Diencephalic Syndrome Due to Optic Pathway Gliomas in Pediatric Patients: An Italian Multicenter Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Statistics
3. Results
3.1. Clinical Features at Diagnosis
3.2. Diagnosis
3.3. Treatment and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Häger, A. The diencephalic syndrome of emaciation. A case report. Eur. Neurol. 1972, 7, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Helfferich, J.; Nijmeijer, R.; Brouwer, O.F.; Boon, M.; Fock, A.; Hoving, E.W.; Meijer, L.; den Dunnen, W.F.; de Bont, E.S. Neurofibromatosis type 1 associated low grade gliomas: A comparison with sporadic low grade gliomas. Crit. Rev. Oncol. Hematol. 2016, 104, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquilina, K.; Daniels, D.J.; Spoudeas, H.; Phipps, K.; Gan, H.W.; Boop, F.A. Optic pathway glioma in children: Does visual deficit correlate with radiology in focal exophytic lesions. Child’s Nerv. Syst. 2015, 31, 2041–2049. [Google Scholar] [CrossRef]
- Siedler, D.G.; Beechey, J.C.; Jessup, P.J.; Thani, N.B. Infantile Optic Pathway Glioblastoma. World Neurosurg. 2019, 129, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, T.; Zhong, Y. Demographic and prognostic factors of optic nerve astrocytoma: A retrospective study of surveillance, epidemiology, and end results (SEER). BMC Cancer 2021, 21, 976. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, A.; Brue, C.; Poussaint, T.Y.; Kieran, M.; Pomeroy, S.L.; Goumnerova, L.; Scott, R.M.; Cohen, L.E. Diencephalic syndrome: A cause of failure to thrive and a model of partial growth hormone resistance. Pediatrics 2005, 115, e742–e748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauner, R.; Trivin, C.; Zerah, M.; Souberbielle, J.C.; Doz, F.; Kalifa, C.; Sainte-Rose, C. Diencephalic syndrome due to hypothalamic tumor: A model of the relationship between weight and puberty onset. J. Clin. Endocrinol. Metab. 2006, 91, 2467–2473. [Google Scholar] [CrossRef] [Green Version]
- Gropman, A.L.; Packer, R.J.; Nicholson, H.S.; Vezina, L.G.; Jakacki, R.; Geyer, R.; Olson, J.M.; Phillips, P.; Needle, M.; Broxson, E.H., Jr.; et al. Treatment of diencephalic syndrome with chemotherapy: Growth, tumor response, and long term control. Cancer 1998, 83, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Kilday, J.-P.; Bartels, U.; Huang, A.; Barron, M.; Shago, M.; Mistry, M.; Zhukova, N.; Laperriere, N.; Dirks, P.; Hawkins, C.; et al. Favorable survival and metabolic outcome for children with diencephalic syndrome using a radiation-sparing approach. J. Neurooncol. 2014, 116, 195–204. [Google Scholar] [CrossRef]
- Perilongo, G.; Carollo, C.; Salviati, L.; Murgia, A.; Pillon, M.; Basso, G.; Gardiman, M.; Laverda, A. Diencephalic syndrome and disseminated juvenile pilocyticastrocytomas of the hypothalamic-optic chiasm region. Cancer 1997, 80, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Gnekow, A.K.; Falkenstein, F.; von Hornstein, S.; Zwiener, I.; Berkefeld, S.; Bison, B.; Warmuth-Metz, M.; HernáizDriever, P.; Soerensen, N.; Kortmann, R.-D.; et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012, 14, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Laithier, V.; Grill, J.; Le Deley, M.-C.; Ruchoux, M.-M.; Couanet, D.; Doz, F.; Pichon, F.; Rubie, H.; Frappaz, D.; Vannier, J.-P.; et al. Progression-free survival in children with optic pathway tumors s: Dependence on age and the quality of the response to chemotherapy--results of the first French prospective study for the French Society of Pediatric Oncology. J. Clin. Oncol. 2003, 21, 4572–4578. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Gebhardt, U.; Sterkenburg, A.S.; Warmuth-Metz, M.; Müller, H.L. Diencephalic syndrome in childhood craniopharyngioma—Results of German multicenter studies on 485 long-term survivors of childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 2014, 99, 3972–3977. [Google Scholar] [CrossRef] [Green Version]
- Taylor, T.; Jaspan, T.; Milano, G.; Gregson, R.; Parker, T.; Ritzmann, T.; Benson, C.; Walker, D.; PLAN Study Group. Radiological classification of optic pathway gliomas: Experience of a modified functional classification system. Br. J. Radiol. 2008, 81, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef]
- D’Amico, A.; Rosano, C.; Pannone, L.; Pinna, V.; Assunto, A.; Motta, M.; Ugga, L.; Daniele, P.; Mandile, R.; Mariniello, L.; et al. Clinical variability of neurofibromatosis 1: A modifying role of cooccurring PTPN11 variants and atypical brain MRI findings. Clin. Genet. 2021, 100, 563–572. [Google Scholar] [CrossRef]
- Santoro, C.; Picariello, S.; Palladino, F.; Spennato, P.; Melis, D.; Roth, J.; Cirillo, M.; Quaglietta, L.; D’Amico, A.; Gaudino, G.; et al. Retrospective Multicentric Study on Non-Optic CNS Tumors in Children and Adolescents with Neurofibromatosis Type 1. Cancers 2020, 12, 1426. [Google Scholar] [CrossRef]
- Vlachopapadopoulou, E.; Tracey, K.J.; Capella, M.; Gilker, C.; Matthews, D.E. Increased energy expenditure in a patient with diencephalic syndrome. J. Pediatr. 1993, 122, 922–924. [Google Scholar] [CrossRef]
- Kim, A.; Soo Moon, J.; Yang, H.R.; Chang, J.Y.; Sung Ko, J.; KeeSeo, J. Diencephalic syndrome: A frequently neglected cause of failure to thrive in infants. Korean J. Pediatr. 2015, 58, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Cavicchiolo, M.E.; Opocher, E.; Daverio, M.; Bendini, M.; Viscardi, E.; Bisogno, G.; Perilongo, G.; Da Dalt, L. Diencephalic syndrome as sign of tumor progression in a child with neurofibromatosis type 1 and optic pathway glioma: A case report. Child’s Nerv. Syst. 2013, 29, 1941–1945. [Google Scholar] [CrossRef]
- Ertiaei, A.; Hanaei, S.; Habibi, Z.; Moradi, E.; Nejat, F. Optic Pathway Gliomas: Clinical Manifestation, Treatment, and Follow-Up. Pediatr. Neurosurg. 2016, 51, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rakotonjanahary, J.; De Carli, E.; Delion, M.; Kalifa, C.; Grill, J.; Doz, F.; Leblond, P.; Bertozzi, A.-I.; Rialland, X. Brain Tumor Committee of SFCE. Mortality in Children with Optic Pathway Glioma Treated with Up-Front BB-SFOP Chemotherapy. PLoS ONE 2015, 10, e0127676. [Google Scholar] [CrossRef] [PubMed]
- Poussaint, T.Y.; Barnes, P.D.; Nichols, K.; Anthony, D.C.; Cohen, L.; Tarbell, N.J.; Goumnerova, L. Diencephalic syndrome: Clinical features and imaging findings. Am. J. Neuroradiol. 1997, 18, 1499–1505. [Google Scholar] [PubMed]
- Santoro, C.; Perrotta, S.; Picariello, S.; Scilipoti, M.; Cirillo, M.; Quaglietta, L.; Cinalli, G.; Cioffi, D.; Di Iorgi, N.; Maghnie, M.; et al. Pretreatment Endocrine Disorders Due to Optic Pathway Gliomas in Pediatric Neurofibromatosis Type 1: Multicenter Study. J. Clin. Endocrinol. Metab. 2020, 105, dgaa138. [Google Scholar] [CrossRef]
- Gan, H.-W.; Phipps, K.; Aquilina, K.; Gaze, M.N.; Hayward, R.; Spoudeas, H.A. Neuroendocrine Morbidity After Pediatric Optic Gliomas: A Longitudinal Analysis of 166 Children Over 30 Years. J. Clin. Endocrinol. Metab. 2015, 100, 3787–3799. [Google Scholar] [CrossRef]
- Lodi, M.; Boccuto, L.; Carai, A.; Cacchione, A.; Miele, M.; Colafati, G.S.; Camassei, F.D.; De Palma, L.; De Benedictis, A.; Ferretti, E.; et al. Low-Grade Gliomas in Patients with Noonan Syndrome: Case-Based Review of the Literature. Diagnostics 2020, 10, 582. [Google Scholar] [CrossRef]
- Ranalli, M.; Boni, A.; Caroleo, A.; Del Baldo, G.; Rinelli, M.; Agolini, E.; Rossi, S.; Miele, E.; Colafati, G.S.; Boccuto, L.; et al. Molecular Characterization of Medulloblastoma in a Patient with Neurofibromatosis Type 1: Case Report and Literature Review. Diagnostics 2021, 11, 647. [Google Scholar] [CrossRef]
- Aftab, S.; Dattani, M.T. Pathogenesis of Growth Failure in Rasopathies. Pediatr. Endocrinol. Rev. 2019, 16, 447–458. [Google Scholar] [CrossRef]
- Picariello, S.; Cerbone, M.; D’Arco, F.; Gan, H.; O’Hare, P.; Aquilina, K.; Opocher, E.; Hargrave, D.; Spoudeas, H.A. A 40-Year Cohort Study of Evolving Hypothalamic Dysfunction in Infants and Young Children (<3 years) with Optic Pathway Gliomas. Cancers 2022, 14, 747. [Google Scholar] [CrossRef]
- Packer, R.J.; Kilburn, L. Molecular-Targeted Therapy for Childhood Brain Tumors s: A Moving Target. J. Child Neurol. 2020, 35, 791–798. [Google Scholar] [CrossRef]
- Guido, C.; Baldari, C.; Maiorano, G.; Mastronuzzi, A.; Carai, A.; Quintarelli, C.; De Angelis, B.; Cortese, B.; Gigli, G.; Palamà, I.E. Nanoparticles for Diagnosis and Target Therapy in Pediatric Brain Cancers. Diagnostics 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
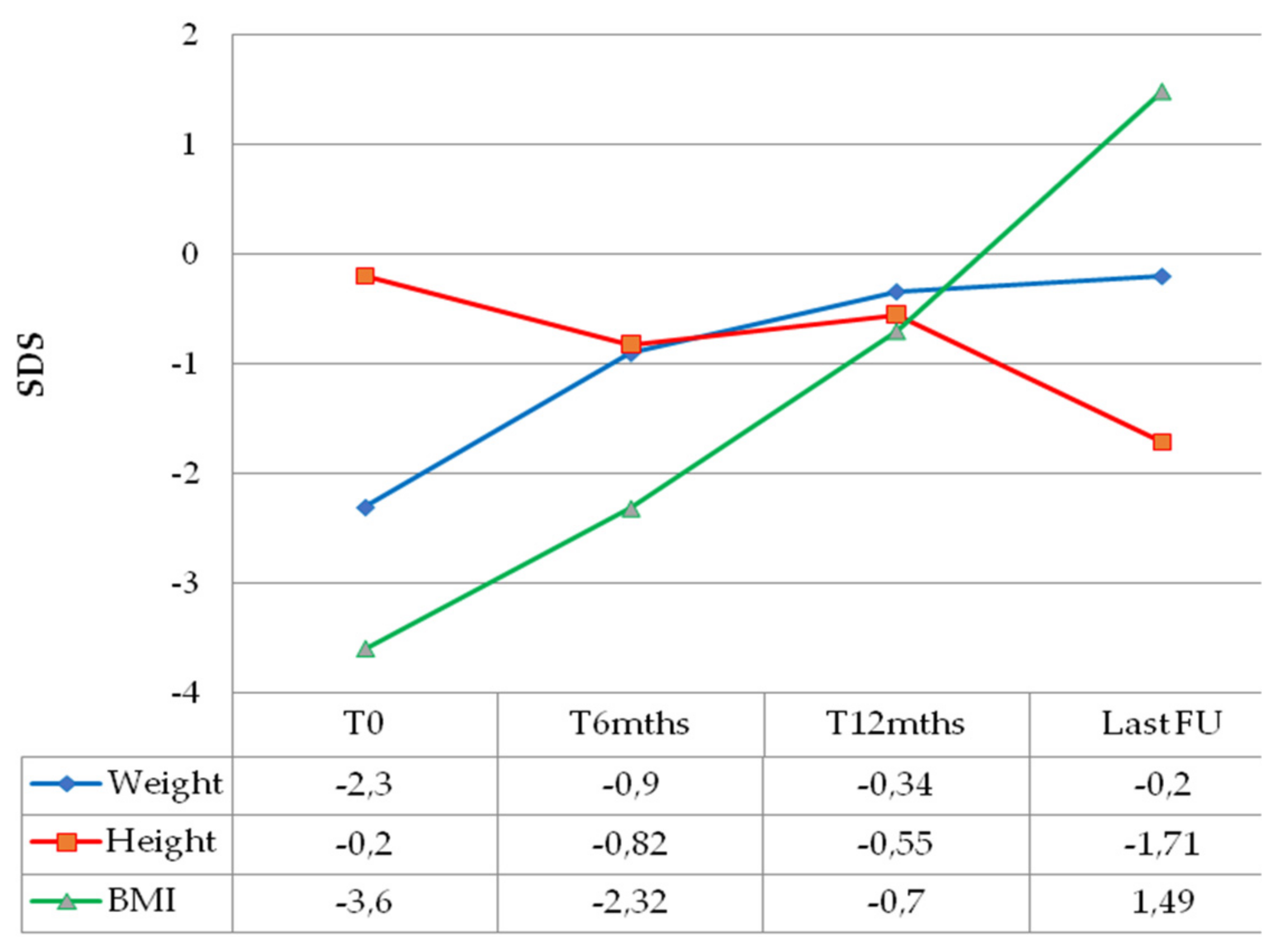

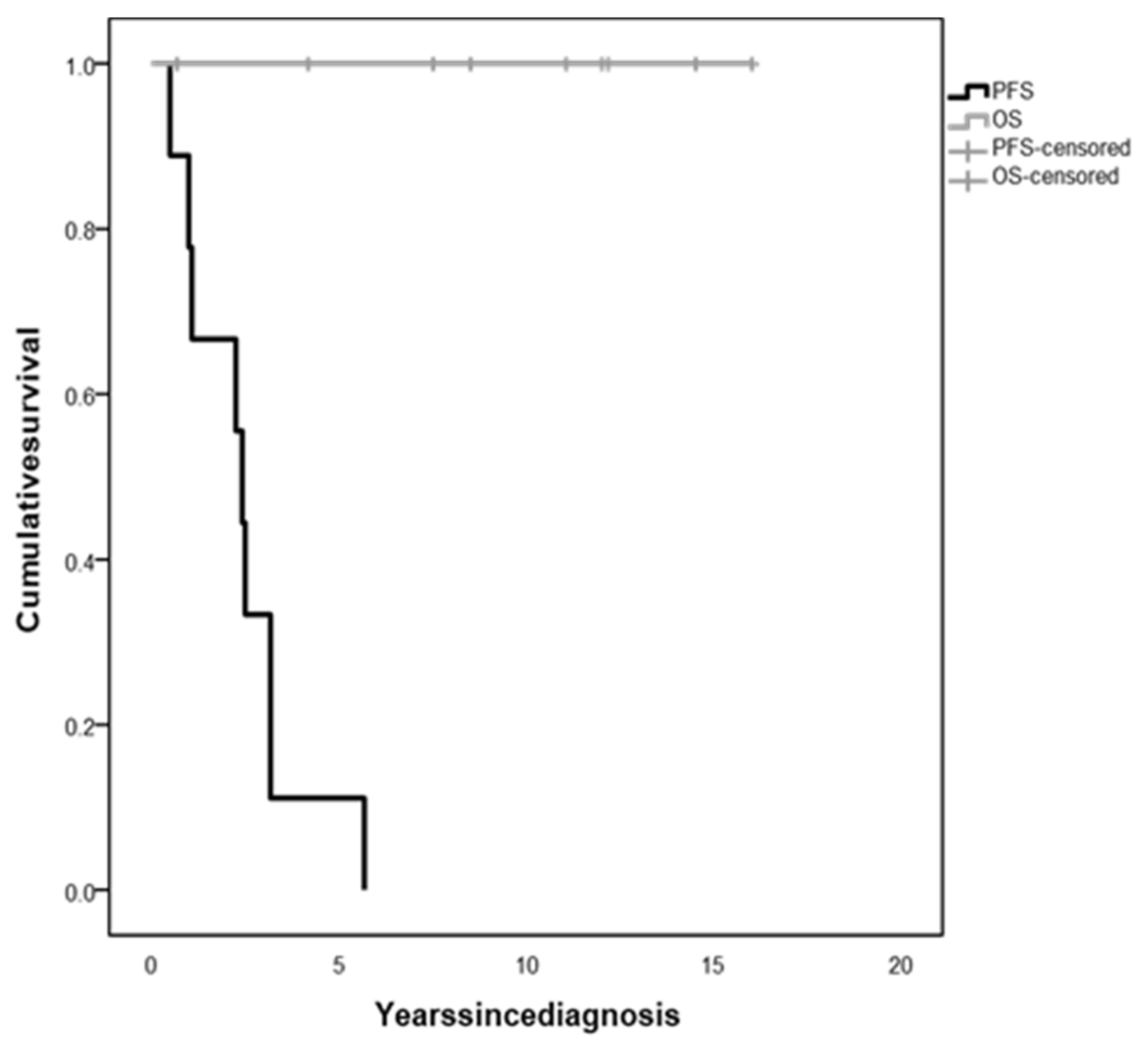
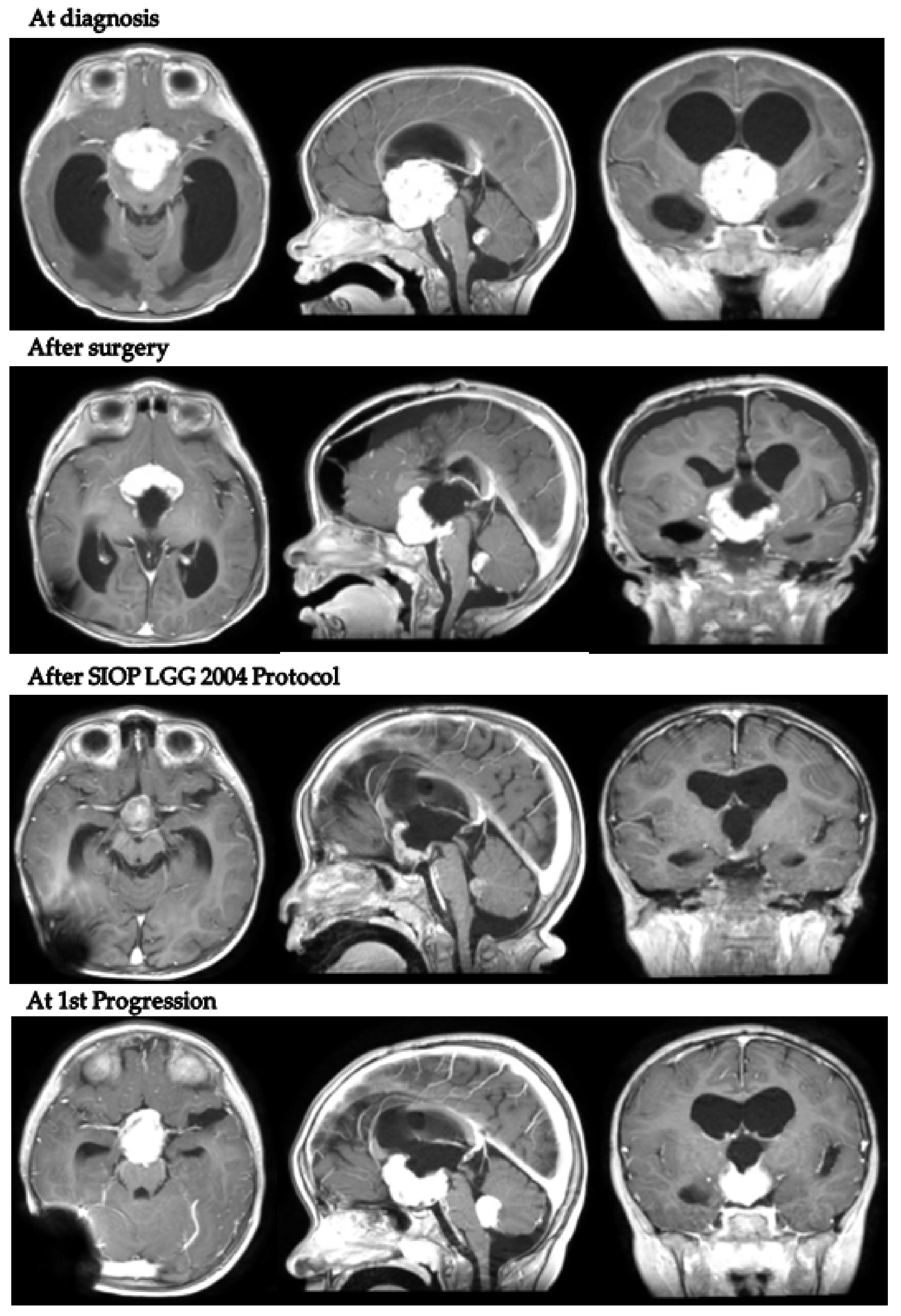
| Sex | Age at OPG Diagnosis (Months) | NF1 Status | PLAN Classification | Histology | BRAF Status | Weight (SDS) | Height (SDS) | BMI (SDS) | Hydrocephalus | Additional Signs/Symptoms | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt1 | M | 3 | Negative | 2a 3b H+ | PXA | N/A | −3.49 | −1.24 | −3.94 | Yes | Nystagmus |
| Pt2 | F | 26 | Positive | 3b H+ | N/A | N/A | −0.76 | 1.13 | −2.05 | No | Nystagmus, strabismus, ataxia, speech delay |
| Pt3 | M | 21 | Positive | 2a H+ | PA | N/A | −1.32 | 0.22 | −2.17 | No | Optic atrophy |
| Pt4 | M | 24 | Positive | 1cL 2bL > R H+ | N/A | N/A | −1.25 | 0.36 | −2.01 | No | Nystagmus, strabismus |
| Pt5 | M | 17 | Negative | 1b 2a H+ LM+ | PXA | V600E mutation | −2.25 | −0.80 | −2.62 | Yes | Strabismus, axial hypotonia, macrocrania |
| Pt6 | M | 7 | Negative | 2bR > L H+ | PA | Wild type | −1.57 | −0.16 | −2.03 | Yes | Nystagmus, strabismus |
| Pt7 | M | 9 | Negative | 2a H+ | PA | Wild type | −2.82 | −0.51 | −3.62 | No | Strabismus |
| Pt8 | F | 14 | Positive + Noonan syndrome | 1aL 2a 3B H+ | PA | Wild type | −4.56 | −2.40 | −4.76 | No | Strabismus |
| Pt9 | F | 13 | Negative | 2aH+ | PXA | BRAF- KIAA1549 fusion | −1.95 | −0.56 | −2.32 | Yes | Nystagmus |
| Patient 1 a | Patient 2 a | Patient 3 a | Patient 4 a | Patient 5 b | Patient 6 b | Patient 7 b | Patient 8 b | Patient 9 b | |
|---|---|---|---|---|---|---|---|---|---|
| Age at last follow-up (years) | 8.42 | 16.17 | 7.58 | 17.17 | 5.50 | 12.00 | 8.75 | 13.00 | 1.60 |
| First-line chemotherapy | SIOP LGG 2004 | SIOP LGG 2004 | SIOP LGG 2004 | HIT-LGG 1996 | SIOP LGG 2004 | SIOP LGG 2004 | SIOP LGG 2004 | SIOP LGG 2004 | SIOP LGG 2004 |
| Number of progressions | 3 | 2 | 4 | 2 | 1 | 5 | 4 | 1 | 1 |
| Chemotherapy at tumor progression | No | N/A | Vinblastine; TMZ | SIOP LGG 2004 | Dabrafenib + trametinib | VCR + CPX + cisplatin | VCR + CPX + cisplatin; bevacizumab + irinotecan | VCR + CPX + cisplatin | Trametinib |
| Current chemotherapy | Off therapy 2012 | Off therapy 2008 | Ongoing | Off therapy 2014 | Ongoing | Off therapy 2018 | Off therapy 2019 | Off therapy 2016 | Ongoing |
| Radiotherapy | No | No | No | No | No | Yes | Yes | No | No |
| Number of neurosurgeries | 6 | 1 | 1 | 1 | 3 | 8 | 3 | 3 | 2 |
| Year of last neurosurgery | 2014 | 2006 | 2016 | 2005 | 2019 | 2018 | 2019 | 2019 | 2021 |
| Progression time (years) | 3.17 | 1.08 | 25 | 5.67 | 2.42 | 3.17 | 2.5 | 1.00 | 0.5 |
| Follow-up (years) | 8.2 | 15 | 5.8 | 15.2 | 4 | 11.3 | 7.9 | 11.7 | 1 |
| Endocrine morbidity | ACTHD, TSHD, CDI | CPP, TSHD, GHD, GnD, obesity | ACTHD, TSHD, CDI | CPP, TSHD, obesity | None | TSHD, GHD, obesity | CDI, TSHD, CPP | CPP | No |
| Neurological outcome | Psychomotor delay | Normal | Learning difficulties | Normal | Speech disorder | Seizures, psychomotor delay | Normal | Learning difficulties, hemiparesis | Normal |
| Visual outcome Right eye Left eye | NLP NLP | NLP NLP | NLP LP | NLP NLP | 2/10 4/10 | 1/12 8/10 | LP 10/10 | 4/10 4/10 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Martino, L.; Picariello, S.; Triarico, S.; Improda, N.; Spennato, P.; Capozza, M.A.; Grandone, A.; Santoro, C.; Cioffi, D.; Attinà, G.; et al. Diencephalic Syndrome Due to Optic Pathway Gliomas in Pediatric Patients: An Italian Multicenter Study. Diagnostics 2022, 12, 664. https://doi.org/10.3390/diagnostics12030664
De Martino L, Picariello S, Triarico S, Improda N, Spennato P, Capozza MA, Grandone A, Santoro C, Cioffi D, Attinà G, et al. Diencephalic Syndrome Due to Optic Pathway Gliomas in Pediatric Patients: An Italian Multicenter Study. Diagnostics. 2022; 12(3):664. https://doi.org/10.3390/diagnostics12030664
Chicago/Turabian StyleDe Martino, Lucia, Stefania Picariello, Silvia Triarico, Nicola Improda, Pietro Spennato, Michele Antonio Capozza, Anna Grandone, Claudia Santoro, Daniela Cioffi, Giorgio Attinà, and et al. 2022. "Diencephalic Syndrome Due to Optic Pathway Gliomas in Pediatric Patients: An Italian Multicenter Study" Diagnostics 12, no. 3: 664. https://doi.org/10.3390/diagnostics12030664
APA StyleDe Martino, L., Picariello, S., Triarico, S., Improda, N., Spennato, P., Capozza, M. A., Grandone, A., Santoro, C., Cioffi, D., Attinà, G., Cinalli, G., Ruggiero, A., & Quaglietta, L. (2022). Diencephalic Syndrome Due to Optic Pathway Gliomas in Pediatric Patients: An Italian Multicenter Study. Diagnostics, 12(3), 664. https://doi.org/10.3390/diagnostics12030664







