Parkinson’s Disease Symptoms Associated with Developing On-State Axial Symptoms Early after Subthalamic Deep Brain Stimulation. Diagnostics. 2022; 12(4):1001. https://doi.org/10.3390/diagnostics12041001
Chicago/Turabian StyleFernández-Pajarín, Gustavo, Ángel Sesar, José Luis Relova, Begoña Ares, Isabel Jiménez, Miguel Gelabert-González, Eduardo Arán, and Alfonso Castro. 2022. "Parkinson’s Disease Symptoms Associated with Developing On-State Axial Symptoms Early after Subthalamic Deep Brain Stimulation" Diagnostics 12, no. 4: 1001. https://doi.org/10.3390/diagnostics12041001
APA StyleFernández-Pajarín, G., Sesar, Á., Relova, J. L., Ares, B., Jiménez, I., Gelabert-González, M., Arán, E., & Castro, A. (2022). Parkinson’s Disease Symptoms Associated with Developing On-State Axial Symptoms Early after Subthalamic Deep Brain Stimulation. Diagnostics, 12(4), 1001. https://doi.org/10.3390/diagnostics12041001






