The Significance of Systemic Immune-Inflammatory Index for Mortality Prediction in Diabetic Patients Treated with Off-Pump Coronary Artery Bypass Surgery
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. All Groups
3.2. Diabetic Group
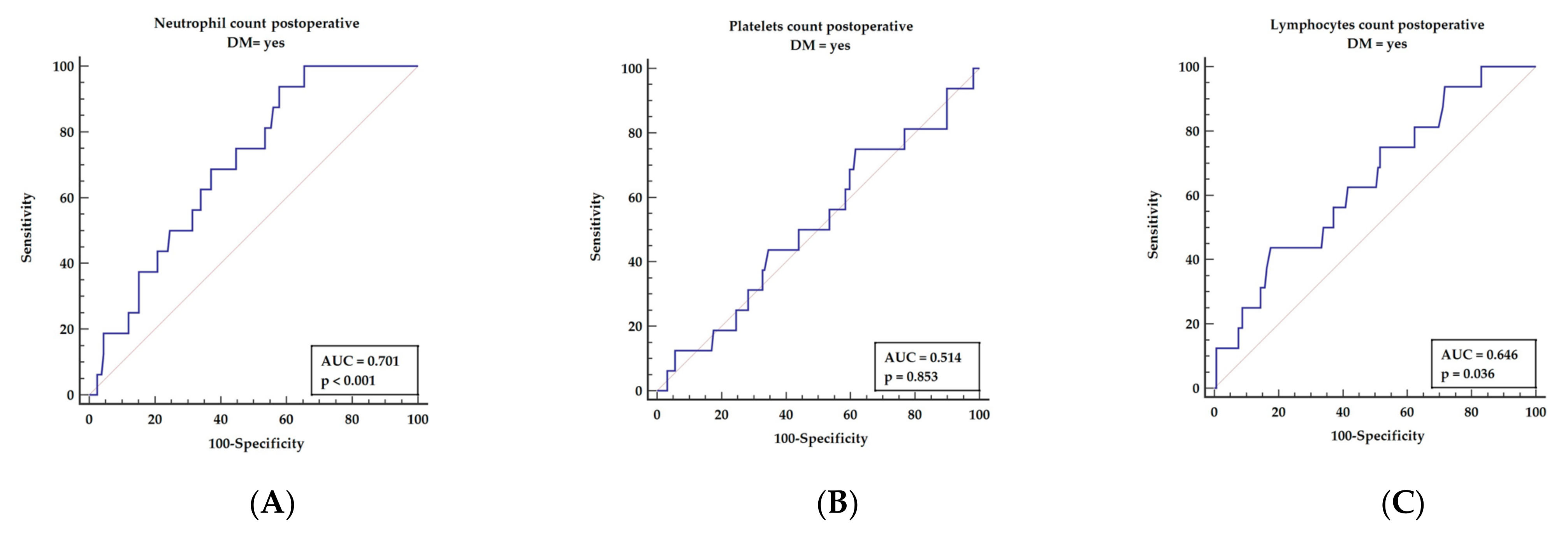
3.3. Receiver Operator Characteristics for Postoperative Inflammatory Markers Revealed in Multivariable Analysis
3.4. Receiver Operator Curve for Postoperative Inflammatory Markers, including Components of the SII
3.5. Univariable Analysis
3.6. Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef]
- Bornfeldt, K.E. Does Elevated Glucose Promote Atherosclerosis? Pros and Cons. Circ. Res. 2016, 119, 190–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herder, C.; Schamarek, I.; Nowotny, B.; Carstensen-Kirberg, M.; Straßburger, K.; Nowotny, P.; Kannenberg, J.M.; Strom, A.; Püttgen, S.; Müssig, K.; et al. Inflammatory markers are associated with cardiac autonomic dysfunction in recent-onset type 2 diabetes. Heart 2017, 103, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Pickup, J.C.; Mattock, M.B.; Chusney, G.D.; Burt, D. NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997, 40, 1286–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, H.A.; Davis-Gorman, G.; Goldman, S.; Copeland, J.G.; McDonagh, P.F. Proinflammatory cytokines are increased in type 2 diabetic women with cardiovascular disease. J. Diabetes Complicat. 2004, 18, 343–351. [Google Scholar] [CrossRef]
- Darakjian, L.; Deodhar, M.; Turgeon, J.; Michaud, V. Chronic Inflammatory Status Observed in Patients with Type 2 Diabetes Induces Modulation of Cytochrome P450 Expression and Activity. Int. J. Mol. Sci. 2021, 22, 4967. [Google Scholar] [CrossRef]
- Park, K.H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Nocuń-Wasilewska, K.; Zwolińska, D.; Zubkiewicz-Kucharska, A.; Polak-Jonkisz, D. Evaluation of Vascular Endothelial Function in Children with Type 1 Diabetes Mellitus. J. Clin. Med. 2021, 10, 5065. [Google Scholar] [CrossRef]
- Maschirow, L.; Khalaf, K.; Al-Aubaidy, H.A.; Jelinek, H.F. Inflammation, coagulation, endothelial dysfunction andoxidative stress in prediabetes—biomarkers as a possible tool for early disease detection for rural screening. Clin. Biochem. 2015, 48, 581–585. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Shim, J.K.; Youn, Y.N.; Song, J.W.; Lee, H.; Kwak, Y.L. Influence of preoperative hemoglobin A1c on early outcomes in patients with diabetes mellitus undergoing off-pump coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2020, 159, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Camici, P.G.; Bairey Merz, C.N. Coronary microvascular dysfunction: Anupdate. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserman, D.H.; Wang, T.J.; Brown, N.J. The Vasculature in Prediabetes. Circ. Res. 2018, 122, 1135–1150. [Google Scholar] [CrossRef]
- Gonzales-Martinez, S.; Tabuena, N.O.; Baranera, M.M.; Marti-Sauri, I.; Moll, J.L.; García, M.Á.M.; Grau, N.B.; Zurdo, J.M.P. Inflammatory markers as predictors of postoperative adverse outcome in octogenarian surgical patients: An observational prospective study. Cirugía Española 2015, 93, 166–173. [Google Scholar] [CrossRef]
- Sapienza, P.; Mingoli, A.; Borrelli, V.; Grande, R.; Sterpetti, A.V.; Biacchi, D.; Ferrer, C.; Rubino, P.; Serra, R.; Tartaglia, E. Different inflammatory cytokines release after open and endovascular reconstructions influences wound healing. Int. Wound J. 2019, 16, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention 2019, 14, 1435–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vejlstrup, A.; Møller, A.M.; Nielsen, C.H.; Damgaard, D. Release of active peptidylarginine deiminase into the circulation during acute inflammation induced by coronary artery bypass surgery. J. Inflamm. Res. 2019, 12, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Greilich, P.E.; Brouse, C.F.; Rinder, H.M.; Jessen, M.E.; Rinder, C.S.; Eberhart, R.C.; Whitten, C.W.; Smith, B.R. Monocyte activation in on-pump versus off-pump coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 2008, 22, 361–368. [Google Scholar] [CrossRef]
- Gaudino, M.; Angelini, G.D.; Antoniades, C.; Bakaeen, F.; Benedetto, U.; Calafiore, A.M.; Di Franco, A.; Di Mauro, M.; Fremes, S.E.; Girardi, L.N.; et al. Off-Pump Coronary Artery Bypass Grafting: 30 Years of Debate. J. Am. Heart Assoc. 2018, 7, e009934–e009949. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Kandula, P.; Hix, J.K.; Thakar, C.V. Off-pump coronary artery bypass surgery and acute kidney injury: A meta-analysis of randomized and observational studies. Am. J. Kidney Dis. 2009, 54, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ruzzeh, S.; Nakamura, K.; Athanasiou, T.; Modine, T.; George, S.; Yacoub, M.; Ilsley, C.; Amrani, M. Does off-pump coronary artery bypass (OPCAB) surgery improve the outcome in high-risk patients: A comparative study of 1398 high-risk patients. Eur. J. Cardiothorac. Surg. 2003, 23, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Urbanowicz, T.; Michalak, M.; Olasińska-Wiśniewska, A.; Witkowska, A.; Rodzki, M.; Błażejowska, E.; Gąsecka, A.; Perek, B.; Jemielity, M. Monocyte-to-Lymphocyte Ratio as a Predictor of Worse Long-Term Survival after Off-Pump Surgical Revascularization-Initial Report. Medicina (Kaunas) 2021, 57, 1324. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Michalak, M.; Mikołajewska, W.; Rodzki, M.; Perek, B.; Olasińska-Wiśniewska, A.; Bociański, M.; Jemielity, M. Mean platelet volume as a simple marker of repeated coronary artery intervention after off-pump technique (OPCAB) procedures—Initial report. Kardiochirurgia I Torakochirurgia Pol./Pol. J. Thorac. Cardiovasc. Surg. 2021, 18, 231–235. [Google Scholar] [CrossRef]
- Jędrychowska, M.; Januszek, R.; Wańha, W.; Malinowski, K.P.; Kunik, P.; Trznadel, A.; Bartuś, J.; Staszczak, B.; Januszek, S.M.; Kameczura, T.; et al. Long-Term Prognostic Significance of High-Sensitive Troponin I Increase during Hospital Stay in Patients with Acute Myocardial Infarction and Non-Obstructive Coronary Arteries. Medicina (Kaunas) 2020, 56, 432. [Google Scholar] [CrossRef] [PubMed]
- Mol, K.H.J.M.; Hoeks, S.E.; Liem, V.G.B.; Stolker, R.J.; van Lier, F. Postoperative troponin release is associated with major adverse cardiovascular events in the first year after noncardiac surgery. Int. J. Cardiol. 2019, 280, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Aljure, O.D.; Fabbro, M., 2nd. Cardiopulmonary Bypass and Inflammation: The Hidden Enemy. J. Cardiothorac. Vasc. Anesth. 2019, 33, 346–347. [Google Scholar] [CrossRef] [Green Version]
- Lockwood, G. Bypass and inflammation. Perfusion 2017, 32, 90–91. [Google Scholar] [CrossRef] [Green Version]
- Perek, B.; Misterski, M.; Stachowiak, W.; Buczkowski, P.; Stefaniak, S.; Puślecki, M.; Urbanowicz, T.; Budniak, W.; Jemielity, M. The impact of coronary artery disease severity on late survival after combined aortic valve replacement and coronary artery bypass grafting—Experience of a single cardiac surgery center. Kardiochir. Torakochirurgia Pol. 2014, 11, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Tribak, M.; Konaté, M.; Ould Hbib, B.; Konan, P.; Mahfoudi, L.; Hassani, A.E.; Daouda, A.; Lachhab, F.; Bendagha, N.; Soufiani, A.; et al. Impact de l’annuloplastie mitrale combinée à la revascularisation chirurgicale dans l’insuffisance mitrale ischémique [Impact of mitral annuloplasty combined with surgical revascularization in ischemic mitral regurgitation]. Ann. Cardiol. Angeiol. (Paris) 2018, 67, 25–31. [Google Scholar] [CrossRef]
- Keskin, M.; Öcal, L.; Cerşit, S.; Yılmaz, C.; Küp, A.; Çelik, M.; Doğan, S.; Koyuncu, A.; Kaya, A.; Turkmen, M.M. The Predictive Role of a Novel Risk Index in Patients Undergoing Carotid Artery Stenting: Systemic Immune-Inflammation Index. J. Stroke Cerebrovasc. Dis. 2021, 30, 105955–105963. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, T.; Chen, L.; Jin, T.; Sheng, Y.; Wu, G.; Zong, G. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron. Artery Dis. 2021, 32, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, P.; Rozalski, M.; Wilczynski, M.; Golanski, J. Systemic immune-inflammation index (SII) and neutrophil to lymphocyte ratio (NLR) are useful markers for assessing effects of anti-inflammatory diet in patients before coronary artery bypass grafting. Rocz. Panstw. Zakl. Hig. 2021, 72, 327–335. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, R.; Ikram, M.A.; Voortman, T.; van Eijck, C.H.J.; Stricker, B.H. Reference values for white blood-cell- based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep. 2018, 8, 10566–10573. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Staburzyńska-Migaj, E.; Pawłowska, M.; Żabicki, B.; Michalak, M.; Filipiak, M.; Grajek, S.; Jemielity, M. EuroSCORE is a predictor of postoperative pericardial effusion following heart transplantation. Ann. Transplant. 2015, 20, 193–197. [Google Scholar]
- Milutinovic, A.V.; Krasic, S.D.; Zivkovic, I.S.; Cirkovic, A.M.; Lokas, S.Z.; Jovanovic, M.M.; Milojevic, P.S.; Peric, M.S. Prediction value of EuroSCORE II in total arterial revascularization and its usage in the evaluation of postoperative complications: Single-center experience. Asian Cardiovasc. Thorac. Ann. 2021, 29, 903–909. [Google Scholar] [CrossRef]
- Paone, S.; Baxter, A.A.; Hulett, M.D.; Poon, I.K.H. Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell. Mol. Life Sci. 2019, 76, 1093–1106. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Urbanowicz, T.K.; Michalak, M.; Gąsecka, A.; Olasińska-Wiśniewska, A.; Perek, B.; Rodzki, M.; Bociański, M.; Jemielity, M. A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting. J. Clin. Med. 2021, 10, 3032. [Google Scholar] [CrossRef]
- Siennicka, A.; Kłysz, M.; Adamska, M.; Chełstowski, K.; Biskupski, A.; Jastrzębska, M. Assessment of Platelet Reactivity and Inflammatory Markers in Coronary Artery Bypass Graft Patients Treated with Acetylsalicylic Acid with Flavonoid Supplementation. Molecules 2021, 26, 7486. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Michalak, M.; Gąsecka, A.; Perek, B.; Rodzki, M.; Bociański, M.; Straburzyńska-Migaj, E.; Jemielity, M. Postoperative Neutrophil to Lymphocyte Ratio as an Overall Mortality Midterm Prognostic Factor following OPCAB Procedures. Clin. Pract. 2021, 11, 587–597. [Google Scholar] [CrossRef] [PubMed]



| Parameters | DM Group (No. = 175) | Non-DM Group (No. = 335) | p |
|---|---|---|---|
| Demographical data: | |||
| 1. Age (years) (median (Q1–Q3)) | 67 (61–73) | 64 (59–70) | 0.002 * |
| 2. Gender (F (%)/M (%)) | 42 (24%)/133 (76%) | 57 (17%)/278 (83%) | 0.058 |
| Comorbidities: | |||
| 1. Arterial hypertension (median (Q1–Q3)) | 36 (21%) | 97 (29%) | 0.041 * |
| 2. COPD (median (Q1–Q3)) | 12 (97%) | 32 (10%) | 0.716 |
| 3. Hypercholesterolemia (median (Q1–Q3)) | 77 (44%) | 219 (65%) | <0.001 * |
| 4. PAD (median (Q1–Q3)) | 27 (15%) | 52 (16%) | 0.967 |
| Echocardiographic estimation of LVEF: | |||
| 1. Preoperative (%) (median (Q1–Q3)) | 55 (50–60) | 55 (50–60) | 0.349 |
| 2. Postoperative (%) (median (Q1–Q3)) | 55 (50–60) | 60 (50–60) | 0.773 |
| Parameters | DM Group (No. = 175) | Non-DM Group (No. = 335) | p |
|---|---|---|---|
| Preoperative laboratory results: | |||
| A. Whole blood count: | |||
| 1. WBC × 109/L (median (Q1–Q3)) | 7.7 (6.5–9.3) | 7.8 (6.4–9.2) | 0.979 |
| 2. Lymphocyte × 109/L (median (Q1–Q3)) | 1.8 (1.5–2.3) | 1.8 (1.4–2.2) | 0.415 |
| 3. Neutrophils × 109/L (median (Q1–Q3)) | 5.0 (4.0–6.2) | 5.1 (4.0–6.3) | 0.748 |
| 4. Monocytes × 109/L (median (Q1–Q3)) | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | 0.828 |
| 5. Hemoglobin × 109/L (median (Q1–Q3)) | 8.6 (8–9.2) | 8.7 (8.3–9.3) | 0.147 |
| 6. Platelets × 103/μL (median (Q1–Q3)) | 225 (189–270) | 228 (192–264) | 0.819 |
| B. Hematologic indexes: | |||
| 1. SIRI (median (Q1–Q3)) | 1.3 (0.8–1.9) | 1.3 (0.9–1.9) | 0.483 |
| 2. SII (median (Q1–Q3)) | 634 (431–916) | 619 (425–914) | 0.649 |
| 3. AISI (median (Q1–Q3)) | 280 (178–470) | 287 (172–440) | 0.764 |
| C. Myocardial injury: | |||
| Troponin on admission × 109/L (median (Q1–Q3)) | 0.01 (0.01–0.03) | 0.01 (0.01–0.02) | 0.527 |
| D. Kidney function: | |||
| Creatinine × 109/L (median (Q1–Q3)) | 86 (76–106) | 85 (71–99) | 0.096 |
| Postoperative laboratory results (24 h): | |||
| A. Whole blood count: | |||
| 7. WBC × 109/L (median (Q1–Q3)) | 8.6 (6.9–10.3) | 8.4 (7–10.4) | 0.925 |
| 8. Lymphocyte × 109/L (median (Q1–Q3)) | 1.9 (1.5–2.5) | 1.9 (1.5–2.5) | 0.983 |
| 9. Neutrophils × 109/L (median (Q1–Q3)) | 5.1 (3.8–6.6) | 4.9 (3.7–6.5) | 0.613 |
| 10. Monocytes × 109/L (median (Q1–Q3)) | 0.84 (0.7–1.1) | 0.9 (0.7–1.1) | 0.587 |
| 11. Hemoglobin mmol/L (median (Q1–Q3)) | 6.9 (6.6–7.3) | 6.8 (6.5–7.2) | 0.082 |
| 12. Platelets × 103/μL (median (Q1–Q3)) | 273 (227–341) | 283 (229–350) | 0.662 |
| B. Hematologic indexes: | |||
| 4. SIRI (median (Q1–Q3)) | 4.3 (2.6–7.1) | 4.2 (2.8–6.0) | 0.556 |
| 5. SII (median (Q1–Q3)) | 699 (507–1062) | 741 (497–1096) | 0.846 |
| 6. AISI (median (Q1–Q3)) | 616 (391–1030) | 634 (390–1088) | 0.729 |
| C. Myocardial injury: | |||
| Troponin on admission μmg/L (median (Q1–Q3)) | 1.5 (0.7–3.5) | 1.6 (0.7–3.8) | 0.615 |
| D. Kidney function: | |||
| Creatinine mmol/L (median (Q1–Q3)) | 96 (79–124) | 86 (71–107) | 0.048 * |
| Number of performed grafts (median (Q1–Q3)) | 2.3 (2.0–2.6) | 2.3 (2.1–2.5) | 0.782 |
| All-cause mortality during observation time (%) | 16 (9%) | 27 (8%) | 0.817 |
| Parameters | DM Deaths (No. = 16) | DM Survivors (No. = 159) | p |
|---|---|---|---|
| Age (years) (median (Q1–Q3)) | 70 (65–76) | 67 (61–73) | 0.097 |
| Gender (F (%)/M (%)) | 5 (31%)/11 (69%) | 37 (23%)/122 (77%) | 0.476 |
| Comorbidities: | |||
| 1. Arterial hypertension (%) | 2 (13%) | 34 (21%) | 0.402 |
| 2. COPD (%) | 4 (25%) | 11 (7%) | 0.014 * |
| 3. Stroke (%) | 6 (38%) | 7 (4%) | <0.001 * |
| 4. Hypercholesterolemia (%) | 6 (38%) | 71 (45%) | 0.583 |
| 5. PAD (%) | 6 (38%) | 21 (13%) | 0.010 * |
| Preoperative: | |||
| 1. WBC × 109/L (median (Q1–Q3)) | 7.6 (6.9–8.8) | 7.73 (6.5–9.3) | 0.709 |
| 2. Lymphocytes × 109/L (median (Q1–Q3)) | 1.6 (1.1–2.0) | 1.8 (1.5–2.3) | 0.105 |
| 3. Neutrophils × 109/L (median (Q1–Q3)) | 5.5 (4.4–6.5) | 5 (4.0–6.2) | 0.399 |
| 4. Monocytes × 109/L (median (Q1–Q3)) | 0.5 (0.3–0.5) | 0.5 (0.4–0.6) | 0.589 |
| 5. Hemoglobin mmol/L (median (Q1–Q3)) | 8.4 (7.5–9.4) | 8.6 (8.1–9.2) | 0.409 |
| 6. Platelets × 103/μL (median (Q1–Q3)) | 234 (209–278) | 224 (189–270) | 0.566 |
| Postoperative (24 h): | |||
| 1. WBC × 109/L (median (Q1–Q3)) | 9.3 (7.6–12) | 8.4 (6.7–10) | 0.107 |
| 2. Lymphocytes × 109/L (median (Q1–Q3)) | 1.7 (1.2–2.1) | 1.9 (1.6–2.5) | 0.055 |
| 3. Neutrophils × 109/L (median (Q1–Q3)) | 6.2 (5–7.9) | 5 (3.7–6.4) | 0.007 * |
| 4. Monocytes × 109/L (median (Q1–Q3)) | 1 (0.6–1.1) | 0.8 (0.7–1.1) | 0.798 |
| 5. Hemoglobin mmol/L (median (Q1–Q3)) | 6.9 (6.6–7.2) | 6.9 (6.6–7.3) | 0.881 |
| 6. Platelets × 103/L (median (Q1–Q3)) | 271 (231–327) | 273 (227–341) | 0.854 |
| Preoperative indexes: | |||
| 1. SIRI (median (Q1–Q3)) | 1.5 (1–2.2) | 1.2 (0.8–1.9) | 0.223 |
| 2. SII (median (Q1–Q3)) | 839 (611–1068) | 617 (422–904) | 0.059 |
| 3. AISI (median (Q1–Q3)) | 355 (217–537) | 267 (172–450) | 0.183 |
| Postoperative indexes: | |||
| 1. SIRI (median (Q1–Q3)) | 5.5 (3.5–9.6) | 4.1 (2.6–6.7) | 0.150 |
| 2. SII (median (Q1–Q3)) | 1097 (679–1956) | 686 (495–1015) | 0.008 * |
| 3. AISI (median (Q1–Q3)) | 1094 (495–1704) | 602 (358–978) | 0.045 * |
| Troponin-I: | |||
| 1. On admission mcg/L (median (Q1–Q3)) | 0.01 (0.01–0.01) | 0.01 (0.01–0.03) | 0.867 |
| 2. Maximum mcg/L (median (Q1–Q3)) | 2.6 (0.6–5.0) | 1.5 (0.7–3.1) | 0.539 |
| Creatinine | |||
| 1. Preoperative mmol/L (median (Q1–Q3)) | 97 (83–136) | 86 (75–102) | 0.232 |
| 2. Postoperative mmol/L (median (Q1–Q3)) | 102 (84–145) | 96 (78–116) | 0.351 |
| LVEF: | |||
| 1. Preoperative (%) (median (Q1–Q3)) | 55 (50–60) | 47 (43–51) | 0.504 |
| 2. Postoperative (%) (median (Q1–Q3)) | 55 (45–55) | 60 (50–60) | 0.091 |
| Parameter | HR | 95% CI | p-Value |
|---|---|---|---|
| Demographical and clinical: | |||
| 1. Age | 1.09 | 1.01–1.17 | 0.027 * |
| 2. Stroke | 7.25 | 2.47–21.27 | <0.001 * |
| 3. PAD | 4.35 | 1.51–12.55 | 0.050 * |
| Preoperative laboratory parameters: | |||
| 1. SIRI | 1.03 | 0.79–1.34 | 0.830 |
| 2. SII | 1.00 | 1.00–1.00 | 0.555 |
| 3. SII > 665 | 2.45 | 0.77–7.85 | 0.131 |
| 4. AISI | 1.00 | 0.99–1.01 | 0.896 |
| Preoperative laboratory parameters: | |||
| 1. Neutrophils postoperatively | 1.24 | 1.05–1.46 | 0.010 * |
| 2. SIRI | 1.07 | 0.94–1.20 | 0.299 |
| 3. SII | 3.43 | 1.00–1.00 | 0.001 * |
| 4. SII > 952 | 5.23 | 1.64–16.68 | 0.005 * |
| 5. AISI | 1.00 | 0.99–1.00 | 0.091 |
| 6. AISI > 1030 | 4.00 | 1.39–11.55 | 0.010 * |
| Postoperative LVEF | 0.90 | 0.86–0.95 | <0.001 * |
| Parameter | HR | 95% CI | p-Value |
|---|---|---|---|
| Clinical characteristics: | |||
| 1. Stroke | 3.39 | 1.06–10.91 | 0.040 * |
| 2. PAD | 3.83 | 1.27–11.56 | 0.017 * |
| Laboratory results: | |||
| SII post > 952 | 3.44 | 1.02–11.66 | 0.047 * |
| Echocardiographic: | |||
| LVEF < 45% postoperatively | 4.11 | 1.21–13.95 | 0.023 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowicz, T.; Michalak, M.; Al-Imam, A.; Olasińska-Wiśniewska, A.; Rodzki, M.; Witkowska, A.; Haneya, A.; Buczkowski, P.; Perek, B.; Jemielity, M. The Significance of Systemic Immune-Inflammatory Index for Mortality Prediction in Diabetic Patients Treated with Off-Pump Coronary Artery Bypass Surgery. Diagnostics 2022, 12, 634. https://doi.org/10.3390/diagnostics12030634
Urbanowicz T, Michalak M, Al-Imam A, Olasińska-Wiśniewska A, Rodzki M, Witkowska A, Haneya A, Buczkowski P, Perek B, Jemielity M. The Significance of Systemic Immune-Inflammatory Index for Mortality Prediction in Diabetic Patients Treated with Off-Pump Coronary Artery Bypass Surgery. Diagnostics. 2022; 12(3):634. https://doi.org/10.3390/diagnostics12030634
Chicago/Turabian StyleUrbanowicz, Tomasz, Michał Michalak, Ahmed Al-Imam, Anna Olasińska-Wiśniewska, Michał Rodzki, Anna Witkowska, Assad Haneya, Piotr Buczkowski, Bartłomiej Perek, and Marek Jemielity. 2022. "The Significance of Systemic Immune-Inflammatory Index for Mortality Prediction in Diabetic Patients Treated with Off-Pump Coronary Artery Bypass Surgery" Diagnostics 12, no. 3: 634. https://doi.org/10.3390/diagnostics12030634
APA StyleUrbanowicz, T., Michalak, M., Al-Imam, A., Olasińska-Wiśniewska, A., Rodzki, M., Witkowska, A., Haneya, A., Buczkowski, P., Perek, B., & Jemielity, M. (2022). The Significance of Systemic Immune-Inflammatory Index for Mortality Prediction in Diabetic Patients Treated with Off-Pump Coronary Artery Bypass Surgery. Diagnostics, 12(3), 634. https://doi.org/10.3390/diagnostics12030634







