New Intraoperative Imaging Tools and Image-Guided Surgery in Gastric Cancer Surgery
Abstract
:1. Introduction
2. Perfusion Assessment with Indocyanine Green (ICG) Fluorescence Imaging (FI)
3. Intraoperative Guidance and Tumor Localization by Fluorescent Marking Clips
4. Lymph Node Mapping with ICG-FI in Gastric Carcinoma
5. Detection of Micrometastases in the Liver and Peritoneal Carcinomatosis
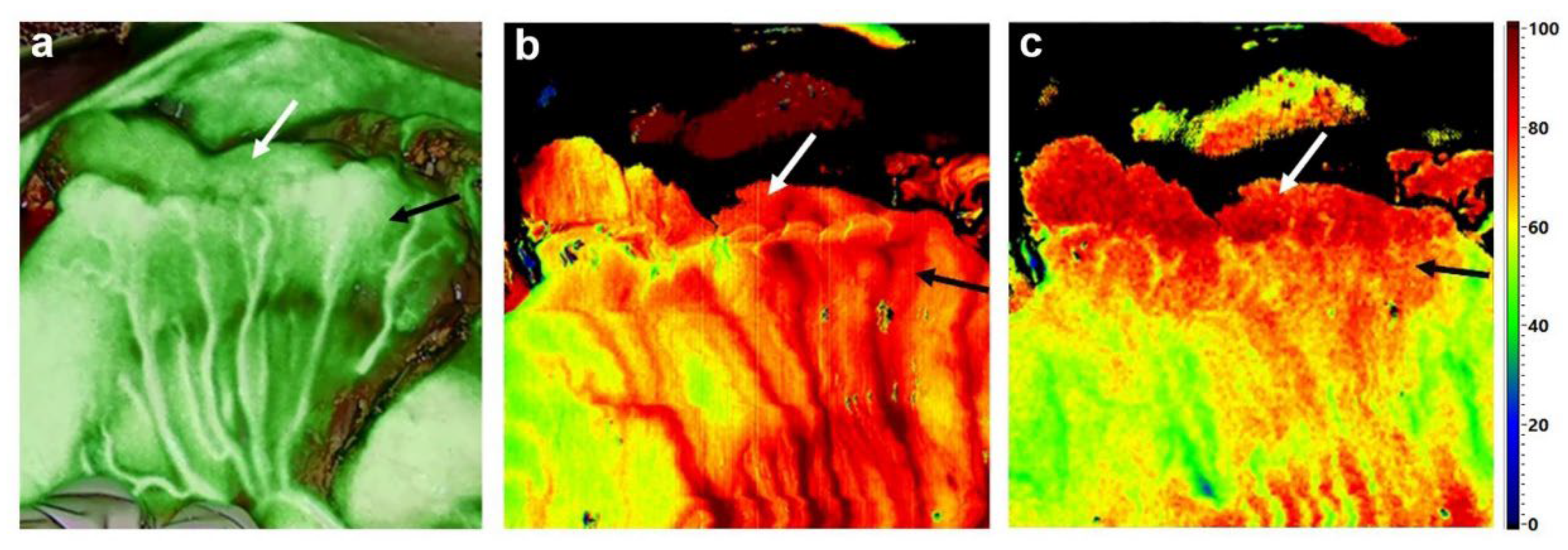
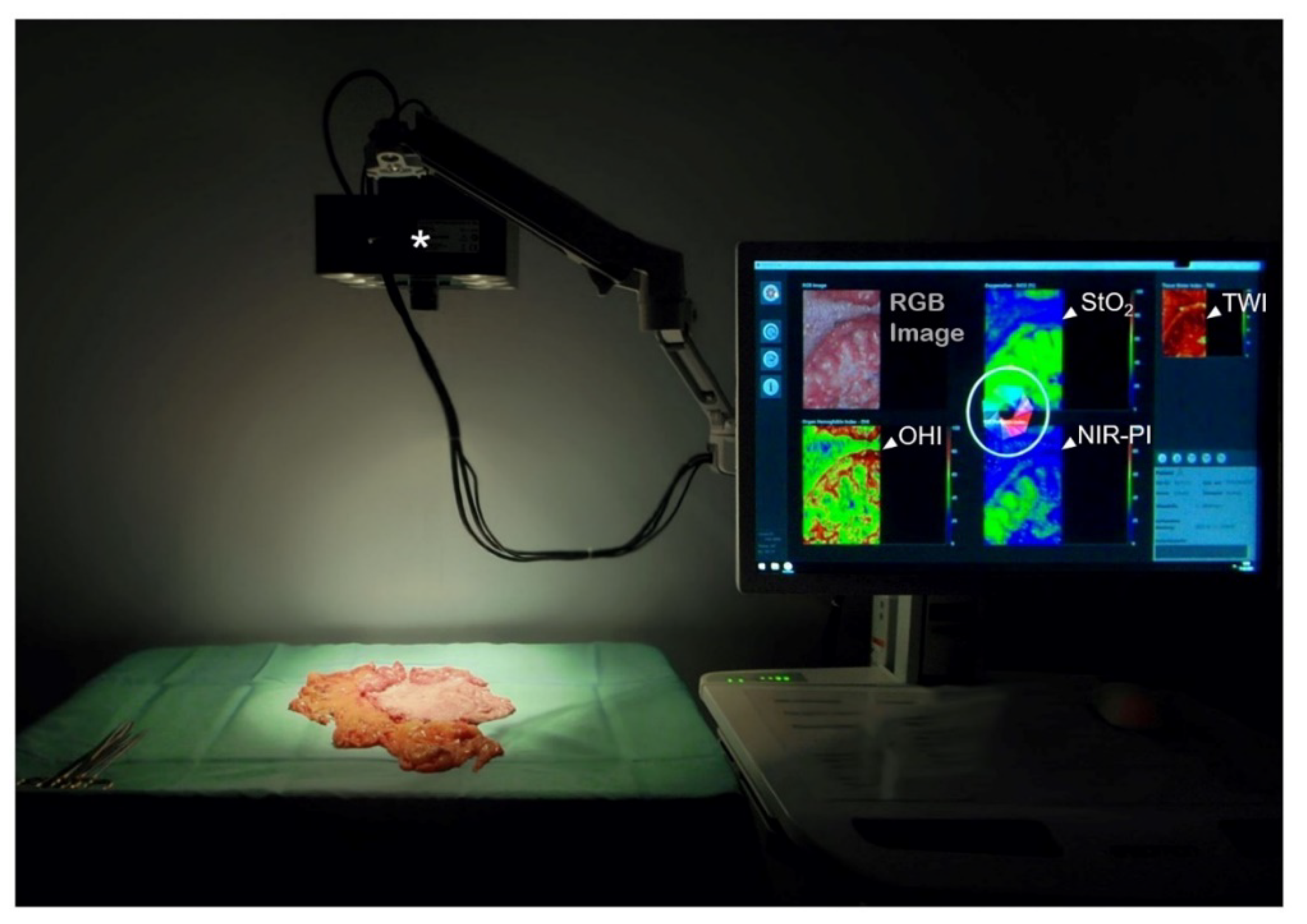
6. Hyperspectral Imaging (HSI)
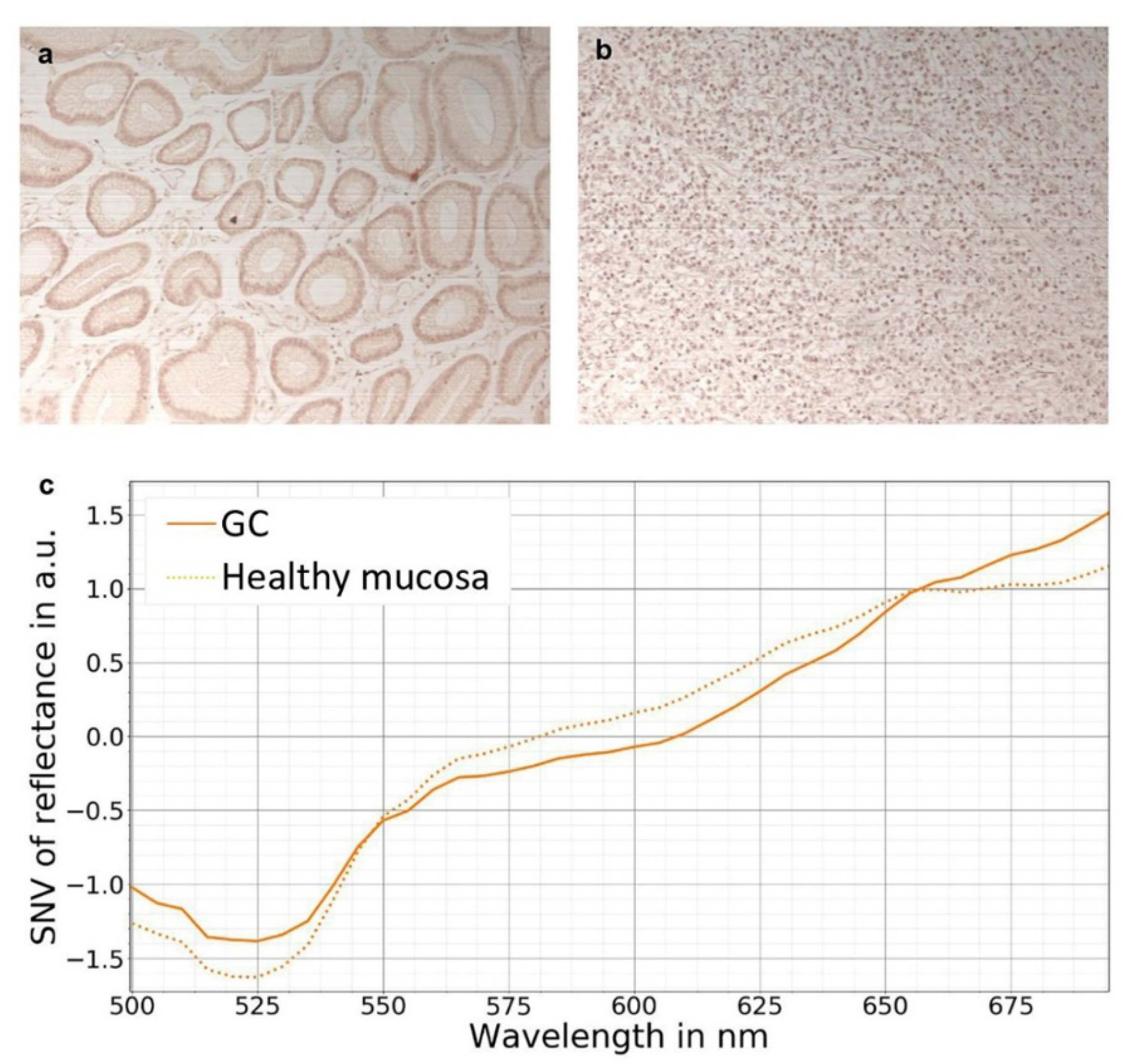
7. Hyperspectral Imaging in Gastric Cancer
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chalopin, C.; Maktabi, M.; Köhler, H.; Cervantes-Sanchez, F.; Pfahl, A.; Jansen-Winkeln, B.; Mehdorn, M.; Barberio, M.; Gockel, I.; Melzer, A. Intraoperative Imaging for Procedures of the Gastrointestinal Tract. In Innovative Endoscopic and Surgical Technology in the GI Tract; Horgan, S., Fuchs, K.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 365–379. ISBN 978-3-030-78217-7. [Google Scholar]
- Jafari, M.D.; Wexner, S.D.; Martz, J.E.; McLemore, E.C.; Margolin, D.A.; Sherwinter, D.A.; Lee, S.W.; Senagore, A.J.; Phelan, M.J.; Stamos, M.J. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): A multi-institutional study. J. Am. Coll. Surg. 2015, 220, 82–92.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Liang, L.; Liu, L.; Zhu, Z. Does intraoperative indocyanine green fluorescence angiography decrease the incidence of anastomotic leakage in colorectal surgery? A systematic review and meta-analysis. Int. J. Colorectal Dis. 2020, 36, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Arezzo, A.; Bonino, M.A.; Ris, F.; Boni, L.; Cassinotti, E.; Foo, D.C.C.; Shum, N.F.; Brolese, A.; Ciarleglio, F.; Keller, D.S.; et al. Intraoperative use of fluorescence with indocyanine green reduces anastomotic leak rates in rectal cancer surgery: An individual participant data analysis. Surg. Endosc. 2020, 34, 4281–4290. [Google Scholar] [CrossRef]
- Kim, M.; Son, S.-Y.; Cui, L.-H.; Shin, H.-J.; Hur, H.; Han, S.-U. Real-time Vessel Navigation Using Indocyanine Green Fluorescence during Robotic or Laparoscopic Gastrectomy for Gastric Cancer. J. Gastric Cancer 2017, 17, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, M.; Shuto, K.; Hirano, A.; Kosugi, C.; Narushima, K.; Hosokawa, I.; Fujino, M.; Yamazaki, M.; Shimizu, H.; Koda, K. A Novel Parameter Identified Using Indocyanine Green Fluorescence Angiography may Contribute to Predicting Anastomotic Leakage in Gastric Cancer Surgery. World J. Surg. 2020, 44, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.-J.; Lee, H.-J.; Kim, T.-H.; Choi, Y.-S.; Park, J.-H.; Son, Y.-G.; Suh, Y.-S.; Kong, S.-H.; Yang, H.-K. Efficacy of Assessing Intraoperative Bowel Perfusion with Near-Infrared Camera in Laparoscopic Gastric Cancer Surgery. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lee, H.-J.; Park, J.-H.; Kim, T.-H.; Son, Y.-G.; Huh, Y.-J.; Choi, J.-H.; Kim, S.-H.; Park, J.-H.; Suh, Y.-S.; et al. Clinical Significance of Intra-operative Gastroscopy for Tumor Localization in Totally Laparoscopic Partial Gastrectomy. J. Gastrointest. Surg. 2021, 25, 1134–1146. [Google Scholar] [CrossRef]
- Namikawa, T.; Hashiba, M.; Kitagawa, H.; Mizuta, H.; Uchida, K.; Sato, T.; Kobayashi, M.; Hanazaki, K. Innovative marking method using novel endoscopic clip equipped with fluorescent resin to locate gastric cancer. Asian J. Endosc. Surg. 2021, 14, 254–257. [Google Scholar] [CrossRef]
- Namikawa, T.; Iwabu, J.; Hashiba, M.; Munekage, M.; Uemura, S.; Yamada, T.; Kitagawa, H.; Mizuta, H.; Okamoto, K.; Uchida, K.; et al. Novel endoscopic marking clip equipped with resin-conjugated fluorescent indocyanine green during laparoscopic surgery for gastrointestinal cancer. Langenbecks. Arch. Surg. 2020, 405, 503–508. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Xie, J.-W.; Zhong, Q.; Wang, J.-B.; Lin, J.-X.; Lu, J.; Cao, L.-L.; Lin, M.; Tu, R.-H.; Huang, Z.-N.; et al. Safety and Efficacy of Indocyanine Green Tracer-Guided Lymph Node Dissection During Laparoscopic Radical Gastrectomy in Patients with Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 300–311. [Google Scholar] [CrossRef]
- Cianchi, F.; Indennitate, G.; Paoli, B.; Ortolani, M.; Lami, G.; Manetti, N.; Tarantino, O.; Messeri, S.; Foppa, C.; Badii, B.; et al. The Clinical Value of Fluorescent Lymphography with Indocyanine Green During Robotic Surgery for Gastric Cancer: A Matched Cohort Study. J. Gastrointest. Surg. 2020, 24, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Berlth, F.; Wang, C.; Wang, S.; Choi, J.-H.; Park, S.-H.; Suh, Y.-S.; Kong, S.-H.; Park, D.J.; Lee, H.-J.; et al. Mapping of the perigastric lymphatic network using indocyanine green fluorescence imaging and tissue marking dye in clinically advanced gastric cancer. Eur. J. Surg. Oncol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Chen, Q.-Y.; Huang, X.-B.; Lin, G.-T.; Liu, Z.-Y.; Chen, J.-Y.; Wang, H.-G.; Weng, K.; Li, P.; Xie, J.-W.; et al. Clinical implications of Indocyanine Green Fluorescence Imaging-Guided laparoscopic lymphadenectomy for patients with gastric cancer: A cohort study from two randomized, controlled trials using individual patient data. Int. J. Surg. 2021, 94, 106120. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Kong, S.-H.; Park, J.-H.; Son, Y.-G.; Huh, Y.-J.; Suh, Y.-S.; Lee, H.-J.; Yang, H.-K. Assessment of the Completeness of Lymph Node Dissection Using Near-infrared Imaging with Indocyanine Green in Laparoscopic Gastrectomy for Gastric Cancer. J. Gastric Cancer 2018, 18, 161–171. [Google Scholar] [CrossRef]
- Park, S.-H.; Berlth, F.; Choi, J.-H.; Park, J.-H.; Suh, Y.-S.; Kong, S.-H.; Park, D.J.; Lee, H.-J.; Yang, H.-K. Near-infrared fluorescence-guided surgery using indocyanine green facilitates secure infrapyloric lymph node dissection during laparoscopic distal gastrectomy. Surg. Today 2020, 50, 1187–1196. [Google Scholar] [CrossRef]
- Liu, M.; Xing, J.; Xu, K.; Yuan, P.; Cui, M.; Zhang, C.; Yang, H.; Yao, Z.; Zhang, N.; Tan, F.; et al. Application of Near-Infrared Fluorescence Imaging with Indocyanine Green in Totally Laparoscopic Distal Gastrectomy. J. Gastric Cancer 2020, 20, 290–299. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Zhong, Q.; Li, P.; Xie, J.-W.; Liu, Z.-Y.; Huang, X.-B.; Lin, G.-T.; Wang, J.-B.; Lin, J.-X.; Lu, J.; et al. Comparison of submucosal and subserosal approaches toward optimized indocyanine green tracer-guided laparoscopic lymphadenectomy for patients with gastric cancer (FUGES-019): A randomized controlled trial. BMC Med. 2021, 19, 276. [Google Scholar] [CrossRef]
- Van der Vorst, J.R.; Schaafsma, B.E.; Hutteman, M.; Verbeek, F.P.R.; Liefers, G.-J.; Hartgrink, H.H.; Smit, V.T.H.B.M.; Löwik, C.W.G.M.; van de Velde, C.J.H.; Frangioni, J.V.; et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013, 119, 3411–3418. [Google Scholar] [CrossRef]
- Peloso, A.; Franchi, E.; Canepa, M.C.; Barbieri, L.; Briani, L.; Ferrario, J.; Bianco, C.; Quaretti, P.; Brugnatelli, S.; Dionigi, P.; et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. HPB 2013, 15, 928–934. [Google Scholar] [CrossRef] [Green Version]
- Baiocchi, G.L.; Gheza, F.; Molfino, S.; Arru, L.; Vaira, M.; Giacopuzzi, S. Indocyanine green fluorescence-guided intraoperative detection of peritoneal carcinomatosis: Systematic review. BMC Surg. 2020, 20, 158. [Google Scholar] [CrossRef]
- Panasyuk, S.V.; Yang, S.; Faller, D.V.; Ngo, D.; Lew, R.A.; Freeman, J.E.; Rogers, A.E. Medical hyperspectral imaging to facilitate residual tumor identification during surgery. Cancer Biol. Ther. 2007, 6, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, H.; Uto, K.; Kosugi, Y.; Kojima, K.; Tanaka, N. Cancer detection using infrared hyperspectral imaging. Cancer Sci. 2011, 102, 852–857. [Google Scholar] [CrossRef]
- Kumashiro, R.; Konishi, K.; Chiba, T.; Akahoshi, T.; Nakamura, S.; Murata, M.; Tomikawa, M.; Matsumoto, T.; Maehara, Y.; Hashizume, M. Integrated Endoscopic System Based on Optical Imaging and Hyperspectral Data Analysis for Colorectal Cancer Detection. Anticancer Res. 2016, 36, 3925–3932. [Google Scholar] [PubMed]
- Beaulieu, R.J.; Goldstein, S.D.; Singh, J.; Safar, B.; Banerjee, A.; Ahuja, N. Automated diagnosis of colon cancer using hyperspectral sensing. Int. J. Med. Robot. 2018, 14, e1897. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Winkeln, B.; Holfert, N.; Köhler, H.; Moulla, Y.; Takoh, J.P.; Rabe, S.M.; Mehdorn, M.; Barberio, M.; Chalopin, C.; Neumuth, T.; et al. Determination of the transection margin during colorectal resection with hyperspectral imaging (HSI). Int. J. Colorectal Dis. 2019, 34, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Maktabi, M.; Köhler, H.; Ivanova, M.; Jansen-Winkeln, B.; Takoh, J.; Niebisch, S.; Rabe, S.M.; Neumuth, T.; Gockel, I.; Chalopin, C. Tissue classification of oncologic esophageal resectates based on hyperspectral data. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1651–1661. [Google Scholar] [CrossRef]
- Siddiqi, A.M.; Li, H.; Faruque, F.; Williams, W.; Lai, K.; Hughson, M.; Bigler, S.; Beach, J.; Johnson, W. Use of hyperspectral imaging to distinguish normal, precancerous, and cancerous cells. Cancer 2008, 114, 13–21. [Google Scholar] [CrossRef]
- Lu, G.; Halig, L.; Wang, D.; Qin, X.; Chen, Z.G.; Fei, B. Spectral-spatial classification for noninvasive cancer detection using hyperspectral imaging. J. Biomed. Opt. 2014, 19, 106004. [Google Scholar] [CrossRef] [Green Version]
- Khouj, Y.; Dawson, J.; Coad, J.; Vona-Davis, L. Hyperspectral Imaging and K-Means Classification for Histologic Evaluation of Ductal Carcinoma In Situ. Front. Oncol. 2018, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Holmer, A.; Tetschke, F.; Marotz, J.; Malberg, H.; Markgraf, W.; Thiele, C.; Kulcke, A. Oxygenation and perfusion monitoring with a hyperspectral camera system for chemical based tissue analysis of skin and organs. Physiol. Meas. 2016, 37, 2064–2078. [Google Scholar] [CrossRef]
- Kulcke, A.; Holmer, A.; Wahl, P.; Siemers, F.; Wild, T.; Daeschlein, G. A compact hyperspectral camera for measurement of perfusion parameters in medicine. Biomed. Tech. 2018, 63, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Winkeln, B.; Maktabi, M.; Takoh, J.P.; Rabe, S.M.; Barberio, M.; Köhler, H.; Neumuth, T.; Melzer, A.; Chalopin, C.; Gockel, I. Hyperspektral-Imaging bei gastrointestinalen Anastomosen. Chirurg 2018, 89, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Nouvong, A.; Hoogwerf, B.; Mohler, E.; Davis, B.; Tajaddini, A.; Medenilla, E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care 2009, 32, 2056–2061. [Google Scholar] [CrossRef] [Green Version]
- Jeffcoate, W.J.; Clark, D.J.; Savic, N.; Rodmell, P.I.; Hinchliffe, R.J.; Musgrove, A.; Game, F.L. Use of HSI to measure oxygen saturation in the lower limb and its correlation with healing of foot ulcers in diabetes. Diabet. Med. 2015, 32, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Calin, M.A.; Coman, T.; Parasca, S.V.; Bercaru, N.; Savastru, R.; Manea, D. Hyperspectral imaging-based wound analysis using mixture-tuned matched filtering classification method. J. Biomed. Opt. 2015, 20, 46004. [Google Scholar] [CrossRef] [PubMed]
- Sakota, D.; Nagaoka, E.; Maruyama, O. Hyperspectral imaging of vascular anastomosis associated with blood flow and hemoglobin concentration. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 4246–4249. [Google Scholar] [CrossRef]
- Köhler, H.; Jansen-Winkeln, B.; Maktabi, M.; Barberio, M.; Takoh, J.; Holfert, N.; Moulla, Y.; Niebisch, S.; Diana, M.; Neumuth, T.; et al. Evaluation of hyperspectral imaging (HSI) for the measurement of ischemic conditioning effects of the gastric conduit during esophagectomy. Surg. Endosc. 2019, 33, 3775–3782. [Google Scholar] [CrossRef] [Green Version]
- Jansen-Winkeln, B.; Takoh, J.P.; Chalopin, C.; Maktabi, M.; Lyros, O.; Sucher, R.; Hoffmeister, A.; Teich, N.; Köhler, H.; Gockel, I. Hyperspectral Imaging: A New Intraoperative Tool for Pouch Assessment in Patients Undergoing Restorative Proctocolectomy. Visc. Med. 2021, 37, 426–433. [Google Scholar] [CrossRef]
- Jansen-Winkeln, B.; Germann, I.; Köhler, H.; Mehdorn, M.; Maktabi, M.; Sucher, R.; Barberio, M.; Chalopin, C.; Diana, M.; Moulla, Y.; et al. Comparison of hyperspectral imaging and fluorescence angiography for the determination of the transection margin in colorectal resections-a comparative study. Int. J. Colorectal Dis. 2020, 36, 283–291. [Google Scholar] [CrossRef]
- Sucher, R.; Athanasios, A.; Köhler, H.; Wagner, T.; Brunotte, M.; Lederer, A.; Gockel, I.; Seehofer, D. Hyperspectral Imaging (HSI) in anatomic left liver resection. Int. J. Surg. Case Rep. 2019, 62, 108–111. [Google Scholar] [CrossRef]
- Mehdorn, M.; Köhler, H.; Rabe, S.M.; Niebisch, S.; Lyros, O.; Chalopin, C.; Gockel, I.; Jansen-Winkeln, B. Hyperspectral Imaging (HSI) in Acute Mesenteric Ischemia to Detect Intestinal Perfusion Deficits. J. Surg. Res. 2020, 254, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sucher, R.; Wagner, T.; Köhler, H.; Sucher, E.; Guice, H.; Recknagel, S.; Lederer, A.; Hau, H.M.; Rademacher, S.; Schneeberger, S.; et al. Hyperspectral Imaging (HSI) of Human Kidney Allografts. Ann. Surg. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Holmer, A.; Kämmerer, P.W.; Dau, M.; Grambow, E.; Wahl, P. The ability of hyperspectral imaging to detect perfusion disorders. In Diffuse Optical Spectroscopy and Imaging VI, Proceedings of the European Conferences on Biomedical Optics, Munich, Germany, 25 June 2017; Dehghani, H., Wabnitz, H., Eds.; SPIE: Washington, DC, USA, 2017; p. 1041213. [Google Scholar]
- Köhler, H.; Kulcke, A.; Maktabi, M.; Moulla, Y.; Jansen-Winkeln, B.; Barberio, M.; Diana, M.; Gockel, I.; Neumuth, T.; Chalopin, C. Laparoscopic system for simultaneous high-resolution video and rapid hyperspectral imaging in the visible and near-infrared spectral range. J. Biomed. Opt. 2020, 25, 086004. [Google Scholar] [CrossRef] [PubMed]
- Halicek, M.; Fabelo, H.; Ortega, S.; Callico, G.M.; Fei, B. In-Vivo and Ex-Vivo Tissue Analysis through Hyperspectral Imaging Techniques: Revealing the Invisible Features of Cancer. Cancers 2019, 11, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, A.; Nishikawa, J.; Kiyotoki, S.; Nakamura, M.; Nishimura, J.; Okamoto, T.; Ogihara, H.; Fujita, Y.; Hamamoto, Y.; Sakaida, I. Use of hyperspectral imaging technology to develop a diagnostic support system for gastric cancer. J. Biomed. Opt. 2015, 20, 16017. [Google Scholar] [CrossRef]
- Liu, N.; Guo, Y.; Jiang, H.; Yi, W. Gastric cancer diagnosis using hyperspectral imaging with principal component analysis and spectral angle mapper. J. Biomed. Opt. 2020, 25, 1–9. [Google Scholar] [CrossRef]
- Ishikawa, M.; Okamoto, C.; Shinoda, K.; Komagata, H.; Iwamoto, C.; Ohuchida, K.; Hashizume, M.; Shimizu, A.; Kobayashi, N. Detection of pancreatic tumor cell nuclei via a hyperspectral analysis of pathological slides based on stain spectra. Biomed. Opt. Express 2019, 10, 4568–4588. [Google Scholar] [CrossRef]
- Maktabi, M.; Wichmann, Y.; Köhler, H.; Chalopin, C.; Neumuth, T.; Jansen-Winkeln, B.; Ahle, H.; Lorenz, D.; Bange, M.; Braun, S.; et al. Semi-automatic decision-making process in histopathological specimens from Barrett’s carcinoma patients using hyperspectral imaging (HSI). Curr. Dir. Biomed. Eng. 2020, 6, 261–263. [Google Scholar] [CrossRef]
- Collins, T.; Maktabi, M.; Barberio, M.; Bencteux, V.; Jansen-Winkeln, B.; Chalopin, C.; Marescaux, J.; Hostettler, A.; Diana, M.; Gockel, I. Automatic Recognition of Colon and Esophagogastric Cancer with Machine Learning and Hyperspectral Imaging. Diagnostics 2021, 11, 1810. [Google Scholar] [CrossRef]
- Hu, B.; Du, J.; Zhang, Z.; Wang, Q. Tumor tissue classification based on micro-hyperspectral technology and deep learning. Biomed. Opt. Express 2019, 10, 6370–6389. [Google Scholar] [CrossRef]
- Li, Y.; Deng, L.; Yang, X.; Liu, Z.; Zhao, X.; Huang, F.; Zhu, S.; Chen, X.; Chen, Z.; Zhang, W. Early diagnosis of gastric cancer based on deep learning combined with the spectral-spatial classification method. Biomed. Opt. Express 2019, 10, 4999–5014. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, M.; Kanawade, R.; Klämpfl, F.; Douplik, A.; Mudter, J.; Neurath, M.F.; Albrecht, H. In-vivo multispectral video endoscopy towards in-vivo hyperspectral video endoscopy. J. Biophotonics 2017, 10, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Signoroni, A.; Savardi, M.; Baronio, A.; Benini, S. Deep Learning Meets Hyperspectral Image Analysis: A Multidisciplinary Review. J. Imaging 2019, 5, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knospe, L.; Gockel, I.; Jansen-Winkeln, B.; Thieme, R.; Niebisch, S.; Moulla, Y.; Stelzner, S.; Lyros, O.; Diana, M.; Marescaux, J.; et al. New Intraoperative Imaging Tools and Image-Guided Surgery in Gastric Cancer Surgery. Diagnostics 2022, 12, 507. https://doi.org/10.3390/diagnostics12020507
Knospe L, Gockel I, Jansen-Winkeln B, Thieme R, Niebisch S, Moulla Y, Stelzner S, Lyros O, Diana M, Marescaux J, et al. New Intraoperative Imaging Tools and Image-Guided Surgery in Gastric Cancer Surgery. Diagnostics. 2022; 12(2):507. https://doi.org/10.3390/diagnostics12020507
Chicago/Turabian StyleKnospe, Luise, Ines Gockel, Boris Jansen-Winkeln, René Thieme, Stefan Niebisch, Yusef Moulla, Sigmar Stelzner, Orestis Lyros, Michele Diana, Jacques Marescaux, and et al. 2022. "New Intraoperative Imaging Tools and Image-Guided Surgery in Gastric Cancer Surgery" Diagnostics 12, no. 2: 507. https://doi.org/10.3390/diagnostics12020507
APA StyleKnospe, L., Gockel, I., Jansen-Winkeln, B., Thieme, R., Niebisch, S., Moulla, Y., Stelzner, S., Lyros, O., Diana, M., Marescaux, J., Chalopin, C., Köhler, H., Pfahl, A., Maktabi, M., Park, J.-H., & Yang, H.-K. (2022). New Intraoperative Imaging Tools and Image-Guided Surgery in Gastric Cancer Surgery. Diagnostics, 12(2), 507. https://doi.org/10.3390/diagnostics12020507










