The Value of 18F-FDG-PET-CT Imaging in Treatment Evaluation of Colorectal Liver Metastases: A Systematic Review
Abstract
1. Introduction
2. Method
3. Results
3.1. 18F-FDG-PET-CT Performance after Surgical Resection
Summary
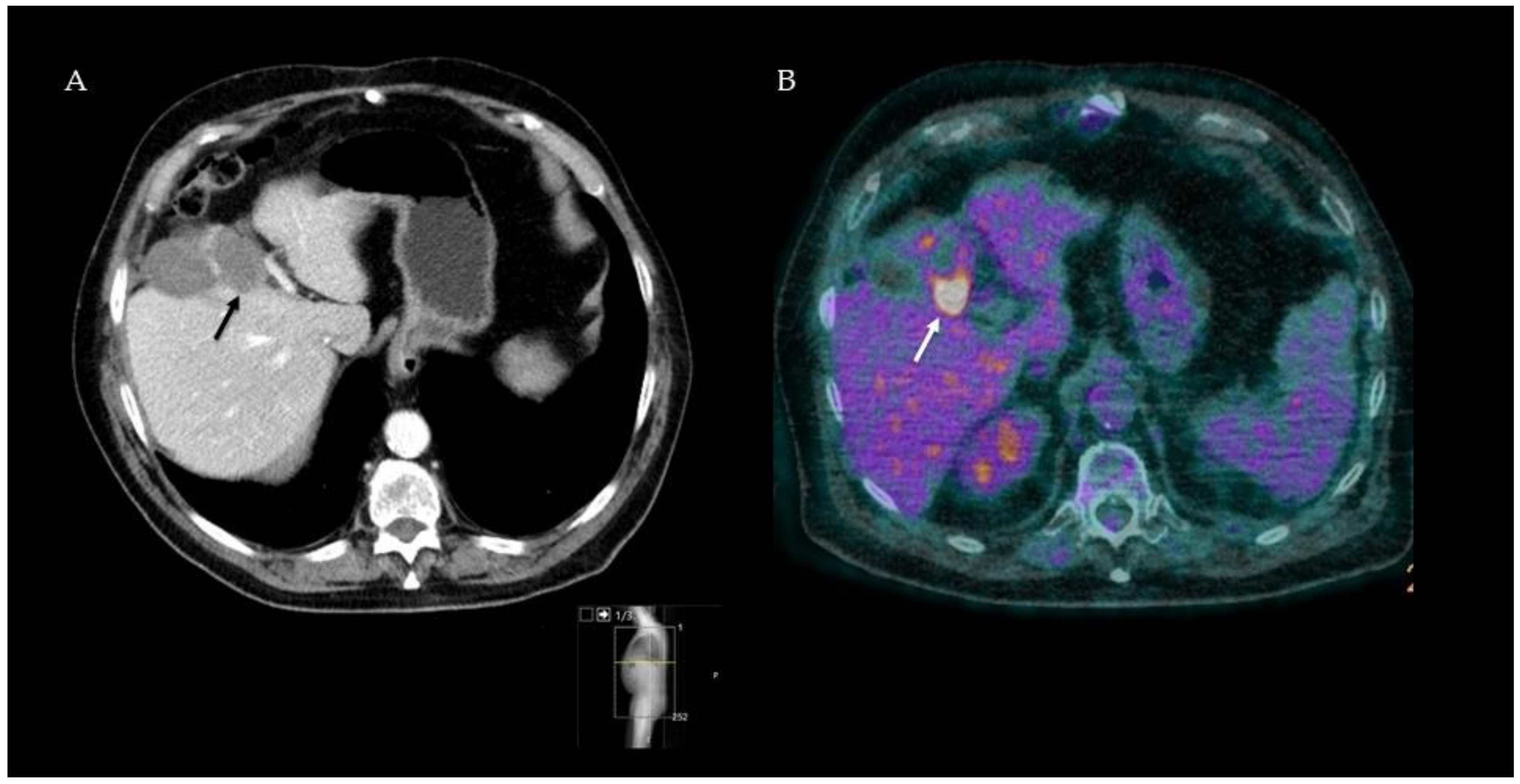
3.2. 18F-FDG-PET-CT after Thermal Ablation
Summary
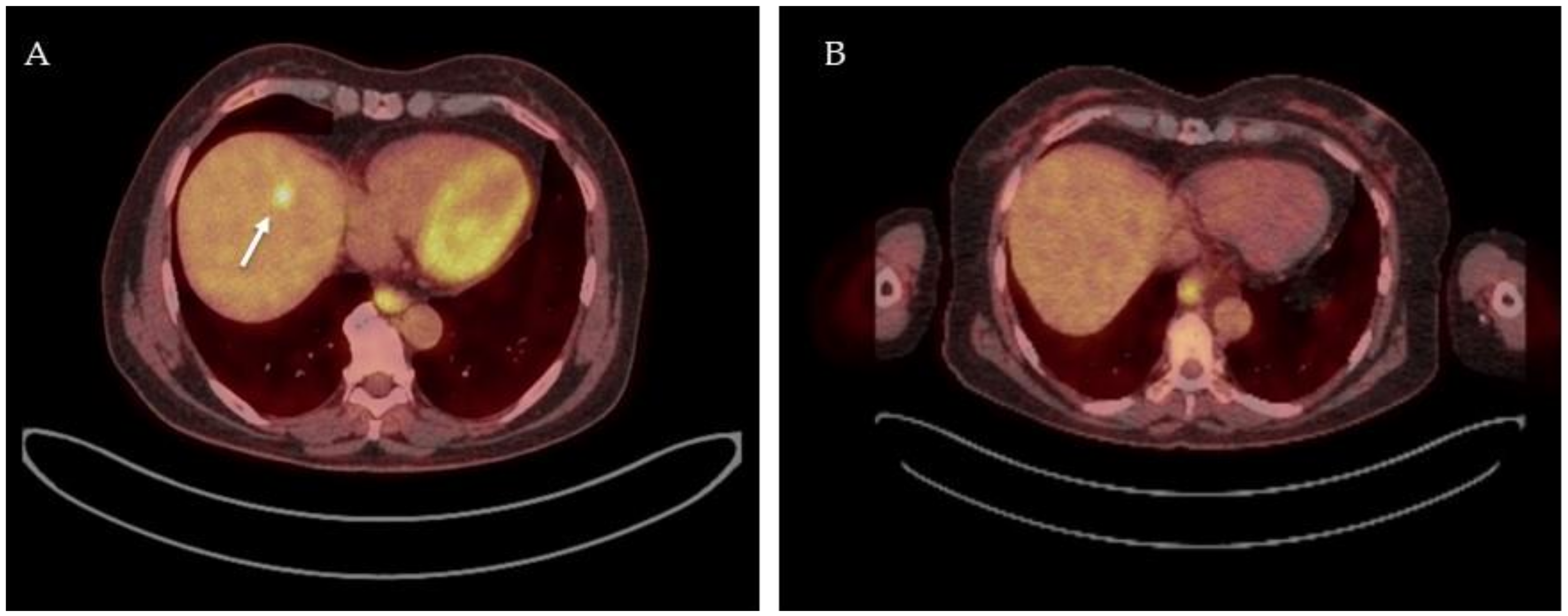
3.3. 18F-FDG-PET-CT as Response-Monitoring Modality after Neoadjuvant Chemotherapy
Summary
3.4. 18F-FDG-PET-CT as Response Monitoring during and after Palliative Chemotherapy
Summary
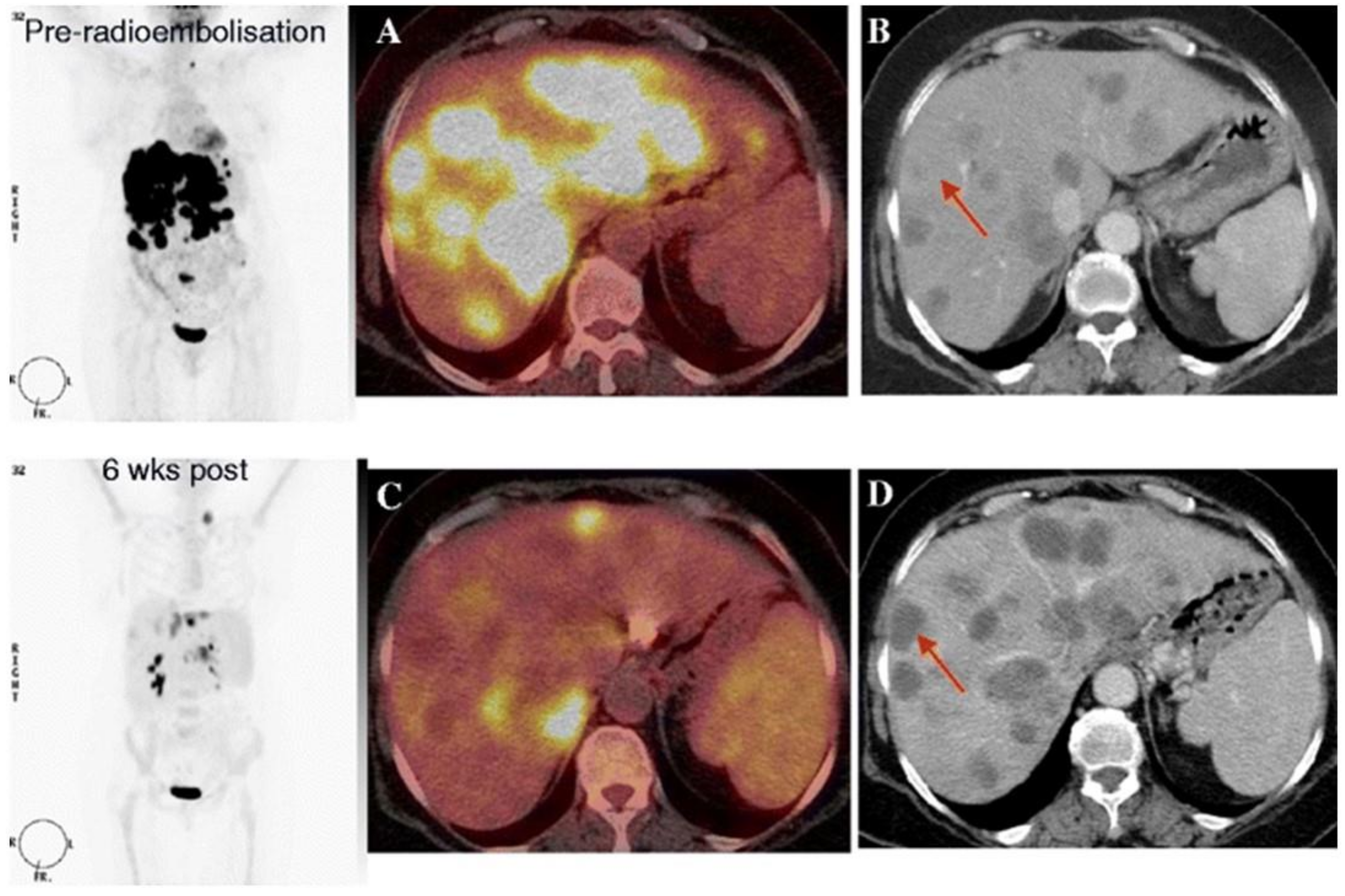
3.5. 18F-FDG-PET-CT after Radioembolization
Summary
3.6. Future Perspectives
3.6.1. Tumor-Targeted PET Tracers
3.6.2. PET-MR Imaging
3.6.3. Radiomics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Puckett, Y.; Nguyen, Q.N. Literature Review of Current Management of Colorectal Liver Metastasis. Cureus 2019, 11, e3940. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, F.B.; Kapiteijn, E.; Ceha, H.M.; Hartgrink, H.H.; Swijnenburg, R.J. Developments in the local treatment of colorectal liver metastases. Ned. Tijdschr. Voor Oncol. 2018, 15, 39–48. [Google Scholar]
- Lee, H.Y.; Woo, I.S. Perioperative Systemic Chemotherapy for Colorectal Liver Metastasis: Recent Updates. Cancers 2021, 13, 4590. [Google Scholar] [CrossRef] [PubMed]
- van den Hoven, A.F.; Rosenbaum, C.E.; Elias, S.G.; de Jong, H.W.; Koopman, M.; Verkooijen, H.M.; Alavi, A.; van den Bosch, M.A.; Lam, M.G. Insights into the Dose-Response Relationship of Radioembolization with Resin 90Y-Microspheres: A Prospective Cohort Study in Patients with Colorectal Cancer Liver Metastases. J. Nucl. Med. 2016, 57, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Tirumani, S.H.; Kim, K.W.; Nishino, M.; Howard, S.A.; Krajewski, K.M.; Jagannathan, J.P.; Cleary, J.M.; Ramaiya, N.H.; Shinagare, A.B. Update on the role of imaging in management of metastatic colorectal cancer. Radiographics 2014, 34, 1908–1928. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Verheul, H.M.W.; Flamen, P.; Rougier, P.; Beets-Tan, R.; Glynne-Jones, R.; Seufferlein, T. Imaging in Colorectal Cancer: Progress and Challenges for the Clinicians. Cancers 2016, 8, 81. [Google Scholar] [CrossRef]
- Hazhirkarzar, B.; Khoshpouri, P.; Shaghaghi, M.; Ghasabeh, M.A.; Pawlik, T.M.; Kamel, I.R. Current state of the art imaging approaches for colorectal liver metastasis. Hepatobiliary Surg. Nutr. 2020, 9, 35–48. [Google Scholar] [CrossRef]
- Asato, N.; Tsurusaki, M.; Sofue, K.; Hieda, Y.; Katsube, T.; Kitajima, K.; Murakami, T. Comparison of gadoxetic acid-enhanced dynamic MR imaging and contrast-enhanced computed tomography for preoperative evaluation of colorectal liver metastases. Jpn. J. Radiol. 2017, 35, 197–205. [Google Scholar] [CrossRef]
- Wong, T.Z.; Khandani, A.H.; Sheikh, A. Chapter 11—Nuclear Medicine. In Clinical Radiation Oncology (Fourth Edition); Gunderson, L.L., Tepper, J.E., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 206–216. [Google Scholar]
- Kim, Y.I.; Lee, H.S.; Choi, J.Y. Prognostic Significance of Pretreatment 18F-FDG PET/CT Volumetric Parameters in Patients With Colorectal Liver Metastasis: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2021, 46, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A. Chapter 2—FDG PET/CT Performance and Reporting. In Fundamentals of Oncologic PET/CT; Ulaner, G.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–8. [Google Scholar]
- Im, H.J.; Bradshaw, T.; Solaiyappan, M.; Cho, S.Y. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One is Better? Nucl. Med. Mol. Imaging 2018, 52, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M.; Erdi, Y.; Akhurst, T.; Mazumdar, M.; Macapinlac, H.A.; Finn, R.D.; Casilla, C.; Fazzari, M.; Srivastava, N.; Yeung, H.W.; et al. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging. The Visual Response Score and the Change in Total Lesion Glycolysis. Clin. Positron Imaging 1999, 2, 159–171. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. 1), 122S–150S. [Google Scholar] [CrossRef]
- Zerizer, I.; Al-Nahhas, A.; Towey, D.; Tait, P.; Ariff, B.; Wasan, H.; Hatice, G.; Habib, N.; Barwick, T. The role of early ¹⁸F-FDG PET/CT in prediction of progression-free survival after ⁹⁰Y radioembolization: Comparison with RECIST and tumour density criteria. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1391–1399. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, B.J.; Kim, H.S.; Kim, J.H. Comparison of the morphologic criteria (RECIST) and metabolic criteria (EORTC and PERCIST) in tumor response assessments: A pooled analysis. Korean J. Intern. Med. 2019, 34, 608–617. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Aarntzen, E.; Heijmen, L.; Oyen, W.J.G. 18F-FDG PET/CT in Local Ablative Therapies: A Systematic Review. J. Nucl. Med. 2018, 59, 551–556. [Google Scholar] [CrossRef]
- Lin, Y.M.; Paolucci, I.; Brock, K.K.; Odisio, B.C. Image-Guided Ablation for Colorectal Liver Metastasis: Principles, Current Evidence, and the Path Forward. Cancers 2021, 13, 3926. [Google Scholar] [CrossRef]
- Viganò, L.; Lopci, E.; Costa, G.; Rodari, M.; Poretti, D.; Pedicini, V.; Solbiati, L.; Chiti, A.; Torzilli, G. Positron Emission Tomography-Computed Tomography for Patients with Recurrent Colorectal Liver Metastases: Impact on Restaging and Treatment Planning. Ann. Surg. Oncol. 2017, 24, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Maffione, A.M.; Lopci, E.; Bluemel, C.; Giammarile, F.; Herrmann, K.; Rubello, D. Diagnostic accuracy and impact on management of 18F-FDG PET and PET/CT in colorectal liver metastasis: A meta-analysis and systematic review. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Samim, M.; Molenaar, I.Q.; Seesing, M.F.J.; van Rossum, P.S.N.; van den Bosch, M.; Ruers, T.J.M.; Borel Rinkes, I.H.M.; van Hillegersberg, R.; Lam, M.; Verkooijen, H.M. The diagnostic performance of 18F-FDG PET/CT, CT and MRI in the treatment evaluation of ablation therapy for colorectal liver metastases: A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Veit, P.; Antoch, G.; Stergar, H.; Bockisch, A.; Forsting, M.; Kuehl, H. Detection of residual tumor after radiofrequency ablation of liver metastasis with dual-modality PET/CT: Initial results. Eur. Radiol. 2006, 16, 80–87. [Google Scholar] [CrossRef]
- Kuehl, H.; Antoch, G.; Stergar, H.; Veit-Haibach, P.; Rosenbaum-Krumme, S.; Vogt, F.; Frilling, A.; Barkhausen, J.; Bockisch, A. Comparison of FDG-PET, PET/CT and MRI for follow-up of colorectal liver metastases treated with radiofrequency ablation: Initial results. Eur. J. Radiol. 2008, 67, 362–371. [Google Scholar] [CrossRef]
- Sahin, D.A.; Agcaoglu, O.; Chretien, C.; Siperstein, A.; Berber, E. The utility of PET/CT in the management of patients with colorectal liver metastases undergoing laparascopic radiofrequency thermal ablation. Ann. Surg. Oncol. 2012, 19, 850–855. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Chang, Z.H.; Lu, Z.M.; Guo, Q.Y. Early PET/CT after radiofrequency ablation in colorectal cancer liver metastases: Is it useful? Chin. Med. J. 2010, 123, 1690–1694. [Google Scholar]
- Nielsen, K.; van Tilborg, A.A.; Scheffer, H.J.; Meijerink, M.R.; de Lange-de Klerk, E.S.; Meijer, S.; Comans, E.F.; van den Tol, M.P. PET-CT after radiofrequency ablation of colorectal liver metastases: Suggestions for timing and image interpretation. Eur. J. Radiol. 2013, 82, 2169–2175. [Google Scholar] [CrossRef]
- Cornelis, F.; Sotirchos, V.; Violari, E.; Sofocleous, C.T.; Schoder, H.; Durack, J.C.; Siegelbaum, R.H.; Maybody, M.; Humm, J.; Solomon, S.B. 18F-FDG PET/CT Is an Immediate Imaging Biomarker of Treatment Success after Liver Metastasis Ablation. J. Nucl. Med. 2016, 57, 1052–1057. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Petre, E.N.; Vakiani, E.; Klimstra, D.; Durack, J.C.; Gonen, M.; Osborne, J.; Solomon, S.B.; Sofocleous, C.T. Immediate Postablation 18F-FDG Injection and Corresponding SUV Are Surrogate Biomarkers of Local Tumor Progression After Thermal Ablation of Colorectal Carcinoma Liver Metastases. J. Nucl. Med. 2018, 59, 1360–1365. [Google Scholar] [CrossRef]
- Lubezky, N.; Metser, U.; Geva, R.; Nakache, R.; Shmueli, E.; Klausner, J.M.; Even-Sapir, E.; Figer, A.; Ben-Haim, M. The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: Comparison with operative and pathological findings. J. Gastrointest. Surg. 2007, 11, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; De Bruyne, S.; Van Damme, N.; Smeets, P.; Ceelen, W.; Troisi, R.; Laurent, S.; Geboes, K.; Peeters, M.; Goethals, I.; et al. Standardized added metabolic activity (SAM) in ¹⁸F-FDG PET assessment of treatment response in colorectal liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, L.; Aufort, S.; Eberlé, M.C.; Assenat, E.; Ychou, M.; Gallix, B. Assessment of liver metastases from colorectal adenocarcinoma following chemotherapy: SPIO-MRI versus FDG-PET/CT. Radiol. Med. 2010, 115, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- García Vicente, A.M.; Domínguez Ferreras, E.; Sánchez Pérez, V.; Poblete García, V.M.; Villa Guzmán, J.C.; Jiménez Aragón, F.; Pineda Pineda, M.D.; Molino Trinidad, C.; Soriano Castrejón, Á. Response assessment of colorectal liver metastases with contrast enhanced CT/18F-FDG PET. Eur. J. Radiol. 2013, 82, e255–e261. [Google Scholar] [CrossRef]
- Burger, I.A.; Schwarz, E.I.; Samarin, A.; Breitenstein, S.; Weber, A.; Hany, T.F. Correlation between therapy response assessment using FDG PET/CT and histopathologic tumor regression grade in hepatic metastasis of colorectal carcinoma after neoadjuvant therapy. Ann. Nucl. Med. 2013, 27, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, Y.; Yoshioka, R.; Gonoi, W.; Sugawara, T.; Yoshida, S.; Hashimoto, M.; Shindoh, J. Fluorine-18-fluorodeoxyglucose positron emission tomography as an objective substitute for CT morphologic response criteria in patients undergoing chemotherapy for colorectal liver metastases. Abdom. Radiol. 2018, 43, 1152–1158. [Google Scholar] [CrossRef]
- Tan, M.C.; Linehan, D.C.; Hawkins, W.G.; Siegel, B.A.; Strasberg, S.M. Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J. Gastrointest. Surg. 2007, 11, 1112–1119. [Google Scholar] [CrossRef]
- De Bruyne, S.; Van Damme, N.; Smeets, P.; Ferdinande, L.; Ceelen, W.; Mertens, J.; Van de Wiele, C.; Troisi, R.; Libbrecht, L.; Laurent, S.; et al. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br. J. Cancer 2012, 106, 1926–1933. [Google Scholar] [CrossRef]
- Lastoria, S.; Piccirillo, M.C.; Caracò, C.; Nasti, G.; Aloj, L.; Arrichiello, C.; de Lutio di Castelguidone, E.; Tatangelo, F.; Ottaiano, A.; Iaffaioli, R.V.; et al. Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab. J. Nucl. Med. 2013, 54, 2062–2069. [Google Scholar] [CrossRef][Green Version]
- Heijmen, L.; ter Voert, E.E.; Oyen, W.J.; Punt, C.J.; van Spronsen, D.J.; Heerschap, A.; de Geus-Oei, L.F.; van Laarhoven, H.W. Multimodality imaging to predict response to systemic treatment in patients with advanced colorectal cancer. PLoS ONE 2015, 10, e0120823. [Google Scholar] [CrossRef]
- Skougaard, K.; Johannesen, H.H.; Nielsen, D.; Schou, J.V.; Jensen, B.V.; Høgdall, E.V.; Hendel, H.W. CT versus FDG-PET/CT response evaluation in patients with metastatic colorectal cancer treated with irinotecan and cetuximab. Cancer Med. 2014, 3, 1294–1301. [Google Scholar] [CrossRef]
- Nemeth, Z.; Wijker, W.; Lengyel, Z.; Hitre, E.; Borbely, K. Metabolic Parameters as Predictors for Progression Free and Overall Survival of Patients with Metastatic Colorectal Cancer. Pathol. Oncol. Res. 2020, 26, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.W.H.; Lam, K.O.; An, H.; Cheung, G.T.C.; Lau, J.K.S.; Choy, T.S.; Lee, V.H.F. Long-term outcomes and recurrence pattern of 18F-FDG PET-CT complete metabolic response in the first-line treatment of metastatic colorectal cancer: A lesion-based and patient-based analysis. BMC Cancer 2018, 18, 776. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.H.; Im, S.A.; Han, S.W.; Goo, J.M.; Willmann, J.K.; Lee, E.S.; Eo, J.S.; Paeng, J.C.; Han, J.K.; et al. Intermodality comparison between 3D perfusion CT and 18F-FDG PET/CT imaging for predicting early tumor response in patients with liver metastasis after chemotherapy: Preliminary results of a prospective study. Eur. J. Radiol. 2012, 81, 3542–3550. [Google Scholar] [CrossRef] [PubMed]
- Correa-Gallego, C.; Gavane, S.; Grewal, R.; Cercek, A.; Klimstra, D.S.; Gewirtz, A.N.; Kingham, T.P.; Fong, Y.; DeMatteo, R.P.; Allen, P.J.; et al. Prospective evaluation of 18F-fluorodeoxyglucose positron emission tomography in patients receiving hepatic arterial and systemic chemotherapy for unresectable colorectal liver metastases. HPB 2015, 17, 644–650. [Google Scholar] [CrossRef][Green Version]
- Soydal, C.; Kucuk, O.N.; Gecim, E.I.; Bilgic, S.; Elhan, A.H. The prognostic value of quantitative parameters of 18F-FDG PET/CT in the evaluation of response to internal radiation therapy with yttrium-90 in patients with liver metastases of colorectal cancer. Nucl. Med. Commun. 2013, 34, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Sabet, A.; Meyer, C.; Aouf, A.; Sabet, A.; Ghamari, S.; Pieper, C.C.; Mayer, K.; Biersack, H.J.; Ezziddin, S. Early post-treatment FDG PET predicts survival after 90Y microsphere radioembolization in liver-dominant metastatic colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 370–376. [Google Scholar] [CrossRef]
- Shady, W.; Sotirchos, V.S.; Do, R.K.; Pandit-Taskar, N.; Carrasquillo, J.A.; Gonen, M.; Sofocleous, C.T. Surrogate Imaging Biomarkers of Response of Colorectal Liver Metastases After Salvage Radioembolization Using 90Y-Loaded Resin Microspheres. AJR Am. J. Roentgenol. 2016, 207, 661–670. [Google Scholar] [CrossRef]
- Shady, W.; Kishore, S.; Gavane, S.; Do, R.K.; Osborne, J.R.; Ulaner, G.A.; Gonen, M.; Ziv, E.; Boas, F.E.; Sofocleous, C.T. Metabolic tumor volume and total lesion glycolysis on FDG-PET/CT can predict overall survival after (90)Y radioembolization of colorectal liver metastases: A comparison with SUVmax, SUVpeak, and RECIST 1.0. Eur. J. Radiol. 2016, 85, 1224–1231. [Google Scholar] [CrossRef]
- Jongen, J.M.J.; Rosenbaum, C.; Braat, M.; van den Bosch, M.; Sze, D.Y.; Kranenburg, O.; Borel Rinkes, I.H.M.; Lam, M.; van den Hoven, A.F. Anatomic versus Metabolic Tumor Response Assessment after Radioembolization Treatment. J. Vasc. Interv. Radiol. 2018, 29, 244–253. [Google Scholar] [CrossRef]
- Sager, S.; Akgün, E.; Uslu-Beşli, L.; Asa, S.; Akovali, B.; Sahin, O.; Yeyin, N.; Demir, M.; Abuqbeitah, M.; Gülsen, F.; et al. Comparison of PERCIST and RECIST criteria for evaluation of therapy response after yttrium-90 microsphere therapy in patients with hepatocellular carcinoma and those with metastatic colorectal carcinoma. Nucl. Med. Commun. 2019, 40, 461–468. [Google Scholar] [CrossRef]
- Cuda, T.J.; Riddell, A.D.; Liu, C.; Whitehall, V.L.; Borowsky, J.; Wyld, D.K.; Burge, M.E.; Ahern, E.; Griffin, A.; Lyons, N.J.R.; et al. PET Imaging Quantifying 68Ga-PSMA-11 Uptake in Metastatic Colorectal Cancer. J. Nucl. Med. 2020, 61, 1576–1579. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kim, H.O.; Kim, K.P.; Lee, J.L.; Kim, H.J.; Lee, S.J.; Lee, S.J.; Oh, S.J.; Kim, J.S.; Ryu, J.S.; et al. 3′-Deoxy-3′-18F-fluorothymidine PET for the early prediction of response to leucovorin, 5-fluorouracil, and oxaliplatin therapy in patients with metastatic colorectal cancer. J. Nucl. Med. 2013, 54, 1209–1216. [Google Scholar] [CrossRef][Green Version]
- Siveke, J.T. Fibroblast-Activating Protein: Targeting the Roots of the Tumor Microenvironment. J. Nucl. Med. 2018, 59, 1412–1414. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, L.; Luo, Z.; Hao, B.; Wu, H.; Lin, Q.; Sun, L.; Chen, H. Comparison of 68Ga-FAPI and 18F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology 2021, 298, 393–402. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Koerber, S.A.; Staudinger, F.; Kratochwil, C.; Adeberg, S.; Haefner, M.F.; Ungerechts, G.; Rathke, H.; Winter, E.; Lindner, T.; Syed, M.; et al. The Role of 68Ga-FAPI PET/CT for Patients with Malignancies of the Lower Gastrointestinal Tract: First Clinical Experience. J. Nucl. Med. 2020, 61, 1331–1336. [Google Scholar] [CrossRef]
- Şahin, E.; Elboğa, U.; Çelen, Y.Z.; Sever, Ö.N.; Çayırlı, Y.B.; Çimen, U. Comparison of 68Ga-DOTA-FAPI and 18FDG PET/CT imaging modalities in the detection of liver metastases in patients with gastrointestinal system cancer. Eur. J. Radiol. 2021, 142, 109867. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, J.; Fu, K.; Lin, Q.; Chen, H. 68Ga-FAPI PET/CT in Assessment of Liver Nodules in a Cirrhotic Patient. Clin. Nucl. Med. 2020, 45, e430–e432. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Kratochwil, C.; Cardinale, J.; Finck, R.; Dabir, M.; Novruzov, E.; Watabe, T.; Kramer, V.; Choyke, P.L.; et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers 2021, 13, 4946. [Google Scholar] [CrossRef]
- Beiderwellen, K.; Geraldo, L.; Ruhlmann, V.; Heusch, P.; Gomez, B.; Nensa, F.; Umutlu, L.; Lauenstein, T.C. Accuracy of [18F]FDG PET/MRI for the Detection of Liver Metastases. PLoS ONE 2015, 10, e0137285. [Google Scholar] [CrossRef] [PubMed]
- Reiner, C.S.; Stolzmann, P.; Husmann, L.; Burger, I.A.; Hüllner, M.W.; Schaefer, N.G.; Schneider, P.M.; von Schulthess, G.K.; Veit-Haibach, P. Protocol requirements and diagnostic value of PET/MR imaging for liver metastasis detection. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Brendle, C.; Schwenzer, N.F.; Rempp, H.; Schmidt, H.; Pfannenberg, C.; la Fougère, C.; Nikolaou, K.; Schraml, C. Assessment of metastatic colorectal cancer with hybrid imaging: Comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Meng, X.; Zhang, Y.; Yu, B.; Yuan, J.; Yu, J.; Zhu, H.; Yang, Z. Diagnostic Value of Delayed PET/MR in Liver Metastasis in Comparison With PET/CT. Front. Oncol. 2021, 11, 717687. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.M.; Hur, B.Y.; Joo, I.; Yi, N.J.; Suh, K.S.; Kang, K.W.; Han, J.K. Colorectal Cancer Liver Metastases: Diagnostic Performance and Prognostic Value of PET/MR Imaging. Radiology 2016, 280, 782–792. [Google Scholar] [CrossRef]
- Kirchner, J.; Sawicki, L.M.; Deuschl, C.; Grüneisen, J.; Beiderwellen, K.; Lauenstein, T.C.; Herrmann, K.; Forsting, M.; Heusch, P.; Umutlu, L. 18 F-FDG PET/MR imaging in patients with suspected liver lesions: Value of liver-specific contrast agent Gadobenate dimeglumine. PLoS ONE 2017, 12, e0180349. [Google Scholar] [CrossRef]
- Martin, O.; Schaarschmidt, B.M.; Kirchner, J.; Suntharalingam, S.; Grueneisen, J.; Demircioglu, A.; Heusch, P.; Quick, H.H.; Forsting, M.; Antoch, G.; et al. PET/MRI Versus PET/CT for Whole-Body Staging: Results from a Single-Center Observational Study on 1,003 Sequential Examinations. J. Nucl. Med. 2020, 61, 1131–1136. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Prosch, H.; Beer, L.; Tamandl, D.; Beyer, T.; Hoeller, C.; Berzaczy, D.; Raderer, M.; Preusser, M.; Hochmair, M.; et al. PET/MRI versus PET/CT in oncology: A prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 51–60. [Google Scholar] [CrossRef]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging-“how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Fiz, F.; Viganò, L.; Gennaro, N.; Costa, G.; La Bella, L.; Boichuk, A.; Cavinato, L.; Sollini, M.; Politi, L.S.; Chiti, A.; et al. Radiomics of Liver Metastases: A Systematic Review. Cancers 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- van Helden, E.J.; Vacher, Y.J.L.; van Wieringen, W.N.; van Velden, F.H.P.; Verheul, H.M.W.; Hoekstra, O.S.; Boellaard, R.; Menke-van der Houven van Oordt, C.W. Radiomics analysis of pre-treatment [18F]FDG PET/CT for patients with metastatic colorectal cancer undergoing palliative systemic treatment. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Bak-Fredslund, K.P.; Ashrafinia, S.; Lu, L.; Schmidtlein, C.R.; Subramaniam, R.M.; Morsing, A.; Keiding, S.; Horsager, J.; Munk, O.L. Prognostic modeling for patients with colorectal liver metastases incorporating FDG PET radiomic features. Eur. J. Radiol. 2019, 113, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Barretta, M.L.; Picone, C.; Avallone, A.; Belli, A.; Patrone, R.; Ferrante, M.; Cozzi, D.; Grassi, R.; et al. Radiomics in hepatic metastasis by colorectal cancer. Infect. Agents Cancer 2021, 16, 39. [Google Scholar] [CrossRef]
- de la Pinta, C.; Castillo, M.E.; Collado, M.; Galindo-Pumariño, C.; Peña, C. Radiogenomics: Hunting Down Liver Metastasis in Colorectal Cancer Patients. Cancers 2021, 13, 5547. [Google Scholar] [CrossRef]
- Staal, F.C.R.; van der Reijd, D.J.; Taghavi, M.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; Maas, M. Radiomics for the Prediction of Treatment Outcome and Survival in Patients With Colorectal Cancer: A Systematic Review. Clin. Colorectal. Cancer 2021, 20, 52–71. [Google Scholar] [CrossRef]
- Wesdorp, N.J.; van Goor, V.J.; Kemna, R.; Jansma, E.P.; van Waesberghe, J.; Swijnenburg, R.J.; Punt, C.J.A.; Huiskens, J.; Kazemier, G. Advanced image analytics predicting clinical outcomes in patients with colorectal liver metastases: A systematic review of the literature. Surg. Oncol. 2021, 38, 101578. [Google Scholar] [CrossRef]

| Category | EORTC PET Criteria | PERCIST Criteria | RECIST 1.1 Criteria | Choi Criteria |
|---|---|---|---|---|
| Complete metabolic response | Complete resolution of 18F-FDG uptake | Complete resolution of 18F-FDG uptake | Disappearance of lesions | Disappearance of enhancing lesions |
| Partial metabolic response | SUVmax reduction of >25% | ≥30% decrease in target tumor(s) 18F-FDG SUV | Tumor diameter declined ≥30% | Tumor density decreased ≥15% |
| Stable disease | No CR, PR, or PD | No CR, PR, or PD | No CR, PR, or PD | No CR, PR, or PD |
| Progressive disease | Increase in 18F-FDG uptake in new metastatic lesions; increase in SUVmax > 25 %; visible increase in extent of 18F-FDG uptake (20% in LD) | Over 30% increase in 18F-FDG SUVmax or new 18F-FDG avid lesions | New lesions; increase ≥20% in the sum of the LDs and absolute increase of ≥5 mm | New lesions; increase ≥20% in tumor density |
| Author | Year | Study Type | N | Ablation Technique | Timing of PET-CT | Reference Standard | Median FUP | Results |
|---|---|---|---|---|---|---|---|---|
| Veit et al. [26] | 2005 | Retrospective | 13 | RFA | Baseline; <48 h post-ablation | Clinical parameters; ceCT, PET-CT, and MRI | ±12 months | PET-CT was more accurate for evaluation of the ablation zone than CT alone, although not statistically significant. |
| Kuehl et al. [27] | 2008 | Prospective | 16 | RFA | Baseline; <24 h after ablation 1, 3, 6, and every 6 months post-ablation | Histology; CEA; ceCT | 22 months | PET-CT and MRI have comparable sensitivity and specificity for detection of LR. |
| Sahin et al. [28] | 2012 | Prospective | 82 | RFA | Variable; ordered on specific indication | Clinical parameters; ceCT | 29 months | PET-CT is superior to CT in detecting LR. |
| Liu et al. [29] | 2012 | Prospective | 12 | RFA | Baseline; <24 h after ablation 1, 3, 6, and every 6 months post-ablation | Follow-up imaging, i.e., final PET-CT | NR | Early PET-CT effectively detects and predicts LR. |
| Nielsen et al. [30] | 2013 | Prospective | 79 | RFA | Baseline; <12 months post-ablation | Follow-up imaging | NR | PET-CT accurately predicts LR within 1 year after treatment. |
| Cornelis et al. [31] | 2016 | Retrospective | 21 | MWA, RFA | Baseline; Immediately after ablation | Clinical parameters; ceCT | 1 year | SUV and TRC ratio predict LR. |
| Cornelis et al. [32] | 2018 | Prospective | 39 | MWA, RFA, IE | Baseline; Immediately after ablation | ceCT | 22.5 months | SUV ratios predict LR in patients with negative biopsies. |
| Author | Year | Study Type | N | Timing of PET-CT | Reference Standard | Median FUP | Results |
|---|---|---|---|---|---|---|---|
| Lubezky et al. [33] | 2007 | Prospective | 75 | Baseline After completion of chemotherapy | Histopathology | NR | Sensitivity for detection of residual disease after chemotherapy was 65% for CT and 49% for PET-CT. |
| Mertens et al. [34] | 2013 | Prospective | 18 | Baseline After completion of chemotherapy | Histopathology | 53 months | Follow-up SUVmax, SAM, and ΔSAM were prognostic for PFS and OS. |
| Bacigalupo et al. [35] | 2010 | Retrospective | 19 | After completion of chemotherapy | Surgical exploration, IOUS, and histopathology | 13 months | Overall per-lesion sensitivity to detect residual disease was 92% for SPIO-MRI and 52% for PET-CT. |
| García Vicente et al. [36] | 2013 | Prospective | 19 | Baseline After 4 cycles | CT and histopathology | 6 months | Sensitivity for detection of residual disease was 38% for PET, 91% for ceCT, and 95% for PET-CT; specificity was 100% for all modalities. |
| Burger et al. [37] | 2013 | Retrospective | 23 | Baseline After completion of chemotherapy | Histopathology | NR | ΔSUVmax > 41% was significantly correlated with TRG. |
| Nishioka et al. [38] | 2018 | Retrospective | 34 | After completion of chemotherapy | Histopathology | NR | A moderate correlation (r = 0.660) between SUVmean and tumor viability was found. However, for the prediction of tumor viability ≤10% SUVmean and SUVmax were accurate predictors (AUC 0.916 and 0.887, respectively). |
| Tan et al. [39] | 2007 | Prospective | 14 | Baseline After completion of chemotherapy | Histopathology | NR | 29 of 34 (85%) lesions displaying CMR showed viable tumor cells at histopathology. |
| De Bruyne et al. [40] | 2012 | Prospective | 19 | Baseline After completion of chemotherapy | Histopathology | 31 months | Low follow-up SUVmax as well as quantitative DCE-MRI parameters were prognostic factors for PFS. |
| Lastoria et al. [41] | 2013 | Prospective | 33 | Baseline After 1 cycle | RECIST and histopathology | 30 months | ΔSUVmax and ΔTLG were significantly predictive for PFS and OS. |
| Author | Year | Study Type | N | Timing of PET-CT | Reference Standard | Median FUP | Results |
|---|---|---|---|---|---|---|---|
| Heijmen et al. [42] | 2015 | Prospective | 39 | Baseline After 1 week After 3 cycles | RECIST | 16 months | Pretreatment, high SUVmax, high TLG, low ADC, and high T2* were associated with a shorter OS. Low pretreatment ADC value was associated with shorter PFS. |
| Skougaard et al. [43] | 2014 | Prospective | 61 | Baseline After every 4 cycles | RECIST | NR | OS was significantly longer for patients with a PMR compared with patients with SMD; no significant difference was found for patients with PR compared with patients with SD. |
| Nemeth et al. [44] | 2020 | Prospective | 53 | Baseline After 2 cycles | EORTC | 24 months | SAM2 and NSAM2 are significant predictors for PFS and OS. |
| Chiu et al. [45] | 2018 | Retrospective | 40 | Baseline Every 3 months after completion of chemotherapy | RECIST | 47 months | OS was longer in patients with CMR compared with patients with PMD (HR 5.329). |
| Kim et al. [46] | 2012 | Prospective | 17 | Baseline After 1 cycle | RECIST | NR | A significant difference in baseline SUVmean, ΔTLG30, and ΔMTV30 was found between responders and non-responders. |
| Correa-Gallego et al. [47] | 2015 | Prospective | 49 | Baseline After 3 cycles After 6 cycles | RECIST and histopathology | 38 months | No correlation between PET-parameters and PFS and OS was found. |
| Author | Year | Study Type | N | Timing of PET-CT | Imaging Evaluation Parameters | Reference Standard | Results |
|---|---|---|---|---|---|---|---|
| Zerizer et al. [17] | 2012 | Retrospective | 25 | Baseline; 6–8 weeks after RE | ΔSUVmax and LTD | ceCT: RECIST 1.1 and Choi criteria | ΔSUVmax was a significant predictor of PFS, while response assessed by RECIST and tumor attenuation did not predict PFS. |
| Soydal et al. [48] | 2013 | Retrospective | 35 | Baseline; 6 weeks after RE | ΔTLG, ΔFTV and ΔSUVmax | OS | ΔTLG was not a significant predictor of OS. |
| Sabet et al. [49] | 2015 | Retrospective | 51 | Baseline; 4 weeks after RE | ≥50% ΔTLR | OS | A decrease of ≥50% of TLG was a significant predictor of prolonged OS. |
| Shady et al. [50] | 2016 | Retrospective | 25 | Baseline; <10 weeks after RE | EORTC PET criteria, Choi criteria, tumor attenuation criteria | ceCT: RECIST 1.1 | Response determined by EORTC PET criteria, Choi criteria, and tumor attenuation criteria were predictors of hepatic PFS. |
| Shady et al. [51] | 2016 | Retrospective | 49 | Baseline; <12 weeks after RE | ΔSUVmax; ΔSUVpeak; ΔMTV; ΔTLG | ceCT: RECIST 1.1 | Response by ≥30% ΔMTV and ΔTLG were significantly correlated with OS, whereas response by ΔSUVmax, ΔSUVpeak, and RECIST did not correlate with OS. |
| Jongen et al. [52] | 2018 | Prospective | 38 | Baseline; 1 month after RE; 3 months after RE | ΔLTD; ΔTLG | MRI: RECIST 1.1 | ΔTLG was more sensitive than ΔLTD for prediction of OS. |
| Sager et al. [53] | 2019 | Retrospective | 19 | Baseline; 6 weeks after RE | Mean tumor volume; µMTV | CT and/or MRI: RECIST 1.1 | PERCIST criteria are more reliable than RECIST criteria for treatment response evaluation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bijlstra, O.D.; Boreel, M.M.E.; van Mossel, S.; Burgmans, M.C.; Kapiteijn, E.H.W.; Oprea-Lager, D.E.; Rietbergen, D.D.D.; van Velden, F.H.P.; Vahrmeijer, A.L.; Swijnenburg, R.-J.; et al. The Value of 18F-FDG-PET-CT Imaging in Treatment Evaluation of Colorectal Liver Metastases: A Systematic Review. Diagnostics 2022, 12, 715. https://doi.org/10.3390/diagnostics12030715
Bijlstra OD, Boreel MME, van Mossel S, Burgmans MC, Kapiteijn EHW, Oprea-Lager DE, Rietbergen DDD, van Velden FHP, Vahrmeijer AL, Swijnenburg R-J, et al. The Value of 18F-FDG-PET-CT Imaging in Treatment Evaluation of Colorectal Liver Metastases: A Systematic Review. Diagnostics. 2022; 12(3):715. https://doi.org/10.3390/diagnostics12030715
Chicago/Turabian StyleBijlstra, Okker D., Maud M. E. Boreel, Sietse van Mossel, Mark C. Burgmans, Ellen H. W. Kapiteijn, Daniela E. Oprea-Lager, Daphne D. D. Rietbergen, Floris H. P. van Velden, Alexander L. Vahrmeijer, Rutger-Jan Swijnenburg, and et al. 2022. "The Value of 18F-FDG-PET-CT Imaging in Treatment Evaluation of Colorectal Liver Metastases: A Systematic Review" Diagnostics 12, no. 3: 715. https://doi.org/10.3390/diagnostics12030715
APA StyleBijlstra, O. D., Boreel, M. M. E., van Mossel, S., Burgmans, M. C., Kapiteijn, E. H. W., Oprea-Lager, D. E., Rietbergen, D. D. D., van Velden, F. H. P., Vahrmeijer, A. L., Swijnenburg, R.-J., Mieog, J. S. D., & de Geus-Oei, L.-F. (2022). The Value of 18F-FDG-PET-CT Imaging in Treatment Evaluation of Colorectal Liver Metastases: A Systematic Review. Diagnostics, 12(3), 715. https://doi.org/10.3390/diagnostics12030715









