Evaluation of a Generative Adversarial Network to Improve Image Quality and Reduce Radiation-Dose during Digital Breast Tomosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. DBT
2.2. Phantom Specifications
2.3. Radiation-Dose Measurement
2.4. Pix2pix
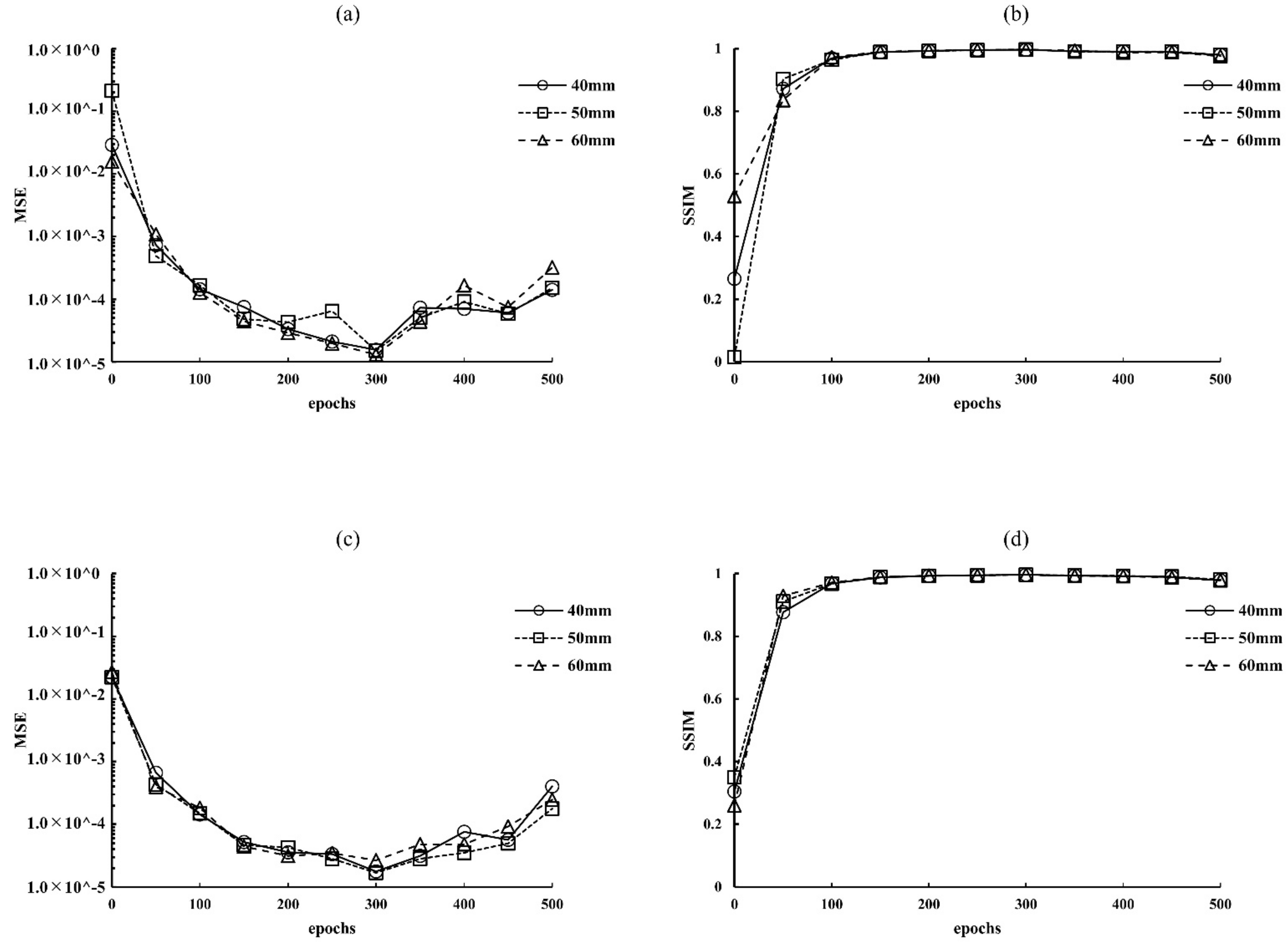
2.5. Optimization Parameters for Epochs
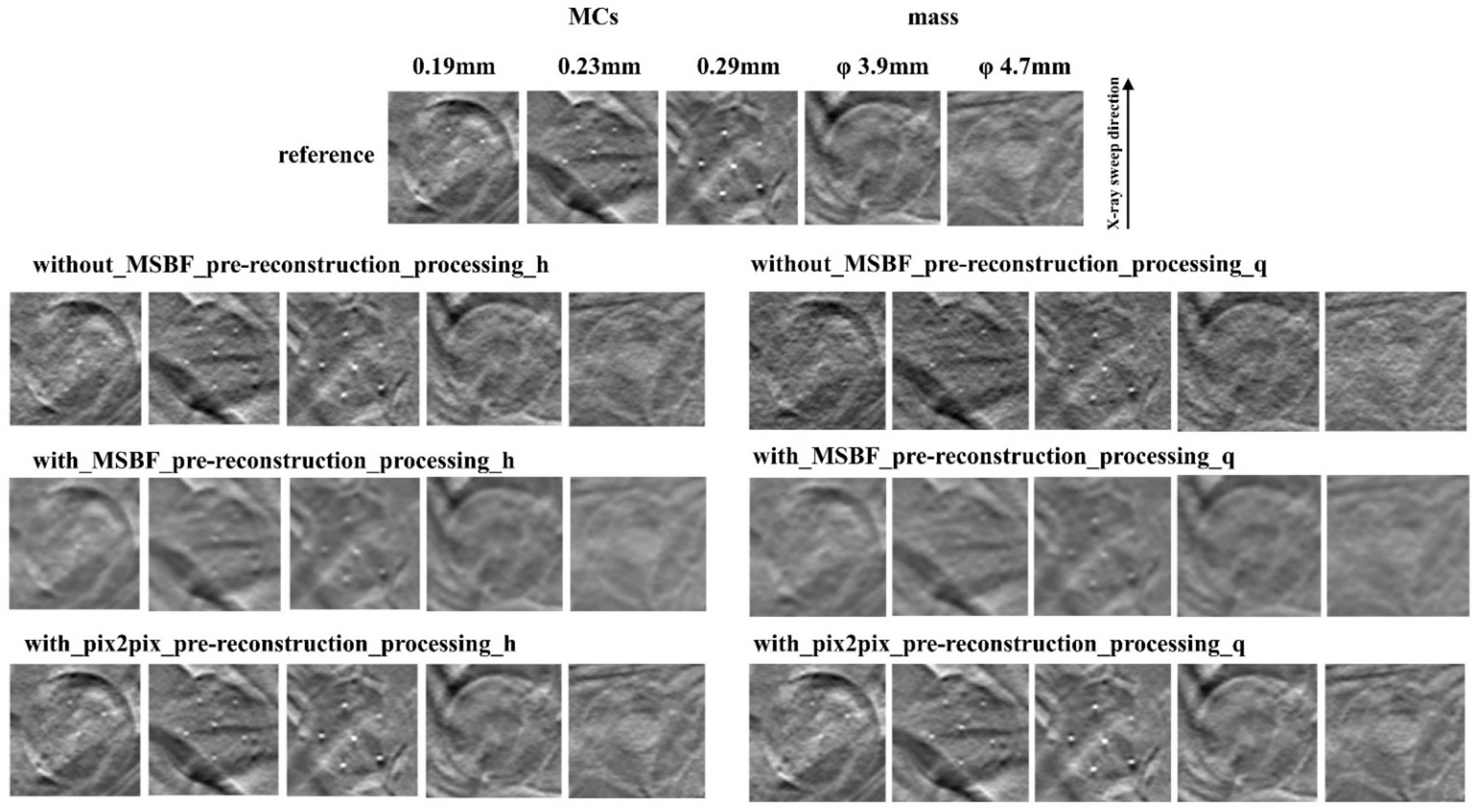
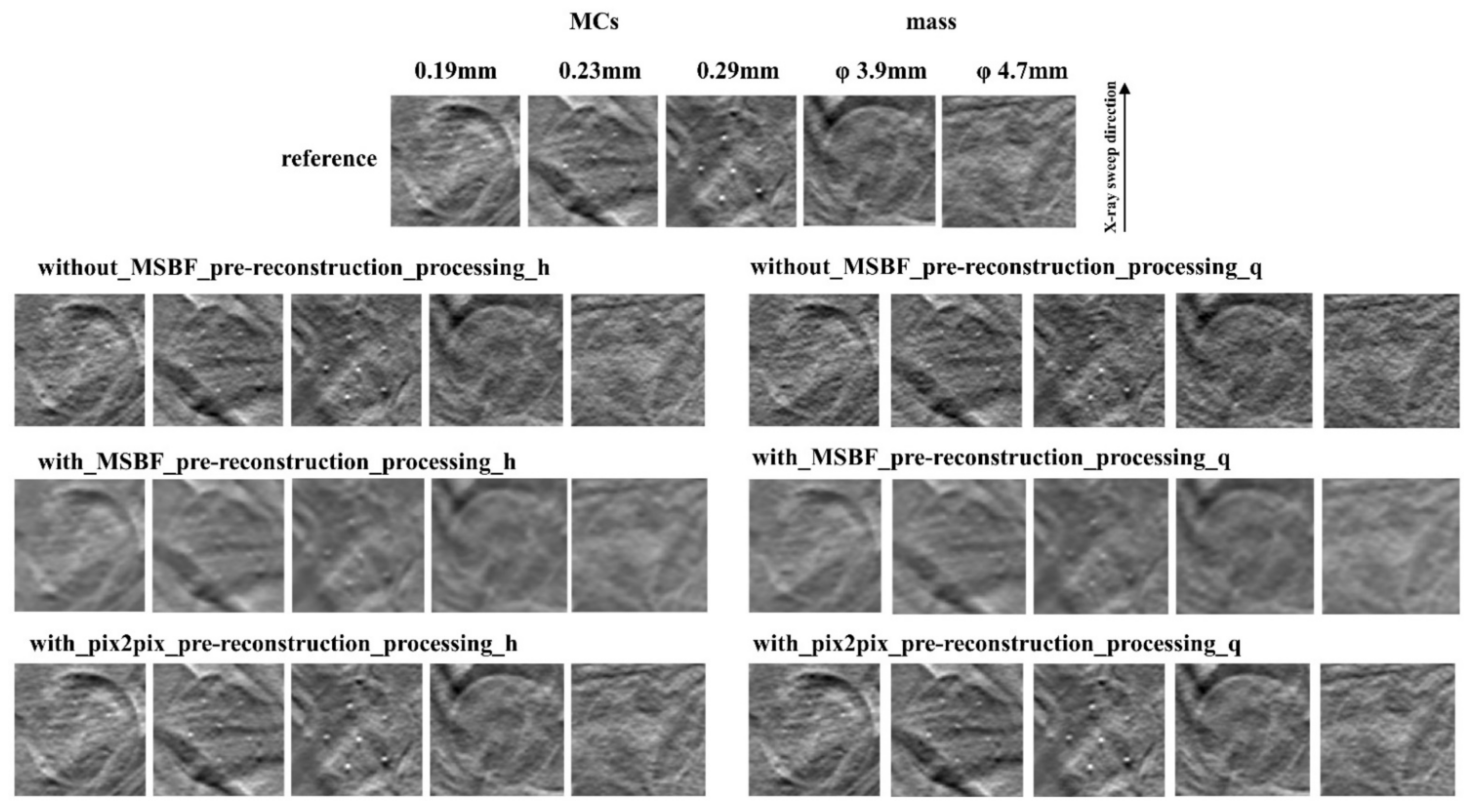
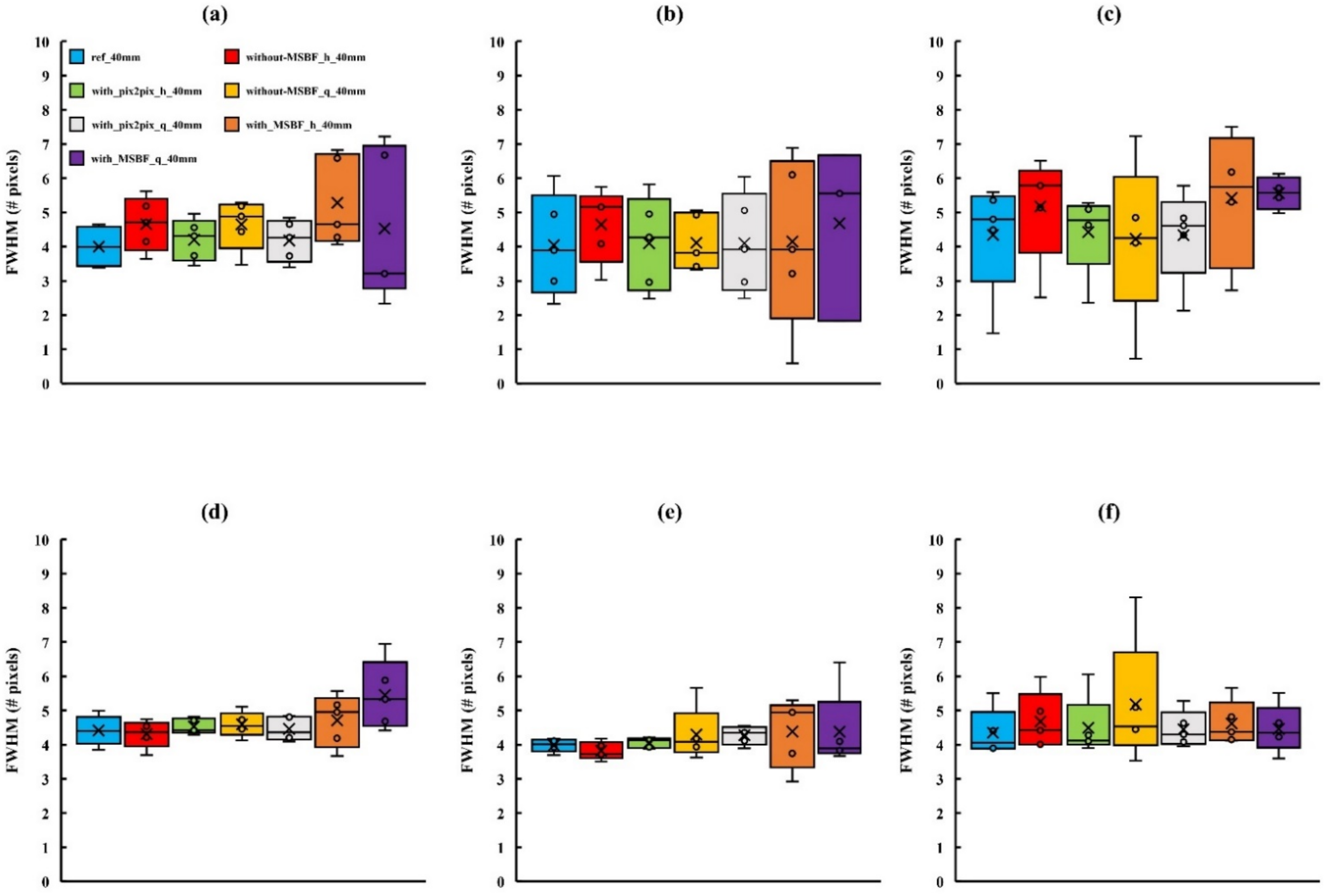
2.6. Evaluation of Image Quality
3. Results
3.1. Optimization Parameters
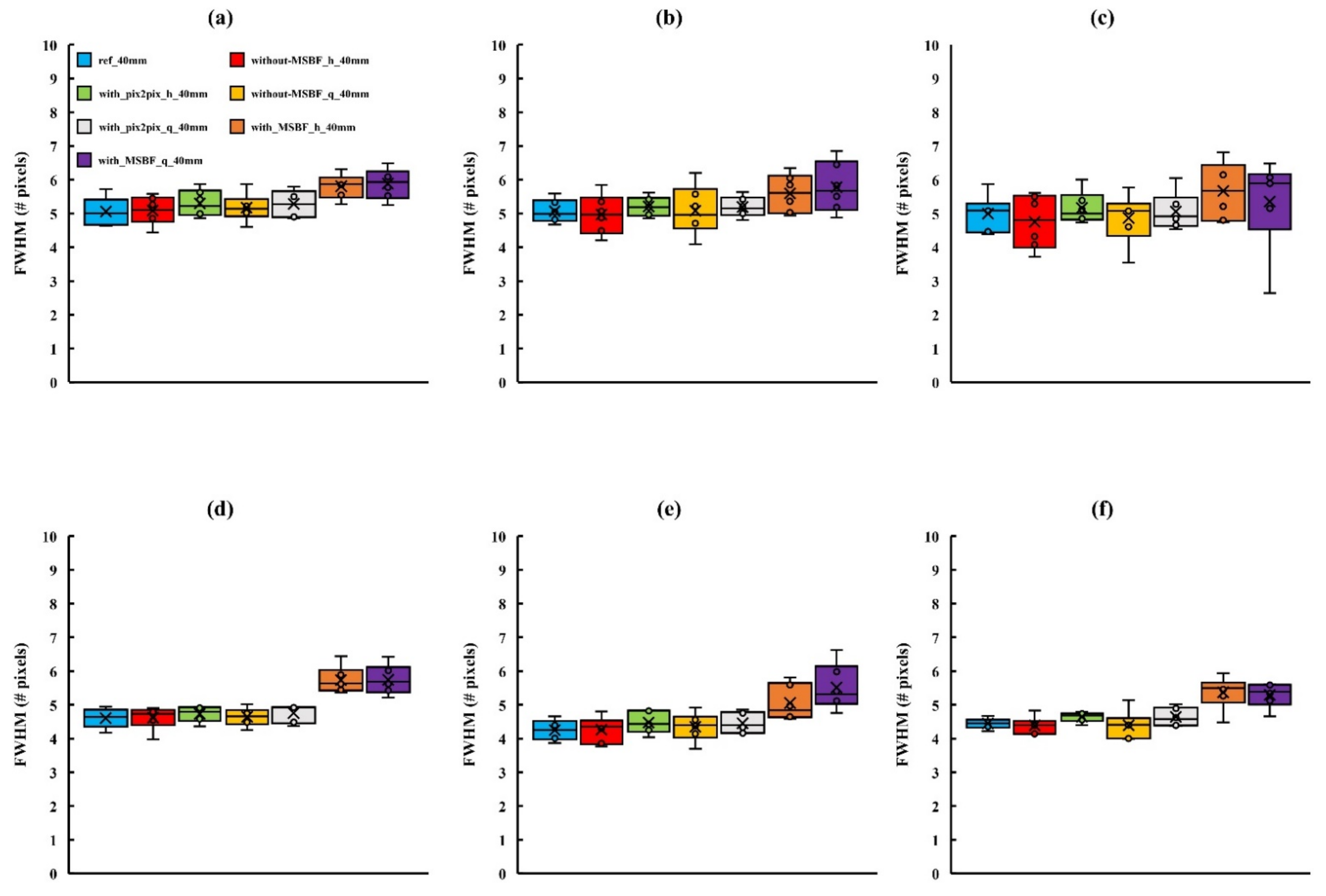
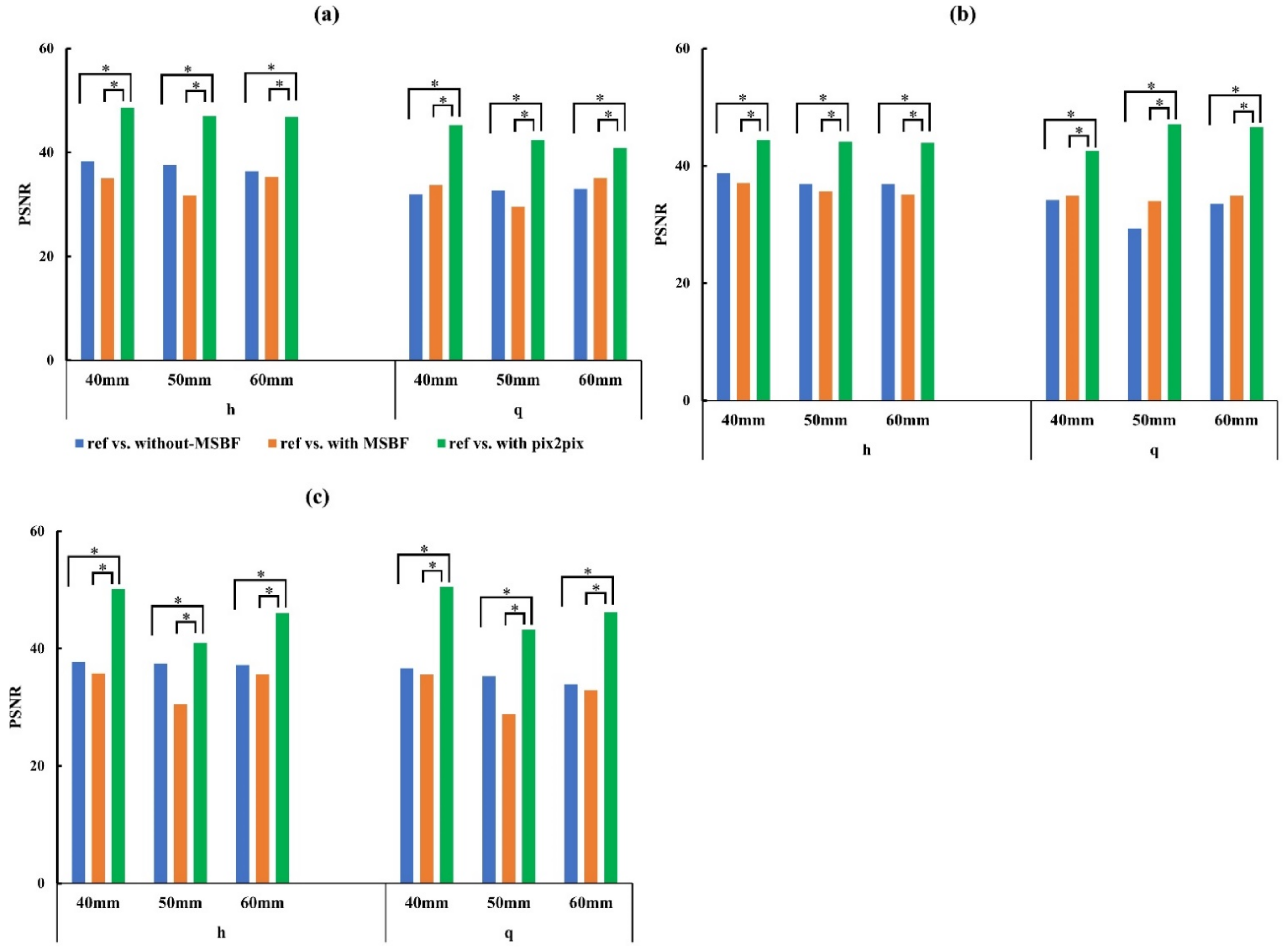
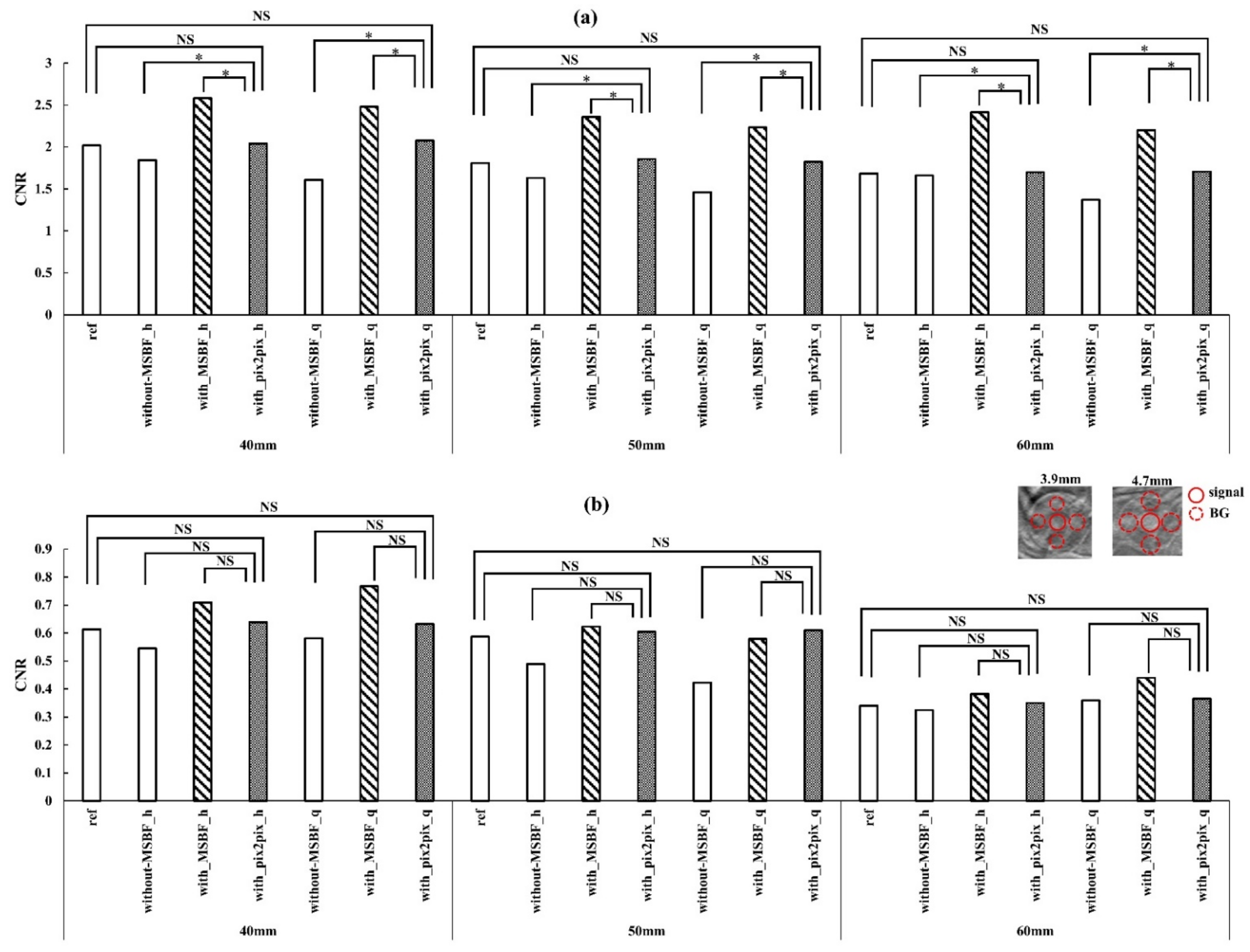
3.2. Image Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Network | Block | Count | Channel | Kernel | Stride | Padding | Activation |
|---|---|---|---|---|---|---|---|
| Generator | encoder | 1 | 128 | 4 × 4 | 2 | same | LRelu |
| 2 | 256 | 1 | LRelu + BN | ||||
| 3 | 512 | ||||||
| 4 | 1024 | ||||||
| 5 | 1024 | ||||||
| 6 | 1024 | ||||||
| 7 | 1024 | ||||||
| decoder | 1 | 1024 | 4 × 4 | 2 | same | LRelu + BN | |
| 2 | 1024 + 1024 # | ||||||
| 3 | 1024 + 1024 # | ||||||
| 4 | 1024 + 1024 # | ||||||
| 5 | 512 + 512 # | ||||||
| 6 | 256 + 256 # | ||||||
| 7 | 128 + 128 # | ||||||
| 1 | - | - | - | Tanh | |||
| Discriminator | 1 | 128 | 4 × 4 | 2 | 1 | LRelu | |
| 2 | 256 | LRelu + BN | |||||
| 3 | 512 | ||||||
| 4 | 1024 | ||||||
| 5 | 1 | 1 | 0 | Sigmoid |
References
- Skaane, P.; Bandos, A.I.; Gullien, R.; Eben, E.B.; Ekseth, U.; Haakenaasen, U.; Izadi, M.; Jebsen, I.N.; Jahr, G.; Krager, M.; et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013, 267, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Machida, H.; Yuhara, T.; Mori, T.; Ueno, E.; Moribe, Y.; Sabol, J.M. Optimizing parameters for flat-panel detector digital tomosynthesis. Radiographics 2010, 30, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Stewart, A.; Stanton, M.; McCauley, T.; Phillips, W.; Kopans, D.B.; Moore, R.H.; Eberhard, J.W.; Opsahl-Ong, B.; Niklason, L.; et al. Tomographic mammography using a limited number of low-dose cone-beam projection images. Med. Phys. 2003, 30, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Helvie, M.A.; Roubidoux, M.A.; Zhang, Y.; Carson, P.L.; Chan, H.P. Tomosynthesis mammography vs conventional mammography: Lesion detection and reader reference. Initial experience. In Proceedings of the Radiological Society of North America 92nd Scientific Assembly and Annual Meeting, Chicago, IL, USA, 26 November–1 December 2003; p. 335. [Google Scholar]
- Sechopoulos, I.; Bliznakova, K.; Fei, B. Power spectrum analysis of the X-ray scatter signal in mammography and breast tomosynthesis projections. Med. Phys. 2013, 40, 101905. [Google Scholar] [CrossRef]
- Sechopoulos, I. A review of breast tomosynthesis. Part I. The image acquisition process. Med. Phys. 2013, 40, 014301. [Google Scholar] [CrossRef]
- Sechopoulos, I. A review of breast tomosynthesis. Part II. Image reconstruction, processing and analysis, and advanced applications. Med. Phys. 2013, 40, 014302. [Google Scholar] [CrossRef]
- Gur, D.; Zuley, M.L.; Anello, M.I.; Rathfon, G.Y.; Chough, D.M.; Ganott, M.A.; Hakim, C.M.; Wallace, L.; Lu, A.; Bandos, A.I. Dose reduction in digital breast tomosynthesis (DBT) screening using synthetically reconstructed projection images: An observer performance study. Acad. Radiol. 2012, 19, 166–171. [Google Scholar] [CrossRef]
- Wu, T.; Moore, R.H.; Rafferty, E.A.; Kopans, D.B. A comparison of reconstruction algorithms for breast tomosynthesis. Med. Phys. 2004, 31, 2636–2647. [Google Scholar] [CrossRef]
- Gordon, R.; Bender, R.; Herman, G.T. Algebraic reconstruction techniques (ART) for three-dimensional electron microscopy and x-ray photography. J. Theor. Biol. 1970, 29, 471–481. [Google Scholar] [CrossRef]
- Dobbins, J.T., 3rd; Godfrey, D.J. Digital x-ray tomosynthesis: Current state of the art and clinical potential. Phys. Med. Biol. 2003, 48, R65–R106. [Google Scholar] [CrossRef]
- Bleuet, P.; Guillemaud, R.; Magnin, I.; Desbat, L. An adapted fan volume sampling scheme for 3D algebraic reconstruction in linear tomosynthesis. IEEE Trans. Nucl. Sci. 2002, 49, 2366–2372. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, J.; Moore, R.; Rafferty, E.; Kopans, D. Digital tomosynthesis mammography using a parallel maximum-likelihood reconstruction method. Proc. SPIE 2004, 5368, 1–11. [Google Scholar]
- Chen, Y.; Lo, J.Y.; Baker, J.A.; Dobbins, J.T., III. Gaussian Frequency Blending Algorithm with Matrix Inversion Tomosynthesis (MITS) and Filtered Back Projection (FBP) for Better Digital Breast Tomosynthesis Reconstruction. Proc. SPIE 2006, 6142, 122–130. [Google Scholar]
- Sidky, E.Y.; Pan, X. Image reconstruction in circular cone-beam computed tomography by constrained, total-variation minimization. Phys. Med. Biol. 2008, 53, 4777–4807. [Google Scholar] [CrossRef] [PubMed]
- Sidky, E.Y.; Pan, X.; Reiser, I.S.; Nishikawa, R.M.; Moore, R.H.; Kopans, D.B. Enhanced imaging of microcalcifications in digital breast tomosynthesis through improved image-reconstruction algorithms. Med. Phys. 2009, 36, 4920–4932. [Google Scholar] [CrossRef]
- Gomi, T. A Comparison of Reconstruction Algorithms Regarding Exposure Dose Reductions during Digital Breast Tomosynthesis. J. Biomed. Sci. Eng. 2014, 7, 516–525. [Google Scholar] [CrossRef][Green Version]
- Lu, Y.; Chan, H.P.; Wei, J.; Hadjiiski, L.M.; Samala, R.K. Multiscale bilateral filtering for improving image quality in digital breast tomosynthesis. Med. Phys. 2015, 42, 182–195. [Google Scholar] [CrossRef]
- Xu, S.; Lu, J.; Zhou, O.; Chen, Y. Statistical iterative reconstruction to improve image quality for digital breast tomosynthesis. Med. Phys. 2015, 42, 5377–5390. [Google Scholar] [CrossRef]
- Sidky, E.Y.; Reiser, I.S.; Nishikawa, R.; Pan, X. Image reconstruction in digital breast tomosynthesis by total variation minimization. Proc. SPIE 2007, 6510, 651027. [Google Scholar]
- Das, M.; Gifford, H.C.; O’Connor, J.M.; Glick, S.J. Penalized maximum likelihood reconstruction for improved microcalcification detection in breast tomosynthesis. IEEE Trans. Med. Imaging 2011, 30, 904–914. [Google Scholar] [CrossRef]
- Chung, J.; Nagy, J.G.; Sechopoulos, I. Numerical algorithms for polyenergetic digital breast tomosynthesis reconstruction. SIAM J. Imaging Sci. 2010, 3, 133–152. [Google Scholar] [CrossRef]
- Michielsen, K.; Van Slambrouck, K.; Jerebko, A.; Nuyts, J. Patchwork reconstruction with resolution modeling for digital breast tomosynthesis. Med. Phys. 2013, 40, 031105. [Google Scholar] [CrossRef] [PubMed]
- Alex, K.; Ilya, S.; Geoffrey, E.H. ImageNet classification with deep convolutional neural networks. In Proceedings of the 25th International Conference on Neural Information Processing Systems, Red Hook, NY, USA, 3–6 December 2012; Volume 1, pp. 1097–1105. [Google Scholar]
- Phillip, I.; Jun-Yan, Z.; Tinghui, Z.; Alexei, A.E. Image-To-Image Translation With Conditional Adversarial Networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 1125–1134. [Google Scholar]
- Zhu, J.; Park, T.; Isola, P.; Efros, A.A. Unpaired Image-to-Image Translation Using Cycle-Consistent Adversarial Networks. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 2242–2251. [Google Scholar] [CrossRef]
- Gao, M.; Fessler, J.A.; Chan, H.P. Deep Convolutional Neural Network with Adversarial Training for Denoising Digital Breast Tomosynthesis Images. IEEE Trans. Med. Imaging 2021, 40, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Moy, L.; Heller, S.L. Digital Breast Tomosynthesis: Update on Technology, Evidence, and Clinical Practice. Radiographics 2021, 41, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Gomi, T.; Sakai, R.; Hara, H.; Watanabe, Y.; Mizukami, S. Development of a denoising convolutional neural network-based algorithm for metal artifact reduction in digital tomosynthesis for arthroplasty: A phantom study. PLoS ONE 2019, 14, e0222406. [Google Scholar] [CrossRef]
- Gomi, T.; Sakai, R.; Hara, H.; Watanabe, Y.; Mizukami, S. Usefulness of a Metal Artifact Reduction Algorithm in Digital Tomosynthesis Using a Combination of Hybrid Generative Adversarial Networks. Diagnostics 2021, 11, 1629. [Google Scholar] [CrossRef]
- Samala, R.K.; Chan, H.P.; Hadjiiski, L.M.; Helvie, M.A.; Richter, C.; Cha, K. Evolutionary pruning of transfer learned deep convolutional neural network for breast cancer diagnosis in digital breast tomosynthesis. Phys. Med. Biol. 2018, 63, 095005. [Google Scholar] [CrossRef]
- Samala, R.K.; Heang-Ping, C.; Hadjiiski, L.; Helvie, M.A.; Richter, C.D.; Cha, K.H. Breast Cancer Diagnosis in Digital Breast Tomosynthesis: Effects of Training Sample Size on Multi-Stage Transfer Learning Using Deep Neural Nets. IEEE Trans. Med. Imaging 2019, 38, 686–696. [Google Scholar] [CrossRef]
- Pinto, M.C.; Rodriguez-Ruiz, A.; Pedersen, K.; Hofvind, S.; Wicklein, J.; Kappler, S.; Mann, R.M.; Sechopoulos, I. Impact of Artificial Intelligence Decision Support Using Deep Learning on Breast Cancer Screening Interpretation with Single-View Wide-Angle Digital Breast Tomosynthesis. Radiology 2021, 300, 529–536. [Google Scholar] [CrossRef]
- Buda, M.; Saha, A.; Walsh, R.; Ghate, S.; Li, N.; Swiecicki, A.; Lo, J.Y.; Mazurowski, M.A. A Data Set and Deep Learning Algorithm for the Detection of Masses and Architectural Distortions in Digital Breast Tomosynthesis Images. JAMA Netw. Open 2021, 4, e2119100. [Google Scholar] [CrossRef]
- Jiang, G.; Wei, J.; Xu, Y.; He, Z.; Zeng, H.; Wu, J.; Qin, G.; Chen, W.; Lu, Y. Synthesis of Mammogram From Digital Breast Tomosynthesis Using Deep Convolutional Neural Network With Gradient Guided cGANs. IEEE Trans. Med. Imaging 2021, 40, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, H.J. Restoration of Full Data from Sparse Data in Low-Dose Chest Digital Tomosynthesis Using Deep Convolutional Neural Networks. J. Digit. Imaging 2018, 32, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Fessler, J.A.; Chan, H.P. Detector Blur and Correlated Noise Modeling for Digital Breast Tomosynthesis Reconstruction. IEEE Trans. Med. Imaging 2018, 37, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, S.; Karellas, A.; Vedantham, S.; Glick, S.J.; D’Orsi, C.J.; Baker, S.P.; Webber, R.L. Comparison of tomosynthesis methods used with digital mammography. Acad. Radiol. 2000, 7, 1085–1097. [Google Scholar] [CrossRef]
- Mahadevan, R.; Ikejimba, L.C.; Lin, Y.; Samei, E.; Lo, J.Y. A task-based comparison of two reconstruction algorithms for digital breast tomosynthesis. Proc. SPIE 2014, 9033, 9033. [Google Scholar]
- Dance, D.R.; Young, K.C.; van Engen, R.E. Estimation of mean glandular dose for breast tomosynthesis: Factors for use with the UK, European and IAEA breast dosimetry protocols. Phys. Med. Biol. 2011, 56, 453–471. [Google Scholar] [CrossRef]
- Kingma, D.; Adam, J.B. Adam: A method for stochastic optimization. In Proceedings of the International Conference on Learning Representations (ICLR) 2015, San Diego, CA, USA, 7–9 May 2015. [Google Scholar]
- Zhou, W.; Bovik, A.C. A universal image quality index. IEEE Signal. Processing Lett. 2002, 9, 81–84. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image Quality Assessment: From Error Visibility to Structural Similarity. IEEE Trans. Image Processing 2004, 13, 600–612. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015, Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar] [CrossRef]
- Larsen, A.B.L.; Sønderby, S.K.; Winther, O. Autoencoding beyond pixels using a learned similarity metric. In Proceedings of the 33rd International Conference on Machine Learning, PMLR, New York, NY, USA, 20–22 June 2016; Volume 48, pp. 1558–1566. [Google Scholar]



| MCs: 0.196 mm | |||||
|---|---|---|---|---|---|
| Variable | Difference | Standard Error | p | 95% CI * | |
| Lower Limit | Upper Limit | ||||
| Ref vs. Without pre-reconstruction processing | −0.3407 | 0.24671 | 0.513 | −0.9799 | 0.2895 |
| vs. MSBF | −0.6529 | 0.24963 | 0.047 | −1.2996 | −0.0061 |
| vs. pix2pix | −0.0553 | 0.24671 | 0.996 | −0.6945 | 0.5838 |
| Without pre-reconstruction processing vs. Ref | 0.3407 | 0.24671 | 0.513 | −0.2985 | 0.9799 |
| vs. MSBF | −0.3122 | 0.20500 | 0.426 | −0.8433 | 0.2190 |
| vs. pix2pix | 0.2854 | 0.20143 | 0.490 | −0.2365 | 0.8073 |
| MSBF vs. Ref | 0.6529 | 0.24963 | 0.047 | 0.0061 | 1.2996 |
| vs. Without pre-reconstruction processing | 0.3122 | 0.20500 | 0.426 | −0.2190 | 0.8433 |
| vs. pix2pix | 0.5975 | 0.20500 | 0.021 | 0.0664 | 1.1287 |
| Pix2pix vs. Ref | 0.0553 | 0.24671 | 0.996 | −0.5838 | 0.6945 |
| vs. Without pre-reconstruction processing | −0.2854 | 0.20143 | 0.490 | −0.8073 | 0.2365 |
| vs. MSBF | −0.5975 | 0.20500 | 0.021 | −1.1287 | −0.0664 |
| Source of Variation | df * | Sums of Squares | Mean Square | F | p |
| Processing | 2 | 10.394 | 5.197 | 4.269 | 0.015 |
| Dose | 1 | 0.023 | 0.023 | 0.019 | 0.890 |
| Processing × Dose | 2 | 0.127 | 0.063 | 0.052 | 0.949 |
| Error | 199 | 242.237 | 1.217 | - | - |
| MCs: 0.23 mm | |||||
|---|---|---|---|---|---|
| Variable | Difference | Standard Error | p | 95% CI * | |
| Lower Limit | Upper Limit | ||||
| Ref vs. Without pre-reconstruction processing | 0.0363 | 0.16756 | 0.996 | −0.3972 | 0.4697 |
| vs. MSBF | −0.6841 | 0.16835 | 0.000 | −1.1197 | −0.2486 |
| vs. pix2pix | −0.1218 | 0.16756 | 0.886 | −0.5553 | 0.3116 |
| Without pre-reconstruction processing vs. Ref | −0.0363 | 0.16756 | 0.996 | −0.4697 | 0.3972 |
| vs. MSBF | −0.7204 | 0.13778 | 0.000 | −1.0768 | −0.3640 |
| vs. pix2pix | −0.1581 | 0.13681 | 0.656 | −0.5120 | 0.1959 |
| MSBF vs. Ref | 0.6841 | 0.16835 | 0.000 | 0.2486 | 1.1197 |
| vs. Without pre-reconstruction processing | 0.7204 | 0.13778 | 0.000 | 0.3640 | 1.0768 |
| vs. pix2pix | 0.5623 | 0.13778 | 0.000 | 0.2059 | 0.9188 |
| Pix2pix vs. Ref | 0.1218 | 0.16756 | 0.886 | −0.3116 | 0.5553 |
| vs. Without pre-reconstruction processing | 0.1581 | 0.13681 | 0.656 | −0.1959 | 0.5120 |
| vs. MSBF | −0.5623 | 0.13778 | 0.000 | −0.9188 | −0.2059 |
| Source of Variation | df * | Sums of Squares | Mean Square | F | p |
| Processing | 2 | 20.275 | 10.138 | 15.045 | 0.000 |
| Dose | 1 | 0.275 | 0.275 | 0.409 | 0.523 |
| Processing × Dose | 2 | 0.642 | 0.321 | 0.477 | 0.621 |
| Error | 243 | 163.734 | 0.674 | - | - |
| MCs: 0.29 mm | |||||
|---|---|---|---|---|---|
| Variable | Difference | Standard Error | p | 95% CI * | |
| Lower Limit | Upper Limit | ||||
| Ref vs. Without pre-reconstruction processing | 0.0238 | 0.11462 | 0.997 | −0.2728 | 0.3203 |
| vs. MSBF | −0.8083 | 0.11462 | 0.000 | −1.1048 | −0.5118 |
| vs. pix2pix | −0.1958 | 0.11462 | 0.321 | −0.4923 | 0.1007 |
| Without pre-reconstruction processing vs. Ref | −0.0238 | 0.11462 | 0.997 | −0.3203 | 0.2728 |
| vs. MSBF | −0.8321 | 0.09359 | 0.000 | −1.0742 | −0.5900 |
| vs. pix2pix | −0.2196 | 0.09359 | 0.091 | −0.4617 | 0.0225 |
| MSBF vs. Ref | 0.8083 | 0.11462 | 0.000 | 0.5118 | 1.1048 |
| vs. Without pre-reconstruction processing | 0.8321 | 0.09359 | 0.000 | 0.5900 | 1.0742 |
| vs. pix2pix | 0.6125 | 0.09359 | 0.000 | 0.3704 | 0.8546 |
| Pix2pix vs. Ref | 0.1958 | 0.11462 | 0.321 | −0.1007 | 0.4923 |
| vs. Without pre-reconstruction processing | 0.2196 | 0.09359 | 0.091 | −0.0225 | 0.4617 |
| vs. MSBF | −0.6125 | 0.09359 | 0.000 | −0.8546 | −0.3704 |
| Source of Variation | df * | Sums of Squares | Mean Square | F | p |
| Processing | 2 | 26.778 | 13.389 | 42.460 | 0.000 |
| Dose | 1 | 0.092 | 0.092 | 0.291 | 0.590 |
| Processing × Dose | 2 | 0.028 | 0.014 | 0.044 | 0.957 |
| Error | 245 | 77.256 | 0.315 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomi, T.; Kijima, Y.; Kobayashi, T.; Koibuchi, Y. Evaluation of a Generative Adversarial Network to Improve Image Quality and Reduce Radiation-Dose during Digital Breast Tomosynthesis. Diagnostics 2022, 12, 495. https://doi.org/10.3390/diagnostics12020495
Gomi T, Kijima Y, Kobayashi T, Koibuchi Y. Evaluation of a Generative Adversarial Network to Improve Image Quality and Reduce Radiation-Dose during Digital Breast Tomosynthesis. Diagnostics. 2022; 12(2):495. https://doi.org/10.3390/diagnostics12020495
Chicago/Turabian StyleGomi, Tsutomu, Yukie Kijima, Takayuki Kobayashi, and Yukio Koibuchi. 2022. "Evaluation of a Generative Adversarial Network to Improve Image Quality and Reduce Radiation-Dose during Digital Breast Tomosynthesis" Diagnostics 12, no. 2: 495. https://doi.org/10.3390/diagnostics12020495
APA StyleGomi, T., Kijima, Y., Kobayashi, T., & Koibuchi, Y. (2022). Evaluation of a Generative Adversarial Network to Improve Image Quality and Reduce Radiation-Dose during Digital Breast Tomosynthesis. Diagnostics, 12(2), 495. https://doi.org/10.3390/diagnostics12020495






