Abstract
Background: We assessed the SARS-CoV-2 reinfection rate in a large patient cohort, and evaluated the effect of varying time intervals between two positive tests on assumed reinfection rates using viral load data. Methods: All positive SARS-CoV-2 samples collected between 1 March 2020 and 1 August 2021 from a laboratory in the region Kennemerland, the Netherlands, were included. The reinfection rate was analyzed using different time intervals between two positive tests varying between 2 and 16 weeks. SARS-CoV-2 PCR crossing point (Cp) values were used to estimate viral loads. Results: In total, 679,513 samples were analyzed, of which 53,366 tests (7.9%) were SARS-CoV-2 positive. The number of reinfections varied between 260 (0.52%) for an interval of 2 weeks, 89 (0.19%) for 4 weeks, 52 (0.11%) for 8 weeks, and 37 (0.09%) for a minimum interval of 16 weeks between positive tests. The median Cp-value (IQR) in the second positive samples decreased when a longer interval was chosen, but stabilized from week 8 onwards. Conclusions: Although the calculated reinfection prevalence was relatively low (0.11% for the 8-week time interval), choosing a different minimum interval between two positive tests resulted in major differences in reinfection rates. As reinfection Cp-values stabilized after 8 weeks, we hypothesize this interval to best reflect novel infection rather than persistent shedding.
1. Introduction
Since the start of the COVID-19 pandemic in early 2020, SARS-CoV-2 was first expected to induce a monophasic disease with at least transient immunity [1]. However, several reports of SARS-CoV-2 reinfections have been reported since [2], which raised critical questions about how well a first infection protects against reinfection [3,4,5,6]. In addition, the occurrence of SARS-CoV-2 reinfection has implications for epidemiological modelling and public health policies with respect to the distribution of (booster) vaccines, and the influence of circulating new genetic variants of SARS-CoV-2 [7,8,9].
Studies conducted to address the incidence of SARS-CoV-2 reinfections are scarce, with most studies reporting single cases or small case series [6,7,10]. The few larger cohort studies that assessed the incidence of reinfection with SARS-CoV-2 estimated a reinfection rate ranging from 0.10% to 0.26% during varying follow-up periods of 2–9 months [11,12,13]. The interpretation of these reports of SARS-CoV-2 reinfections is hampered by the methodological inconsistencies between studies. For instance, to distinct true reinfections from prolonged viral shedding, viral loads of the first and second positive test may be helpful. However, SARS-CoV-2 viral loads in respiratory samples lack comparability of Ct- or Cp-values derived from different laboratories, as these are assay- and method-specific [14]. This issue complicates the evaluation of SARS-CoV-2 viral loads in respiratory samples derived from potential reinfection patients when multiple laboratories are involved in analyzing these samples, which is often the case [13]. It should also be noted that most studies use different definitions of reinfection: though some choose a stringent definition including the requirement of a negative SARS-CoV-2 PCR result between two positive tests, reporting of COVID-19-related symptoms during both episodes, a minimal viral load, and evidence of genotypic variance between the two viral strains [7], others use only a selection of these [15]. The major difference between studies, though, seems to be the chosen minimum time interval between two positive tests, which ranges from 8 to 82 days between studies [2,7], and which is likely to have a large influence on the reported prevalence of reinfection. One of the reasons for including a time interval criterion is to exclude patients who show prolonged viral shedding, which is thought to disappear after 28 days in most COVID-19 cases [16], but has occasionally been demonstrated up to 63 days after symptom onset [17].
The aim of this study was to assess the incidence of reinfection with SARS-CoV-2, to evaluate the effect of varying time intervals between two positive tests on assumed reinfection rates, and to incorporate viral load data of both the first and second positive test in these analyses in a large cohort of SARS-CoV-2-positive patients tested in a single large regional laboratory in the Netherlands. Thus, we set out to provide an evidence-based time interval to be incorporated in the definition of SARS-CoV-2 reinfection.
2. Methods
2.1. Setting, Study Design and Participants
The Regional Public Health Laboratory Kennemerland, Haarlem, the Netherlands, performs SARS-CoV-2 RT-PCR testing for over 800,000 inhabitants, including health care workers (HCW), patients of four large teaching hospitals, patients of more than 600 GPs, 90 nursing home organizations, and those who are tested because of the presence of symptoms in public health testing facilities set up by the Public Health Services Kennemerland (PHS Kennemerland) and Hollands Noorden (PHS Hollands Noorden). Data from all SARS-CoV-2 RT-PCR results from nasopharyngeal (NP), oropharyngeal (OP), and combined swabs collected between 1 March 2020 and 1 August 2021 were analyzed in the present study. For the patients with more than one positive test result obtained at least one week apart, we retrieved the age, the dates of both positive tests, and the Cp-value of both positive tests from the electronic patient records of the Public Health Laboratory Kennemerland.
2.2. Assays
The SARS-CoV-2 RT-PCR used, which is based on the presence of the E-gene [18], was carried out after a lysis step of the samples. For all swabs, crossing-point (Cp)-values were calculated on Lightcycler 480 1.5.1 software (Roche diagnostics, Basel, Switzerland). We used sequencing data of the Dutch National Institute for Public Health and the Environment to estimate the circulation of specific viral variants in the population during our study period [19].
2.3. Statistical Analysis
Prevalence of reinfection was calculated using four different time intervals between two positive SARS-CoV-2 tests: 2 weeks; 4 weeks; 8 weeks; and 16 weeks. Continuous variables were presented as median with interquartile ranges (IQR), and categorical variables as numbers with percentages. Statistical analyses were performed with R and RStudio (R v 4.0.3), with packages tidyverse and tidymodels.
3. Results
In total, 679,513 samples were collected from 473,411 unique patients, of which 53,366 (7.9%) tests derived from 51,484 unique patients were positive for SARS-CoV-2. In Figure 1, the weekly number of positive tests is presented, as well the cumulative number of reinfections for each chosen time interval. In addition, the proportion of circulating viral variants in the Netherlands during the study period is shown (in background colors). These data show a gradual increase in the number of reinfections over time, with an acceleration in the last weeks when the Delta variant became dominant.
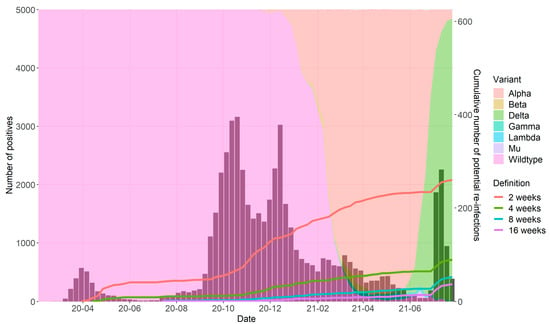
Figure 1.
Cumulative number of reinfections according to different time intervals between two positive tests.
Each colored line corresponds to one specific minimum time interval between two positive tests (2–16 weeks). Bars represent the number of reported SARS-CoV-2 positive tests per week. Background colors show the proportion of the circulating viral variant during the study period.
Table 1 shows, per time interval chosen, the number of reinfections, median age of patients, and Cp-values (indicating viral load) of the first and second positive tests. The number of reinfections decreased from 260 (0.52%) reinfections for a minimum interval of 2 weeks, to 37 (0.09%) reinfections for an interval of 16 weeks. The median age (IQR) of the patients decreased considerably when a longer time interval was chosen: 69.0 (33.5) years for the 2-week interval vs. 24.0 (30.0) years for the 16-week interval. The median Cp-value (IQR) in the second positive samples decreased (reflecting higher viral loads) when a longer interval was chosen: Cp-value 34.1 (6.1) for the 2-week interval vs. 28.7 (6.5) for the 16-week interval.

Table 1.
Number of reinfections according to different definitions.
In Figure 2, the median Cp-values are presented for the first and second positive RT-PCR tests according to reinfection definitions (ranging from 1 to 20 weeks). Whereas 684 “reinfection” cases were included using a minimum of 1 week between positive tests, this number decreased when using stricter definitions (Supplementary Table S1). The first positive PCR had a similar median Cp-value in all case definitions. However, the second positive PCR showed a high median Cp-value when a short interval between positive PCRs was used to define reinfection, suggesting a significant contribution from the detection of persistent shedding (with low viral loads). The Cp-value of the second PCR stabilized when at least 8 weeks between positives was used to define cases, suggesting persistent shedding no longer explained the second positive PCR. Reinfections defined by at least 8 weeks between positive PCRs consistently had higher median Cp-values at the time of reinfection compared to the first episode, indicating lower viral loads than during the first infection.
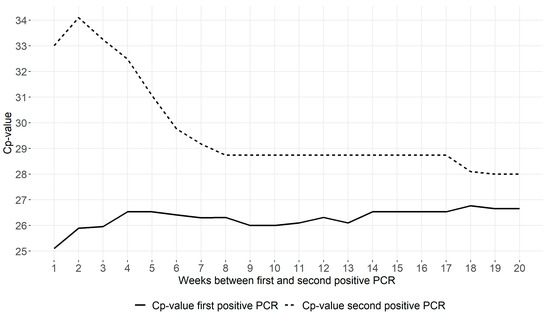
Figure 2.
Median Cp-values of first and second positive RT-PCR tests of reinfection cases according to different time intervals between two positive tests.
The solid line corresponds to the median Cp-value of the first positive test of reinfection patients for increasing minimum time intervals between two positive tests (ranging from 1 to 20 weeks). The dotted line corresponds to the median Cp-value of the second positive test of these reinfection patients.
4. Discussion
Our study shows that it is necessary to establish a consensus on the definition of SARS-CoV-2 reinfection, as this has implications for epidemiological modelling and public health policies [13]. Depending on the time interval chosen, the percentage of reinfection varied between 0.09% and 0.52%. The calculated reinfection prevalence for the 8-week time interval (0.11%) is in line with previous studies with comparable follow-up [11,12,13].
In previous studies, a standardized time interval between two positive tests to define reinfections was lacking [7,11,12,13,15], and a wide range of reinfection rates were reported [11,12,13]. This could possibly (at least partly) be explained by a phenomenon called “prolonged shedding”. In some patients, traces of the viral RNA are detectable for a relatively long period after onset of symptoms, sometimes even when the symptoms have disappeared [17]. Our data showed increasing viral loads in the second test sample when the time interval to define a reinfection was extended, with a plateau in median viral load of an approximate Cp-value of 28.7 reached at 8 weeks since the first positive test. This implicates that the preferred time interval between two positive tests should be at least 8 weeks, in line with previous observations [13], and as suggested by international guidelines [20,21,22].
One of the limitations of our study concerns the possibility of underreporting of reinfections in our population. Large scale public health test facilities became available in the Netherlands in May 2020. During the first COVID-19 wave in the Netherlands, testing was mainly available for health care workers [23]. Therefore, the majority of the SARS-CoV-2 infections that occurred between the start of the pandemic in February 2020 and June 2020 were not registered, and reinfections were not recognized if patients were tested positive in the following months. Another potential reason for underreporting is the fact that secondary infections might be less often reported. Patients who recover from a primary SARS-CoV-2 infection may be less likely to have themselves tested when they develop respiratory symptoms again, assuming that they are immune for SARS-CoV-2. Finally, it should be noted that not all of the collected samples in the regions Kennemerland and Hollands Noorden were analyzed at the Regional Public Health Laboratory Kennemerland and registered in our database. This could have led to an underestimation of the reinfection prevalence in our population. Also, patients might have tested positive outside our region. Although this may have affected the reinfection rates in our study (and comparability with other studies), it does not affect our finding that the preferred time interval between two positive tests should be at least 8 weeks.
Unfortunately, no clinical details were available for the cases, including data on immunocompromising underlying conditions of participants or their vaccination status. This limited the possibility to assess the influence of these factors on the occurrence of re-infection in individual patients, and to accurately distinguish between prolonged shedding and relapse of re-infection cases. In addition, no sequencing results were available for individual samples, making it difficult to establish full certainty of reinfection. However, as (re-)infections can be asymptomatic, clinical details alone do not provide sufficient evidence for reinfection. Sequencing can be performed in individual cases to ascertain that a reinfection is caused by a different variant. This was, however, not feasible on a large scale in our public health setting. However, to the best of our knowledge, we provide the largest dataset on reinfections and Cp-values (or viral loads) performed with a single PCR-technique, enabling comparison between samples. Also, we aimed to establish a simple time-interval-based definition that can be used in all labs that perform SARS-CoV-2 PCR testing, regardless of the availability of rapid sequencing or clinical information.
In conclusion, it is of major importance to establish a consensus on the definition of SARS-CoV-2 reinfections, as the influence of waning immunity, and the circulating and spreading of new SARS-CoV-2 variants on reinfection rates can only be valued when a uniform definition is used between studies. Based on our data, we believe that an 8-week period between two positive PCRs can be used as a definition for reinfection cases in case sequencing, and conclusive clinical information is lacking, thus providing an epidemiological tool for future studies.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/diagnostics12030719/s1, Supplemental Table S1. Number of reinfections according to different definitions, and corresponding Cp-values of first and second positive test.
Author Contributions
S.M.E., B.L.H., A.W., T.W., J.M.P. and D.S. (Dennis Souverein) participated in conceptualization; S.M.E., T.W., I.M., B.L.H., J.S., J.K., M.H., D.S. (Dennis Souverein), J.C.S., F.S.M., J.d.B., A.W. and D.S. (Dominic Snijders) contributed to data collection; S.M.E., T.W., A.W. and D.S. (Dennis Souverein) wrote the original draft; B.L.H., I.M., S.v.L., M.H., E.K. and J.d.B. contributed to reviewing and editing the paper; S.M.E., I.M. and D.S. (Dennis Souverein) performed data curation; and T.W., D.S. (Dennis Souverein) and S.M.E. contributed to data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Medical Ethical Committee of the Amsterdam UMC approved this study on 22 April 2021 (Study number: 2021.0179). The data were anonymized after collection, and analyzed under code. Procedures were in accordance with the General Data Protection Regulation and Good Clinical Practice standards.
Informed Consent Statement
Patient consent was waived by the ethics committee of the Amsterdam UMC.
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
We would like to thank all physicians, nurses, public health testing personnel, laboratory technicians, and administration personnel who have worked hard to provide SARS-CoV-2 testing for a large number of individuals, making it possible to perform these analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gousseff, M.; Penot, P.; Gallay, L.; Batisse, D.; Benech, N.; Bouiller, K.; Collarino, R.; Conrad, A.; Slama, D.; Joseph, C.; et al. Clinical recurrences of COVID-19 symptoms after recovery: Viral relapse, reinfection or inflammatory rebound? J. Infect. 2020, 81, 816–846. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.L.; Hoang, V.T.; Gautret, P. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: A narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Overbaugh, J. Understanding protection from SARS-CoV-2 by studying reinfection. Nat Med. 2020, 26, 1680–1681. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Hung, I.F.; Ip, J.D.; Chu, A.W.; Chan, W.M.; Tam, A.R.; Fong, C.H.; Yuan, S.; Tsoi, H.W.; Ng, A.C.; et al. Coronavirus Disease 2019 (COVID-19) Re-infection by a Phylogenetically Distinct Severe Acute Respiratory Syndrome Coronavirus 2 Strain Confirmed by Whole Genome Sequencing. Clin. Infect. Dis. 2021, 73, e2946–e2951. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Bhoyar, R.C.; Jain, A.; Srivastava, S.; Upadhayay, R.; Imran, M.; Jolly, B.; Divakar, M.K.; Sharma, D.; Sehgal, P.; et al. Asymptomatic Reinfection in 2 Healthcare Workers from India with Genetically Distinct Severe Acute Respiratory Syndrome Coronavirus 2. Clin. Infect. Dis. 2021, 73, e2823–e2825. [Google Scholar] [CrossRef] [PubMed]
- Tillett, R.L.; Sevinsky, J.R.; Hartley, P.D.; Kerwin, H.; Crawford, N.; Gorzalski, A.; Laverdure, C.; Verma, S.C.; Rossetto, C.C.; Jackson, R.D.; et al. Genomic evidence for reinfection with SARS-CoV-2: A case study. Lancet Infect. Dis. 2021, 21, 52–58. [Google Scholar] [CrossRef]
- Sciscent, B.Y.; Eisele, C.D.; Ho, L.; King, S.D.; Jain, R.; Golamari, R.R. COVID-19 reinfection: The role of natural immunity, vaccines, and variants. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Okhuese, A.V. Estimation of the Probability of Reinfection With COVID-19 by the Susceptible-Exposed-Infectious-Removed-Undetectable-Susceptible Model. JMIR Public Health Surveill. 2020, 6, e19097. [Google Scholar] [CrossRef] [PubMed]
- Malkov, E. Simulation of coronavirus disease 2019 (COVID-19) scenarios with possibility of reinfection. Chaos Solitons Fractals 2020, 139, 110296. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Brito, A.F.; Trubin, P.; Lu, P.; Wong, P.; Alpert, T.; Peña-Hernández, M.A.; Haynes, W.; Kamath, K.; Liu, F.; et al. Case Study: Longitudinal immune profiling of a SARS-CoV-2 reinfection in a solid organ transplant recipient. medRxiv 2021. [Google Scholar] [CrossRef]
- Perez, G.; Banon, T.; Gazit, S.; Moshe, S.; Wortsman, B.J.; Grupel, D.; Peretz, A.; Tov, A.B.; Chodick, G.; Mizrahi-Reuveni, M.; et al. A 1 to 1000 SARS-CoV-2 reinfection proportion in members of a large healthcare provider in Israel: A preliminary report. medRxiv 2021. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Coyle, P.; Malek, J.A.; Ahmed, A.A.; Mohamoud, Y.A.; Younuskunju, S.; Ayoub, H.H.; Kanaani, Z.A.; Kuwari, E.A.; et al. SARS-CoV-2 reinfection in a cohort of 43,000 antibody-positive individuals followed for up to 35 weeks. medRxiv 2021. [Google Scholar] [CrossRef]
- Murillo-Zamora, E.; Mendoza-Cano, O.; Delgado-Enciso, I.; Hernandez-Suarez, C.M. Predictors of severe symptomatic laboratory-confirmed SARS-CoV-2 reinfection. Public Health 2021, 193, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.; Peaper, D.R.; She, R.C.; Nolte, F.S.; Wojewoda, C.M.; Anderson, N.W.; Pritt, B.S. College of American Pathologists (CAP) Microbiology Committee Perspective: Caution Must Be Used in Interpreting the Cycle Threshold (Ct) Value. Clin. Infect. Dis. 2021, 72, e685–e686. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, S.; Kotecha, D.; Bird, P.W.; Folwell, A.; Biju, S.; Tang, J.W. Setting the criteria for SARS-CoV-2 reinfection - six possible cases. J. Infect. 2021, 82, 282–327. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widders, A.; Broom, A.; Broom, J. SARS-CoV-2: The viral shedding vs infectivity dilemma. Infect. Dis. Health 2020, 25, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijksinstituut Voor Volksgezondheid en Milieu. Varianten van Het Coronavirus SARS-CoV-2. Available online: https://www.rivm.nl/coronavirus-covid-19/virus/varianten (accessed on 1 October 2021).
- European Centre for Disease Prevention and Control. Surveillance Definitions for COVID-19. 15 March 2021. Stockholm: ECDC. 2021. Available online: https://www.ecdc.europa.eu/en/covid-19/surveillance/surveillance-definitions (accessed on 16 February 2022).
- Centers for Disease Control and Prevention. Investigative Criteria for Suspected Cases of SARS-CoV-2 Reinfection (ICR). Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/invest-criteria.html (accessed on 26 September 2021).
- Rijksinstituut voor Volksgezondheid en Milieu. COVID-19 Richtlijn. Available online: https://lci.rivm.nl/richtlijnen/covid-19 (accessed on 1 October 2021).
- Rijksoverheid. Coronavirus Tijdlijn. 2020. Available online: https://www.rijksoverheid.nl/onderwerpen/coronavirus-tijdlijn (accessed on 19 October 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).