MRI-Based Radiomics Analysis for Intraoperative Risk Assessment in Gravid Patients at High Risk with Placenta Accreta Spectrum
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
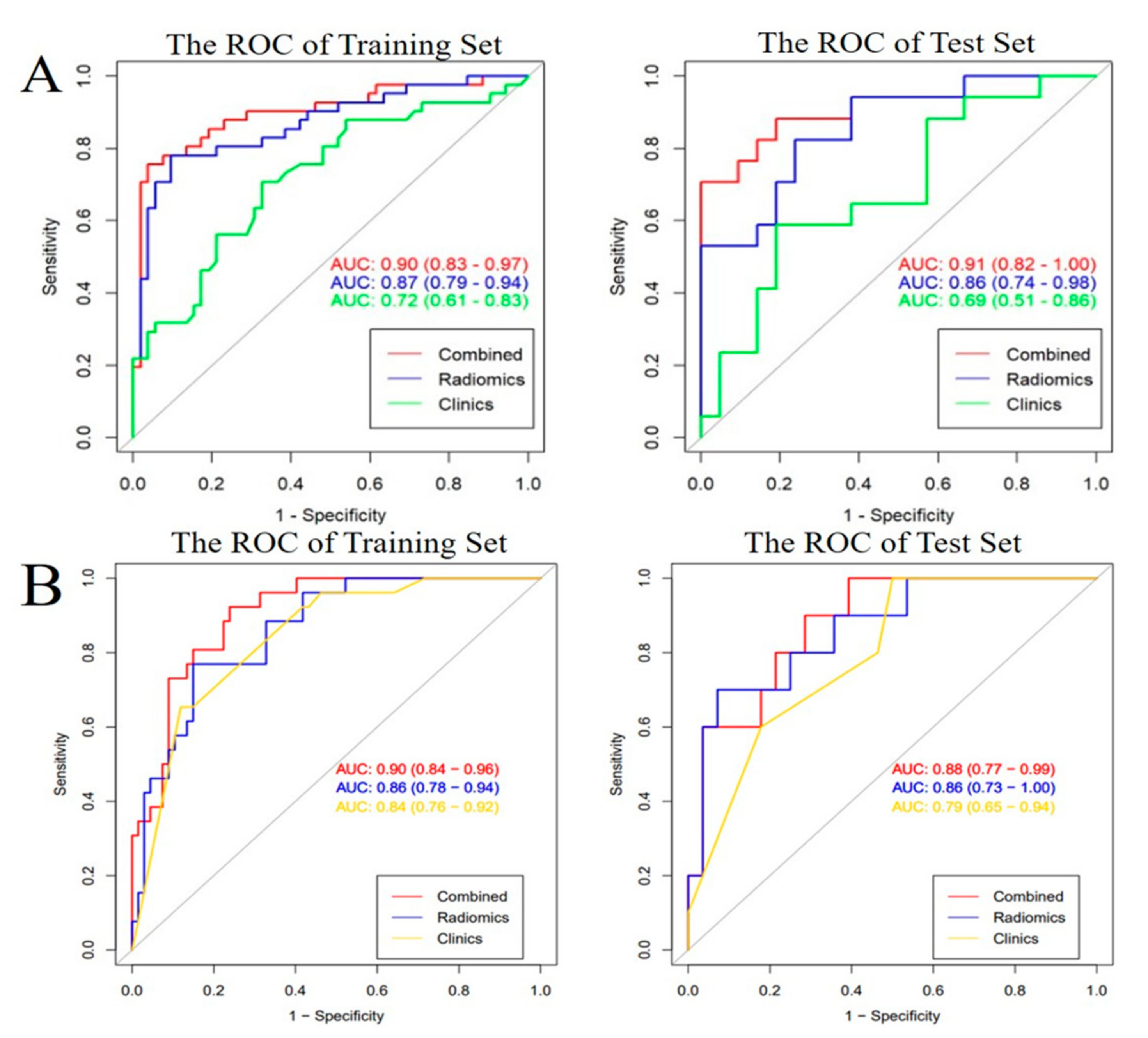
2.2. Features Selection and Development and Validation of Prediction Models
2.3. Predictive Performance of All the Models and Clinical Utility
3. Discussion
3.1. The Status and Related Research of Intraoperative Risk Assessment for Patient
3.2. The Feasibility of MRI-Based Radiomics Analysis for Intraoperative Risk Assessment
3.3. The Necessity of Combined Clinical Variable Analysis for Intraoperative Risk Assessment
3.4. The Clinical Significance of Intraoperative risk Assessment
3.5. Limitation
4. Materials and Methods
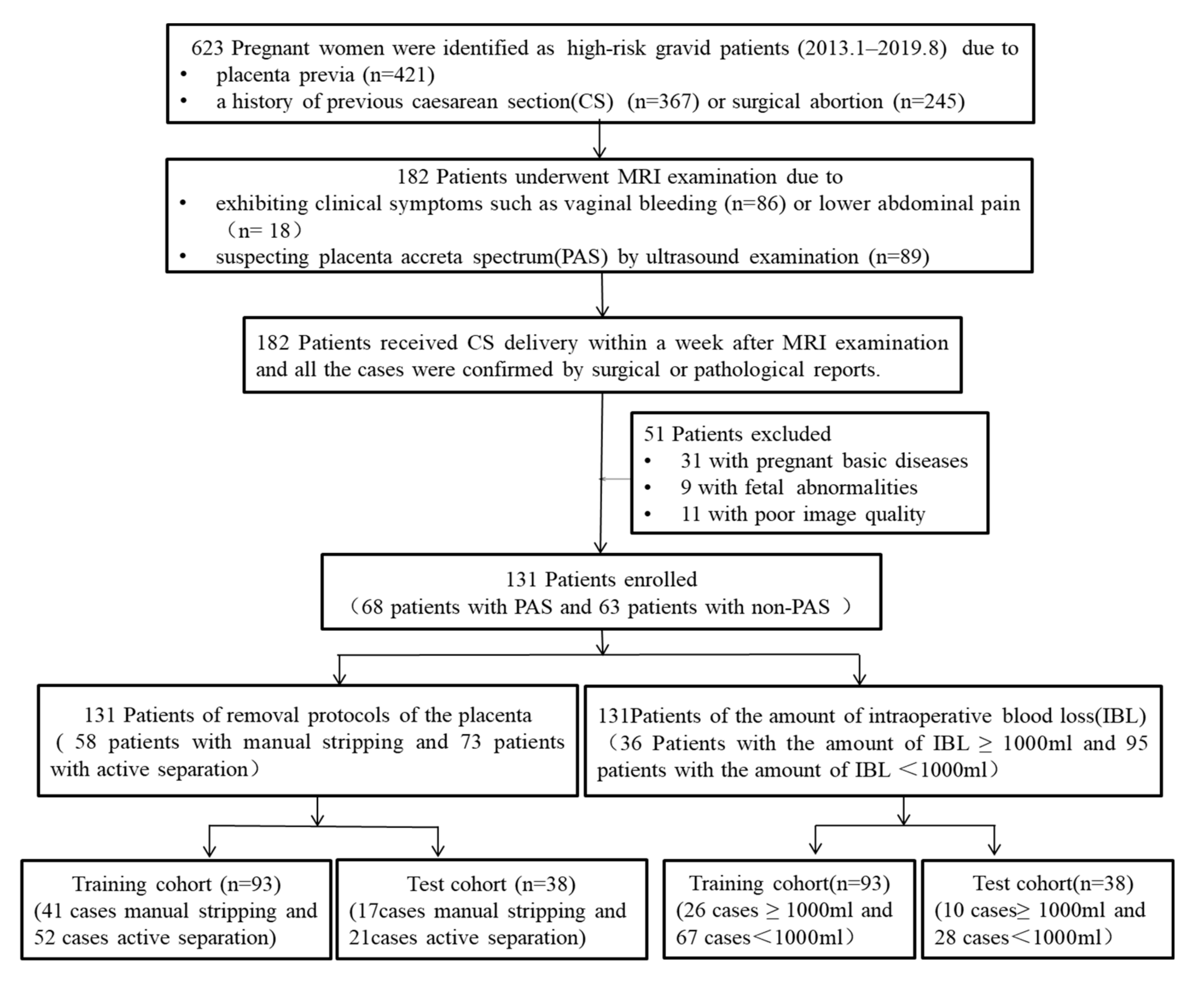
4.1. Patients
4.2. Assessment Standard of Intraoperative Risks
4.3. MRI Imaging
4.4. Radiomics Analysis
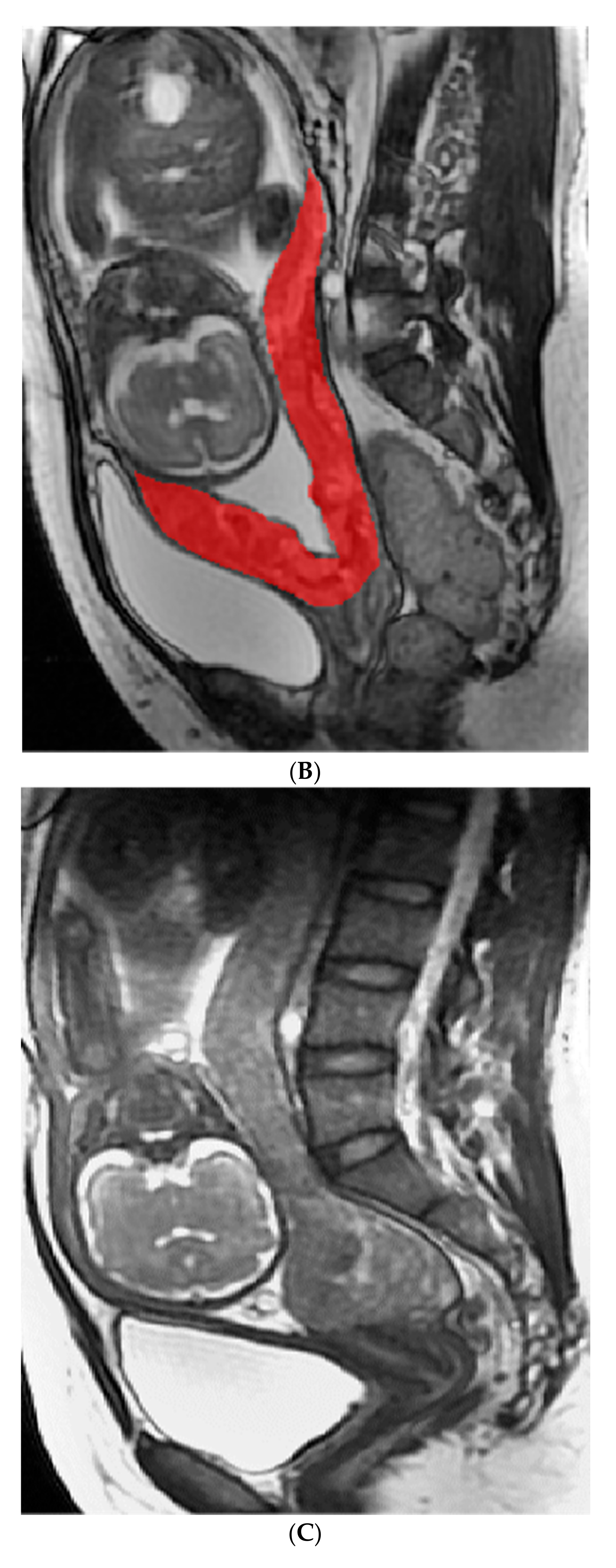
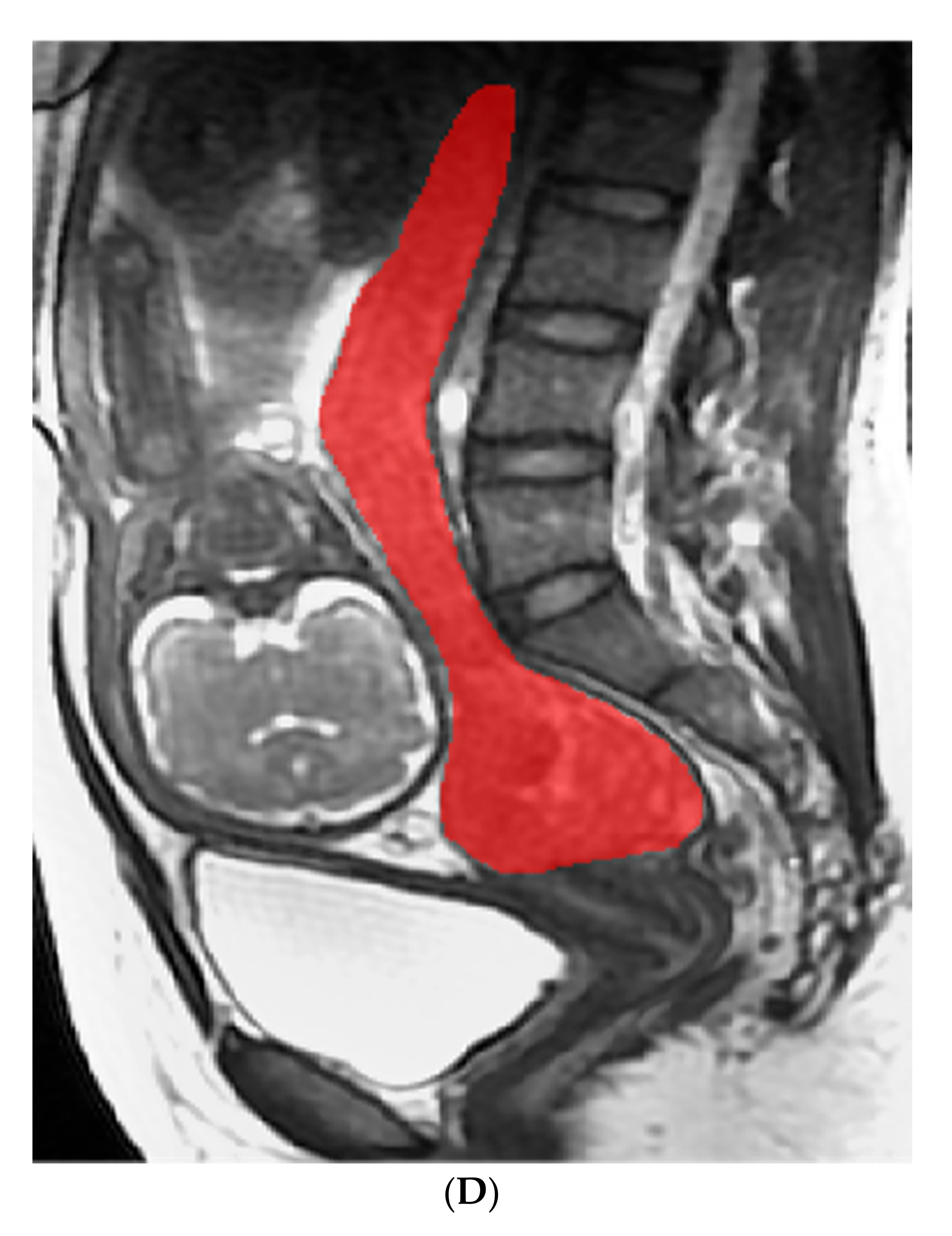
4.4.1. Image Analysis and Segment
4.4.2. Radiomics Feature Extraction, Selection, and Radiomics Score Calculation
4.5. Development of the Radiomics, Clinical, and Clinical-Radiomics Combined Models
4.6. Evaluating the Performance and Utility of All the Models
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silver, R.M.; Branch, D.W. Placenta Accreta Spectrum. N. Engl. J. Med. 2018, 37, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Poder, L.; Bourgioti, C.; Bharwani, N.; Lewis, S.; Kamath, A.; Nougaret, S.; Soyer, P.; Weston, M.; Castillo, R.P.; et al. Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders. Eur. Radiol. 2020, 30, 2604–2615. [Google Scholar] [CrossRef]
- Baldwin, H.J.; Patterson, J.A.; Nippita, T.A.; Torvaldsen, S.; Ibiebele, I.; Simpson, J.M.; Ford, J.B. Maternal and neonatal out-comes following abnormally invasive placenta: A population-based record linkage study. Acta Obstet. Gynecol. Scand. 2017, 96, 1373–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, Z.S.; Eller, A.G.; Bardsley, T.R.; Greene, T.; Varner, M.W.; Silver, R.M. Risk factors for placenta accrete: A large pro-spective cohort. Am. J. Perinatol. 2014, 31, 799–804. [Google Scholar]
- Chu, C.; Liu, M.; Zhang, Y.; Yu, L.; Wang, D.; Gao, C.; Li, W. Quantifying magnetic resonance imaging features to classify pla-centa accreta spectrum (PAS) in high-risk gravid patients. Clin. Imaging 2021, 80, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Carusi, D.A. The Placenta Accreta Spectrum, Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2018, 61, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Hobson, S.R.; Kingdom, J.C.; Murji, A.; Windrim, R.C.; Carvalho, J.; Singh, S.S.; Ziegler, C.; Birch, C.; Frecker, E.; Lim, K.; et al. No. 383-Screening, Diagnosis, and Management of Placenta Accreta Spectrum Disorders. J. Obstet. Gynae-Col. Can. 2019, 41, 1035–1049. [Google Scholar] [CrossRef]
- Einerson, B.D.; Branch, D.W. Surgical Management of Placenta Accreta Spectrum. Clin. Obstet. Gynecol. 2018, 61, 774–782. [Google Scholar] [CrossRef]
- Sentilhes, L.; Kayem, G.; Silver, R.M. Conservative Management of Placenta Accreta Spectrum. Clin. Obstet. Gynecol. 2018, 61, 783–794. [Google Scholar] [CrossRef]
- Baughman, W.C.; Corteville, J.E.; Shah, R.R. Placenta accrete: Spectrum of US and MR imaging findings. Radiographics 2008, 28, 1905–1916. [Google Scholar] [CrossRef] [Green Version]
- Palacios-Jaraquemada, J.M. Diagnosis and management of placenta accreta. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 1133–1148. [Google Scholar] [CrossRef]
- Bour, L.; Place, V.; Bendavid, S.; Fargeaudou, Y.; Portal, J.J.; Ricbourg, A.; Sebbag, D.; Dohan, A.; Vicaut, E.; Soyer, P. Suspected invasive placenta: Evaluation with magnetic resonance imaging. Eur. Radiol. 2014, 24, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shan, R.; Zhao, L.; Song, Q.; Zuo, C.; Zhang, X.; Wang, S.; Shi, H.; Gao, F.; Qian, T.; et al. Invasive placenta previa, Placental bulge with distorted uterine outline and uterine serosal hypervascularity at 1.5T MRI—Use-ful features for differentiating placenta percreta from placenta accreta. Eur. Radiol. 2018, 28, 708–717. [Google Scholar] [CrossRef]
- Bourgioti, C.; Konstantinidou, A.E.; Zafeiropoulou, K.; Antoniou, A.; Fotopoulos, S.; Theodora, M.; Daskalakis, G.; Nikolaidou, M.E.; Tzavara, C.; Letsika, A.; et al. Intraplacental Fetal Vessel Diameter May Help Predict for Placental Invasiveness in Pregnant Women at High Risk for Placenta Accreta Spectrum Disorders. Radiology 2021, 298, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.; Deist, T.M.; Peerlings, J.; de Jong, E.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, C.; Wang, L.; Luo, R.; Li, J.; Zheng, H.; Yin, Q.; Zhang, Z.; Duan, S.; Li, X.; et al. MRI radiomics analysis for predicting preoperative synchronous distant metastasis in patients with rectal cancer. Eur. Radiol. 2019, 29, 4418–4426. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Song, Y.; Yin, X.; Chen, A.; Tang, N.; Prince, M.R.; Yang, G.; Wang, H. Utilization of radiomics to predict long-term outcome of magnetic resonance-guided focused ultrasound ablation therapy in adenomyosis. Eur. Radiol. 2021, 31, 392–402. [Google Scholar] [CrossRef]
- Do, Q.N.; Lewis, M.A.; Xi, Y.; Madhuranthakam, A.J.; Happe, S.K.; Dashe, J.S.; Lenkinski, R.E.; Khan, A.; Twickler, D.M. MRI of the Placenta Accreta Spectrum (PAS) Disorder, Radiomics Analysis Correlates with Surgical and Pathological Outcome. J. Magn. Reson. Imaging 2020, 51, 936–946. [Google Scholar] [CrossRef]
- Wu, Q.; Yao, K.; Liu, Z.; Li, L.; Zhao, X.; Wang, S.; Shang, H.; Lin, Y.; Wen, Z.; Zhang, X.; et al. Radiomics analysis of placenta on T2WI facilitates prediction of postpartum haemorrhage, A multicentre study. Ebiomedicine 2019, 50, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Romeo, V.; Ricciardi, C.; Cuocolo, R.; Stanzione, A.; Verde, F.; Sarno, L.; Improta, G.; Mainenti, P.P.; D’Armiento, M.; Brunetti, A.; et al. Machine learning analysis of MRI-derived texture features to predict placenta accreta spectrum in patients with placenta previa. Magn. Reson. Imaging 2019, 64, 71–76. [Google Scholar] [CrossRef]
- Sun, H.; Qu, H.; Chen, L.; Wang, W.; Liao, Y.; Zou, L.; Zhou, Z.; Wang, X.; Zhou, S. Identification of suspicious invasive placen-tation based on clinical MRI data using textural features and automated machine learning. Eur. Radiol. 2019, 29, 6152–6162. [Google Scholar] [CrossRef]
- Alamo, L.; Anaye, A.; Rey, J.; Denys, A.; Bongartz, G.; Terraz, S.; Artemisia, S.; Meuli, R.; Schmidt, S. Detection of suspected placental invasion by MRI: Do the results depend on observer’ experience? Eur. J. Radiol. 2013, 82, e51–e57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, H.; Xin, Y.; Zhang, C.; Liu, Z.; Han, X.; Liu, Q.; Li, Y.; Huang, Z. Assessment of the massive hemorrhage in placen-ta accreta spectrum with magnetic resonance imaging. Exp. Ther. Med. 2020, 19, 2367–2376. [Google Scholar]
- Romeo, V.; Maurea, S. The new era of advanced placental tissue characterization using MRI texture analysis, Clinical implications. Ebiomedicine 2020, 51, 102588. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Wu, C.; Zheng, H.; Wang, L.; Guan, W.; Duan, S.; Wang, D. Radiogenomics of neuroblastoma in pediatric patients, CT-based radiomics signature in predicting MYCN amplification. Eur. Radiol. 2021, 31, 3080–3089. [Google Scholar] [CrossRef]
- Chen, E.; Mar, W.A.; Horowitz, J.M.; Allen, A.; Jha, P.; Cantrell, D.R.; Cai, K. Texture analysis of placental MRI: Can it aid in the prenatal diagnosis of placenta accreta spectrum? Abdom. Radiol. 2019, 44, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, K.; Morizane, M.; Deguchi, M.; Ebina, Y.; Tanaka, U.; Ueno, Y.; Kitajima, K.; Maeda, T.; Sugimura, K.; Yamada, H. A novel scoring system for predicting adherent placenta in women with placenta previa. Placenta 2018, 64, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauniaux, E.; Hussein, A.M.; Fox, K.A.; Collins, S.L. New evidence-based diagnostic and management strategies for placenta accreta spectrum disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 61, 75–88. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Training Cohort (n = 93) | Testing Cohort (n = 38) | ||||
|---|---|---|---|---|---|---|
| Active Separation (N = 52) | Manual Stripping (N = 41) | p-Value | Active Separation (N = 21) | Manual Stripping (N = 17) | p-Value | |
| Age (mean ± SD) | 31.90 ± 4.53 | 32.76 ± 4.56 | 0.37 | 32.10 ± 4.33 | 33 ± 3.76 | 0.16 |
| Weeks of gestation at time of MRI examination (mean ± SD) | 34.54 ± 3.03 | 30.74 ± 7.37 | <0.001 | 34.86 ± 2.60 | 33.35 ± 4.45 | 0.20 |
| Vaginal bleeding n (%) | 0.27 | 0.04 | ||||
| No | 34 (65.4) | 20 (48.8) | 13 (61.9) | 6 (35.3) | ||
| Minor | 16 (30.8) | 19 (46.3) | 6 (28.6) | 11 (64.7) | ||
| Massive | 2 (3.8) | 2 (4.9) | 2 (9.5) | 0 (0) | ||
| Placenta previa n (%) | <0.001 | 0.20 | ||||
| Low lying | 20 (38.5) | 31 (75.6) | 8 (38.2) | 12 (70.6) | ||
| Marginal | 11 (21.2) | 3 (7.3) | 4 (19) | 2 (11.8) | ||
| Partial | 10 (19.2) | 3 (7.3) | 4 (19) | 2 (11.8) | ||
| Complete | 11 (21.2) | 4 (9.8) | 5 (23.8) | 1 (5.9) | ||
| No. of previous CS n (%) | 0.01 | <0.001 | ||||
| =0 | 36 (69.2) | 18 (43.9) | 1 (85.7) | 6 (35.3) | ||
| =1 | 16 (30.8) | 19 (46.3) | 3 (14.3) | 10 (58.8) | ||
| ≥2 | 0 (0) | 4 (10) | 0 (0) | 1 (5.9) | ||
| No. of previous surgical abortion n (%) | 0.01 | 0.31 | ||||
| =0 | 33 (63.5) | 13 (31.7) | 10 (47.6) | 6 (35.3) | ||
| =1 | 11 (21.2) | 19 (46.3) | 7 (33.3) | 10 (58.8) | ||
| ≥2 | 8 (15.3) | 9 (22) | 4 (19.1) | 1 (5.9) | ||
| Blood loss during surgery (mean ± SD mL) | 471.54 ± 296.74 | 1834.88 ± 1774.03 | <0.001 | 379.52 ± 96.67 | 2047.06 ± 2041.18 | <0.001 |
| PAS | <0.001 | <0.001 | ||||
| No | 37 (71.2) | 3 (7.3) | 20 (95.2) | 3 (17.6) | ||
| Placenta accrete | 15 (28.8) | 10 (24.4) | 1 (4.8) | 5 (29.4) | ||
| Placenta increta | 0 (0) | 24 (58.5) | 0 (0) | 7 (41) | ||
| Placenta percreta | 0 (0) | 4 (9.8) | 0 (0) | 2 (12) | ||
| Characteristics | Training Cohort (n = 93) | Testing Cohort (n = 38) | ||||
|---|---|---|---|---|---|---|
| <1000 mL (N = 67) | ≥1000 mL (N = 26) | p-Value | <1000 mL (N = 28) | ≥1000 mL (N = 10) | p-Value | |
| Age (mean ± SD) | 31.63 ± 4.47 | 32.85 ± 4.30 | 0.34 | 32.86 ± 4.64 | 32.3 ± 3.74 | 0.74 |
| Weeks of gestation at time of MRI examination (mean ± SD) | 34.24 ± 3.80 | 29.88 ± 7.48 | <0.001 | 34 ± 4.38 | 33.25 ± 5.25 | 0.66 |
| Vaginal bleeding n (%) | 0.67 | 0.71 | ||||
| No | 42 (62.7) | 10 (38.5) | 15 (53.6) | 6 (60) | ||
| Minor | 22 (32.8) | 14 (53.8) | 12 (42.9) | 4 (40) | ||
| Massive | 3 (4.5) | 2 (7.7) | 1 (3.6) | 0 (0) | ||
| Placenta previa n (%) | <0.001 | 0.04 | ||||
| Low lying | 26 (38.8) | 24 (92.3) | 13 (46.4) | 8 (80) | ||
| Marginal | 14 (20.9) | 0 (0) | 6 (21.4) | 0 (0) | ||
| Partial | 12 (17.9) | 1 (3.8) | 4 (14.3) | 2 (20) | ||
| Complete | 15 (22.4) | 1 (3.8) | 5 (17.9) | 0 (0) | ||
| No. of previous CS n (%) | <0.001 | 0.01 | ||||
| =0 | 49 (73.1) | 7 (26.9) | 20 (71.4) | 2 (20) | ||
| =1 | 16 (23.9) | 17 (65.4) | 8 (28.6) | 7 (70) | ||
| ≥2 | 2 (3.0) | 2 (7.7) | 0 (0) | 1 (10) | ||
| No. of previous surgical abortion n (%) | 0.02 | 0.09 | ||||
| =0 | 39 (58.2) | 7 (27.0) | 13 (46.4) | 3 (30) | ||
| =1 | 17 (25.4) | 14 (53.8) | 9 (32.1) | 7 (70) | ||
| ≥2 | 11 (16.4) | 5 (19.2) | 6 (21.5) | 0 (0) | ||
| Removal protocols of the placenta | <0.001 | <0.001 | ||||
| Active separation | 48 (71.6) | 3 (11.5) | 21 (75) | 1 (10) | ||
| Manual stripping | 19 (28.4) | 23 (88.5) | 7 (25) | 9 (90) | ||
| PAS | <0.001 | <0.001 | ||||
| No | 48 (71.6) | 1 (3.8) | 14 (50) | 0 (0) | ||
| Placenta accrete | 14 (20.9) | 2 (7.7) | 12 (42.9) | 3 (30) | ||
| Placenta increta | 5 (7.5) | 18 (69.2) | 2 (7.1) | 6 (60) | ||
| Placenta percreta | 0 (0) | 5 (19.2) | 0 (0) | 1 (10) | ||
| Evaluation-Parameters | The Predictive Model for Removal Protocols of The Placenta | The Predictive Model for Intraoperative Blood Loss | ||
|---|---|---|---|---|
| Training Cohort | Test Cohort | Training Cohort | Test Cohort | |
| Radiomics model | ||||
| AUC (95%CI) | 0.87 (0.79~0.94) | 0.86 (0.74~0.98) | 0.86 (0.78~0.94) | 0.86 (0.73~1.0) |
| Accuracy (95%CI) | 0.85 (0.76~0.92) | 0.79 (0.63~0.90) | 0.83 (0.74~0.90) | 0.87 (0.72~0.96) |
| Sensitivity | 0.90 | 0.76 | 0.85 | 0.93 |
| Specificity | 0.78 | 0.82 | 0.77 | 0.70 |
| Pos. Pred. Value | 0.84 | 0.84 | 0.90 | 0.90 |
| Neg. Pred. Value | 0.86 | 0.74 | 0.67 | 0.78 |
| Clinical model | ||||
| AUC (95%CI) | 0.72 (0.61~0.83) | 0.69 (0.51~0.86) | 0.84 (0.76~0.92) | 0.79 (0.65~0.94) |
| Accuracy (95%CI) | 071 (0.54~0.85) | 0.69 (0.58~0.78) | 0.76 (0.60~0.89) | 0.82 (0.72~0.89) |
| Sensitivity | 0.71 | 0.63 | 0.55 | 0.68 |
| Specificity | 0.71 | 0.74 | 0.85 | 0.87 |
| Pos. Pred. Value | 0.59 | 0.71 | 0.6 | 0.65 |
| Neg. Pred. Value | 0.81 | 0.67 | 0.82 | 0.88 |
| Combined model with radiomics and clinics | ||||
| AUC (95%CI) | 0.90 (0.83~0.97) | 0.91 (0.82~1.00) | 0.90 (0.84~0.96) | 0.88 (0.77~0.99) |
| Accuracy (95%CI) | 0.87 (0.79~0.93) | 0.87 (0.72~0.96) | 0.81 (0.77~0.88) | 0.76 (0.60~0.89) |
| Sensitivity | 0.94 | 1.0 | 0.6 | 0.53 |
| Specificity | 0.83 | 0.81 | 0.96 | 0.95 |
| Pos. Pred. Value | 0.76 | 0.71 | 0.92 | 0.9 |
| Neg. Pred. Value | 0.96 | 1.0 | 0.76 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.; Liu, M.; Zhang, Y.; Zhao, S.; Ge, Y.; Li, W.; Gao, C. MRI-Based Radiomics Analysis for Intraoperative Risk Assessment in Gravid Patients at High Risk with Placenta Accreta Spectrum. Diagnostics 2022, 12, 485. https://doi.org/10.3390/diagnostics12020485
Chu C, Liu M, Zhang Y, Zhao S, Ge Y, Li W, Gao C. MRI-Based Radiomics Analysis for Intraoperative Risk Assessment in Gravid Patients at High Risk with Placenta Accreta Spectrum. Diagnostics. 2022; 12(2):485. https://doi.org/10.3390/diagnostics12020485
Chicago/Turabian StyleChu, Caiting, Ming Liu, Yuzhen Zhang, Shuhui Zhao, Yaqiong Ge, Wenhua Li, and Chengjin Gao. 2022. "MRI-Based Radiomics Analysis for Intraoperative Risk Assessment in Gravid Patients at High Risk with Placenta Accreta Spectrum" Diagnostics 12, no. 2: 485. https://doi.org/10.3390/diagnostics12020485
APA StyleChu, C., Liu, M., Zhang, Y., Zhao, S., Ge, Y., Li, W., & Gao, C. (2022). MRI-Based Radiomics Analysis for Intraoperative Risk Assessment in Gravid Patients at High Risk with Placenta Accreta Spectrum. Diagnostics, 12(2), 485. https://doi.org/10.3390/diagnostics12020485






