Prognostic Value of Chemotherapy Response Score (CRS) Assessed on the Adnexa in Ovarian High-Grade Serous Carcinoma: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electronic Search and Study Selection
2.2. Data Extraction
2.3. Risk of Bias Assessment
2.4. Data Analysis
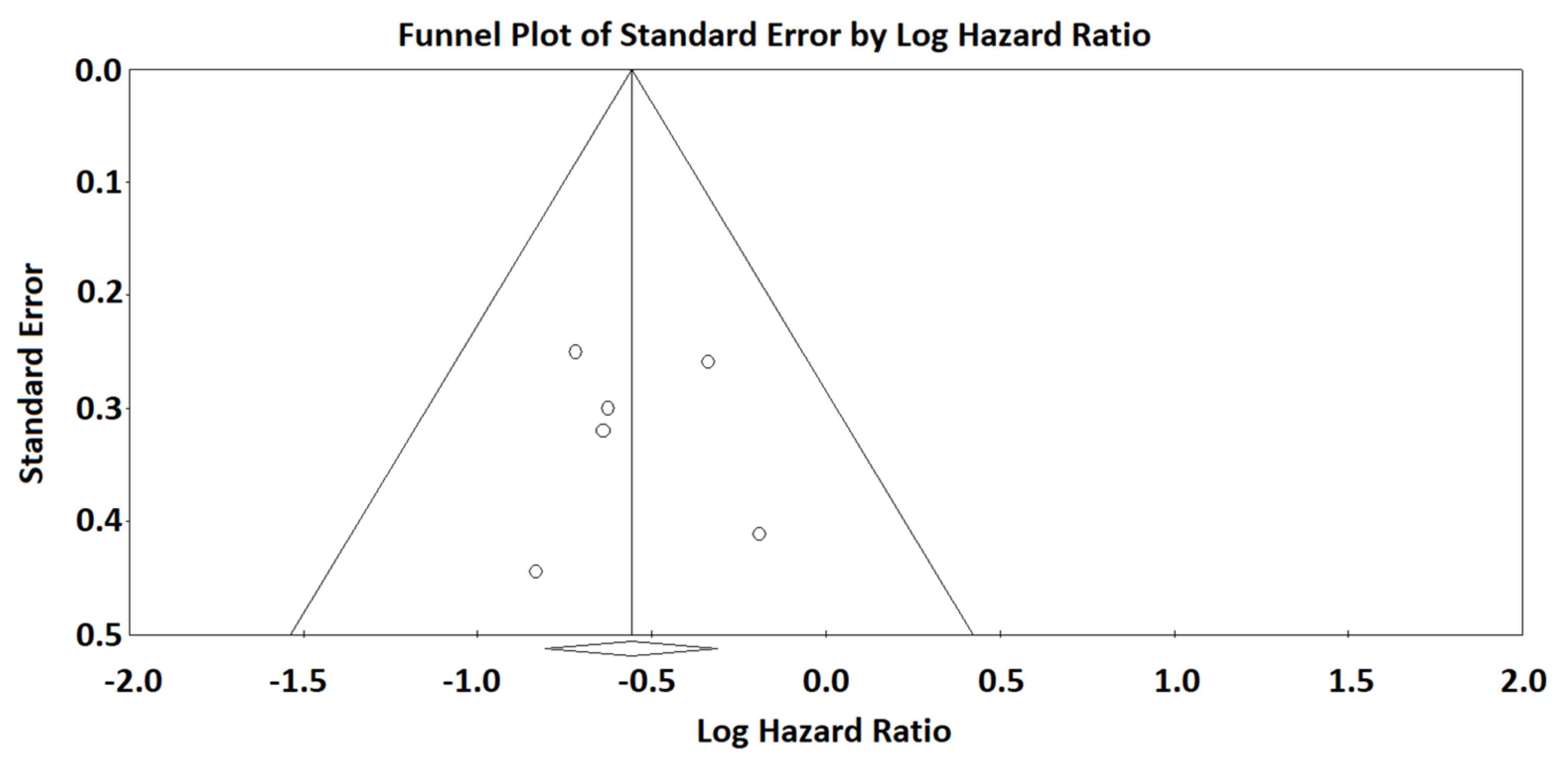
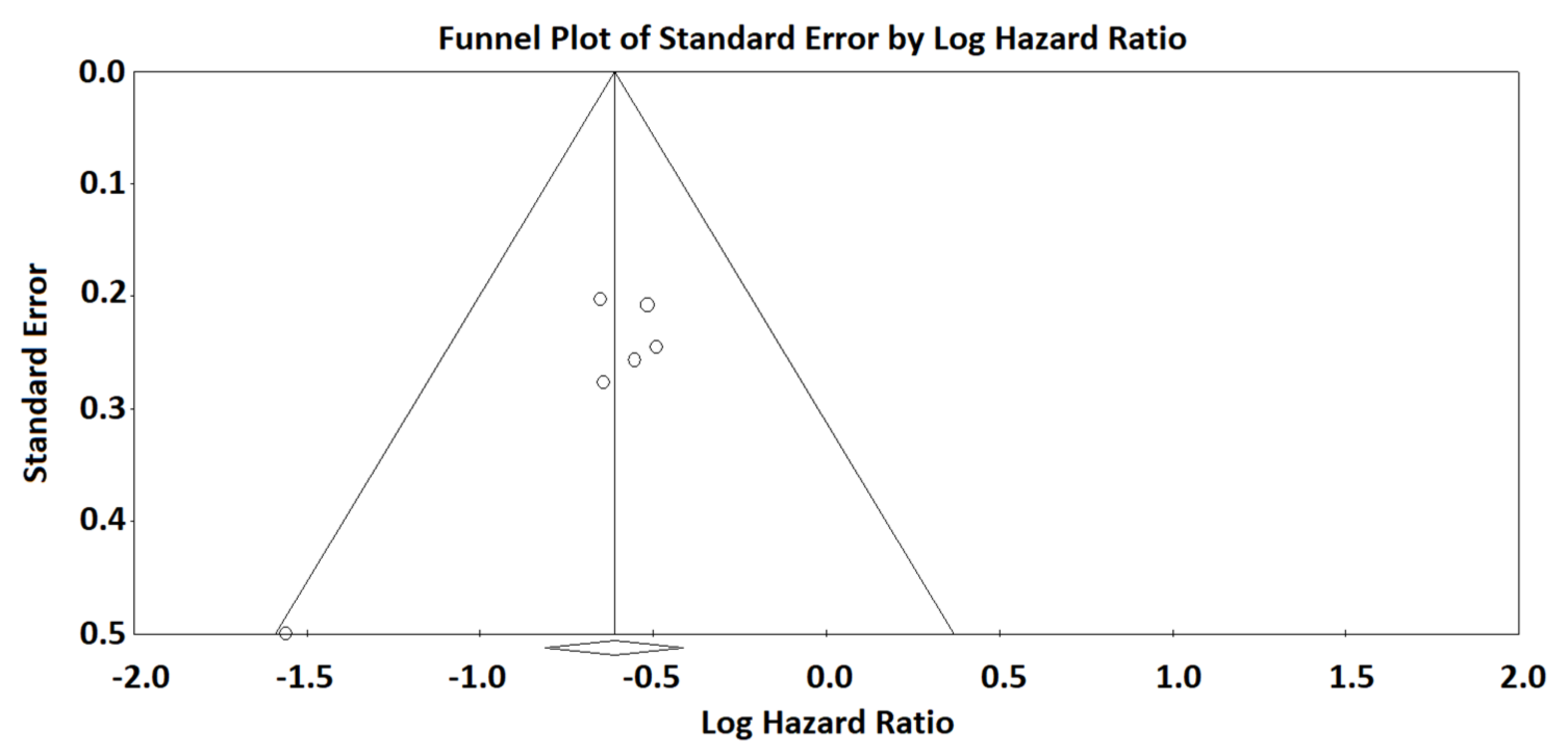
3. Results
Strengths and Limitations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lisio, M.-A.; Fu, L.; Goyeneche, A.; Gao, Z.-H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Zannoni, G.F.; Angelico, G.; Santoro, A. Aberrant non-canonical WNT pathway as key-driver of high-grade serous ovarian cancer development. Virchows Arch. 2020, 477, 321–322. [Google Scholar] [CrossRef]
- D’Alessandris, N.; Travaglino, A.; Santoro, A.; Arciuolo, D.; Scaglione, G.; Raffone, A.; Inzani, F.; Zannoni, G.F. TCGA molecular subgroups of endometrial carcinoma in ovarian endometrioid carcinoma: A quantitative systematic review. Gynecol. Oncol. 2021, 163, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Piermattei, A.; Santoro, A.; Angelico, G.; Inzani, F.; Valente, M.; Pettinato, A.; Vatrano, S.; Scambia, G.; Fraggetta, F.; Zannoni, G.F. Cerebellar Metastasis from Ovarian Carcinoma Harboring PIK3CA-Activating Mutation: A “Clear” Explanation for Unexpected “Vertigo”. Int. J. Gynecol. Pathol. 2020, 39, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- De Haven Brandon, A.; Box, G.; Hallsworth, A.; Court, W.; Matthews, N.; Herodek, B.; Arteagabeitia, A.B.; Valenti, M.; Kirkin, V. Identification of ovarian high-grade serous carcinoma cell lines that show estrogen-sensitive growth as xenografts in immunocompromised mice. Sci. Rep. 2020, 10, 10799. [Google Scholar] [CrossRef]
- Prislei, S.; Martinelli, E.; Zannoni, G.F.; Petrillo, M.; Filippetti, F.; Mariani, M.; Mozzetti, S.; Raspaglio, G.; Scambia, G.; Ferlini, C. Role and prognostic significance of the epithelial-mesenchymal transition factor ZEB2 in ovarian cancer. Oncotarget 2015, 6, 18966–18979. [Google Scholar] [CrossRef] [Green Version]
- D’Andrilli, G.; Masciullo, V.; Bagella, L.; Tonini, T.; Minimo, C.; Zannoni, G.F.; Giuntoli, R.L., 2nd; Carlson, J.A., Jr.; Soprano, D.R.; Soprano, K.J.; et al. Frequent loss of pRb2/p130 in human ovarian carcinoma. Clin. Cancer Res. 2004, 10, 3098–3103. [Google Scholar] [CrossRef] [Green Version]
- Petrillo, M.; Zannoni, G.; Beltrame, L.; Martinelli, E.; DiFeo, A.; Paracchini, L.; Craparotta, I.; Mannarino, L.; Vizzielli, G.; Scambia, G.; et al. Identification of high-grade serous ovarian cancer miRNA species associated with survival and drug response in patients receiving neoadjuvant chemotherapy: A retrospective longitudinal analysis using matched tumor biopsies. Ann. Oncol. 2016, 27, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, A.; Zannoni, G.F.; Travaglia, D.; Petrillo, M.; Scambia, G.; Gallo, D. Prognostic significance of the estrogen receptor beta (ERβ) isoforms ERβ1, ERβ2, and ERβ5 in advanced serous ovarian cancer. Gynecol. Oncol. 2014, 132, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, A.; De Stefano, I.; Vellone, V.G.; Lisi, L.; Bottoni, C.; Scambia, G.; Zannoni, G.F.; Gallo, D. Expression of the Glioma-Associated Oncogene Homolog 1 (Gli1) in Advanced Serous Ovarian Cancer Is Associated with Unfavorable Overall Survival. PLoS ONE 2013, 8, e60145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.-J.; Bristow, R.E. Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: Redefining ‘optimal’ residual disease. Gynecol. Oncol. 2012, 125, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.; Kitchener, H.; Lopes, A.D.B.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Böhm, S.; Faruqi, A.; Said, I.; Lockley, M.; Brockbank, E.; Jeyarajah, A.; Fitzpatrick, A.; Ennis, D.; Dowe, T.; Santos, J.L.; et al. Chemotherapy Response Score: Development and Validation of a System to Quantify Histopathologic Response to Neoadjuvant Chemotherapy in Tubo-Ovarian High-Grade Serous Carcinoma. J. Clin. Oncol. 2015, 33, 2457–2463. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Judge, M.J.; Clarke, B.; Davidson, B.; Gilks, C.B.; Hollema, H.; Ledermann, J.A.; Matias-Guiu, X.; Mikami, Y.; Stewart, C.J.R.; et al. Data set for reporting of ovary, fallopian tube and primary peritoneal carcinoma: Recommendations from the International Collaboration on Cancer Reporting (ICCR). Mod. Pathol. 2015, 28, 1101–1122. [Google Scholar] [CrossRef]
- Ditzel, H.M.; Strickland, K.C.; Meserve, E.E.; Stover, E.; Konstantinopoulos, P.A.; Matulonis, U.A.; Muto, M.G.; Liu, J.F.; Feltmate, C.; Horowitz, N.; et al. Assessment of a Chemotherapy Response Score (CRS) System for Tubo-Ovarian High-Grade Serous Carcinoma (HGSC). Int. J. Gynecol. Pathol. 2019, 38, 230–240. [Google Scholar] [CrossRef]
- Singh, P.; Kaushal, V.; Rai, B.; Rajwanshi, A.; Gupta, N.; Dey, P.; Garg, R.; Rohilla, M.; Suri, V.; Ghoshal, S.; et al. The chemotherapy response score is a useful histological predictor of prognosis in high-grade serous carcinoma. Histopathology 2018, 72, 619–625. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Chung, Y.S.; Na, K.; Kim, H.M.; Park, C.K.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T.; Kim, H.-S. External validation of chemotherapy response score system for histopathological assessment of tumor regression after neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J. Gynecol. Oncol. 2017, 28, e73. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, S.; Polson, A.; Nath, R.; Lane, G.; Sayasneh, A.; Tranoulis, A.; Jakes, A.; Begum, S.; Mehra, G. EP1271 Prognostic implications of histological tumor regression (Böhm´s score) in patients receiving neoadjuvant chemotherapy for high grade serous tubal & ovarian carcinoma. ePoster 2019, 29, A640–A641. [Google Scholar] [CrossRef]
- Böhm, S.; Le, N.; Lockley, M.; Brockbank, E.; Faruqi, A.; Said, I.; Jeyarajah, A.; Wuntakal, R.; Gilks, B.; Singh, N. Histopathologic response to neoadjuvant chemotherapy as a prognostic biomarker in tubo-ovarian high-grade serous carcinoma: Updated Chemotherapy Response Score (CRS) results. Int. J. Gynecol. Cancer 2019, 29, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, E.; Meniawy, T.; Munro, A.; Bulsara, M.; Stewart, C.J.; Tan, A.; Koay, M.E.; Magee, D.; Codde, J.; Tan, J.; et al. Prognostic Role of Histological Tumor Regression in Patients Receiving Neoadjuvant Chemotherapy for High-Grade Serous Tubo-ovarian Carcinoma. Int. J. Gynecol. Cancer 2017, 27, 708–713. [Google Scholar] [CrossRef]
- Cohen, P.A.; Powell, A.; Böhm, S.; Gilks, C.B.; Stewart, C.J.; Meniawy, T.; Bulsara, M.; Avril, S.; Brockbank, E.C.; Bosse, T.; et al. Pathological chemotherapy response score is prognostic in tubo-ovarian high-grade serous carcinoma: A systematic review and meta-analysis of individual patient data. Gynecol. Oncol. 2019, 154, 441–448; Erratum in Gynecol Oncol. 2020, 157, 558–559; Erratum in Gynecol Oncol. 2021, 161, 328–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, A.; Angelico, G.; Piermattei, A.; Inzani, F.; Valente, M.; Arciuolo, D.; Spadola, S.; Mulè, A.; Zorzato, P.; Fagotti, A.; et al. Pathological Chemotherapy Response Score in Patients Affected by High Grade Serous Ovarian Carcinoma: The Prognostic Role of Omental and Ovarian Residual Disease. Front. Oncol. 2019, 9, 778. [Google Scholar] [CrossRef] [Green Version]
- Lawson, B.C.; Euscher, E.D.; Bassett, R.L.; Liu, J.; Ramalingam, P.; Zhong, Y.; Fleming, N.D.; Malpica, A. A 3-Tier Chemotherapy Response Score for Ovarian/Fallopian Tube/Peritoneal High-grade Serous Carcinoma: Is it Clinically Relevant? Am. J. Surg. Pathol. 2020, 44, 206–213. [Google Scholar] [CrossRef]
- Michaan, N.; Chong, W.Y.; Han, N.Y.; Lim, M.C.; Park, S.Y. Prognostic Value of Pathologic Chemotherapy Response Score in Patients With Ovarian Cancer After Neoadjuvant Chemotherapy. Int. J. Gynecol. Cancer 2018, 28, 1676–1682. [Google Scholar] [CrossRef]
- Zannoni, G.F.; Travaglino, A.; Raffone, A.; Arciuolo, D.; D’Alessandris, N.; Scaglione, G.; Tralongo, P.; Inzani, F.; Angelico, G.; Santoro, A. Depth of Stromal Invasion as the Most Prognostically Relevant Regression System in Locally Advanced Cervical Cancer after Neoadjuvant Treatment: A Systematic Review and Meta-Analysis Grading. Diagnostics 2021, 11, 1772. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Santoro, A.; Raimondo, D.; Angelico, G.; Valente, M.; Arciuolo, D.; Scaglione, G.; D’alessandris, N.; Casadio, P.; et al. Clear cell endometrial carcinomas with mismatch repair deficiency have a favorable prognosis: A systematic review and meta-analysis. Gynecol. Oncol. 2021, 162, 804–808. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Lyness, R.W.; Atkinson, R.J.; Dobbs, S.P.; Harley, I.; McClelland, H.R.; Price, J.H. Morphological effects of chemotherapy on ovarian carcinoma. J. Clin. Pathol. 2002, 55, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrington, D.A.; Felix, A.S.; Owda, R.; Suarez, A.A.; Cohen, D.W.; Senter, L.; Copeland, L.J.; Fowler, J.M.; Backes, F.J.; Cohn, D.E.; et al. Pathologic chemotherapy response score in epithelial ovarian cancer: Surgical, genetic, and survival considerations. Surg. Oncol. 2020, 34, 40–45. [Google Scholar] [CrossRef] [PubMed]
- McNulty, M.; Das, A.; Cohen, P.A.; Dean, A. Measuring response to neoadjuvant chemotherapy in high-grade serous tubo-ovarian carcinoma: An analysis of the correlation between CT imaging and chemotherapy response score. Int. J. Gynecol. Cancer 2019, 29, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Angelico, G.; Ieni, A.; Caltabiano, R.; Santoro, A.; Inzani, F.; Spadola, S.; Tuccari, G.; Macrì, A.; Zannoni, G.F. Evaluation of Beta-Catenin Subcellular Localization and Water Channel Protein AQP1 Expression as Predictive Markers of Chemo-Resistance in Ovarian High-Grade Serous Carcinoma: Comparative Study between Preoperative Peritoneal Biopsies and Surgical Samples. Diagnostics 2021, 11, 452. [Google Scholar] [CrossRef]
- Bodnar, L.; Stanczak, A.; Cierniak, S.; Smoter, M.; Cichowicz, M.; Kozlowski, W.; Szczylik, C.; Wieczorek, M.; Lamparska-Przybysz, M. Wnt/β-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancer. J. Ovarian Res. 2014, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Ranieri, G.; Annese, T.; Nico, B. Aquaporins in cancer. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 1550–1553. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Zhu, Z.; Zheng, M.; Wang, D.; Chen, Z.; Sun, H. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? J. Transl. Med. 2015, 13, 96. [Google Scholar] [CrossRef] [Green Version]
- Angelico, G.; Caltabiano, R.; Loreto, C.; Ieni, A.; Tuccari, G.; Ledda, C.; Rapisarda, V. Immunohistochemical Expression of Aquaporin-1 in Fluoro-Edenite-Induced Malignant Mesothelioma: A Preliminary Report. Int. J. Mol. Sci. 2018, 19, 685. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Airley, R.; Hewitt, S.M.; Marples, D. Heterogeneous expression of the aquaporin 1 (AQP1) water channel in tumors of the prostate, breast, ovary, colon and lung: A study using high density multiple human tumor tissue microarrays. Int. J. Oncol. 2005, 26, 1149–1158. [Google Scholar] [CrossRef] [PubMed]


| Study | Country | Sample Size | Period of Enrollment |
|---|---|---|---|
| Bohm 2015 | UK (test cohort) | 62 (test cohort) | 2009–2014 |
| Lee 2017 | Korea | 110 | 2006–2014 |
| Ditzel 2018 | Massachusetts (USA) | 68 (59 adnexal) | 2005–2012 |
| Michaan 2018 | Korea | 132 | 2009–2014 |
| Santoro 2019 | Italy | 161 | 2014–2017 |
| Lawson 2020 | Texas (USA) | 158 | 2013–2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, A.; Travaglino, A.; Inzani, F.; Straccia, P.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Angelico, G.; Piermattei, A.; et al. Prognostic Value of Chemotherapy Response Score (CRS) Assessed on the Adnexa in Ovarian High-Grade Serous Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 633. https://doi.org/10.3390/diagnostics12030633
Santoro A, Travaglino A, Inzani F, Straccia P, Arciuolo D, Valente M, D’Alessandris N, Scaglione G, Angelico G, Piermattei A, et al. Prognostic Value of Chemotherapy Response Score (CRS) Assessed on the Adnexa in Ovarian High-Grade Serous Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(3):633. https://doi.org/10.3390/diagnostics12030633
Chicago/Turabian StyleSantoro, Angela, Antonio Travaglino, Frediano Inzani, Patrizia Straccia, Damiano Arciuolo, Michele Valente, Nicoletta D’Alessandris, Giulia Scaglione, Giuseppe Angelico, Alessia Piermattei, and et al. 2022. "Prognostic Value of Chemotherapy Response Score (CRS) Assessed on the Adnexa in Ovarian High-Grade Serous Carcinoma: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 3: 633. https://doi.org/10.3390/diagnostics12030633
APA StyleSantoro, A., Travaglino, A., Inzani, F., Straccia, P., Arciuolo, D., Valente, M., D’Alessandris, N., Scaglione, G., Angelico, G., Piermattei, A., Cianfrini, F., Raffone, A., & Zannoni, G. F. (2022). Prognostic Value of Chemotherapy Response Score (CRS) Assessed on the Adnexa in Ovarian High-Grade Serous Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics, 12(3), 633. https://doi.org/10.3390/diagnostics12030633








