Deep Learning Approach to Automatize TMTV Calculations Regardless of Segmentation Methodology for Major FDG-Avid Lymphomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Preprocessing
2.3. Ground Truth Generation
2.4. Model Architecture and Training
2.5. Post-Processing
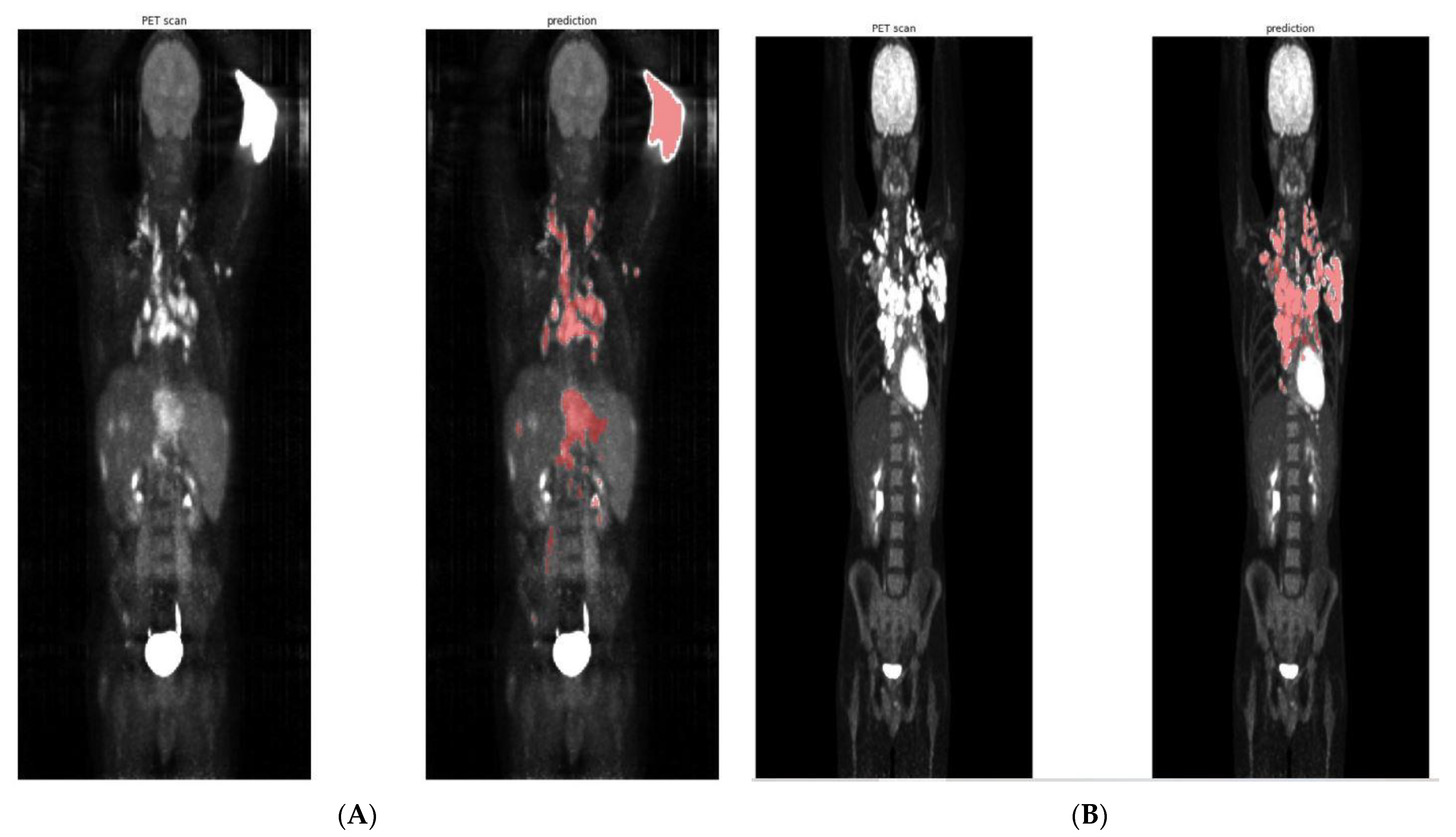
2.6. Predicted TMTV Validations
3. Results
3.1. Training of the Convolutional Neural Network
3.2. Raw TMTV Prediction without Post-Processing
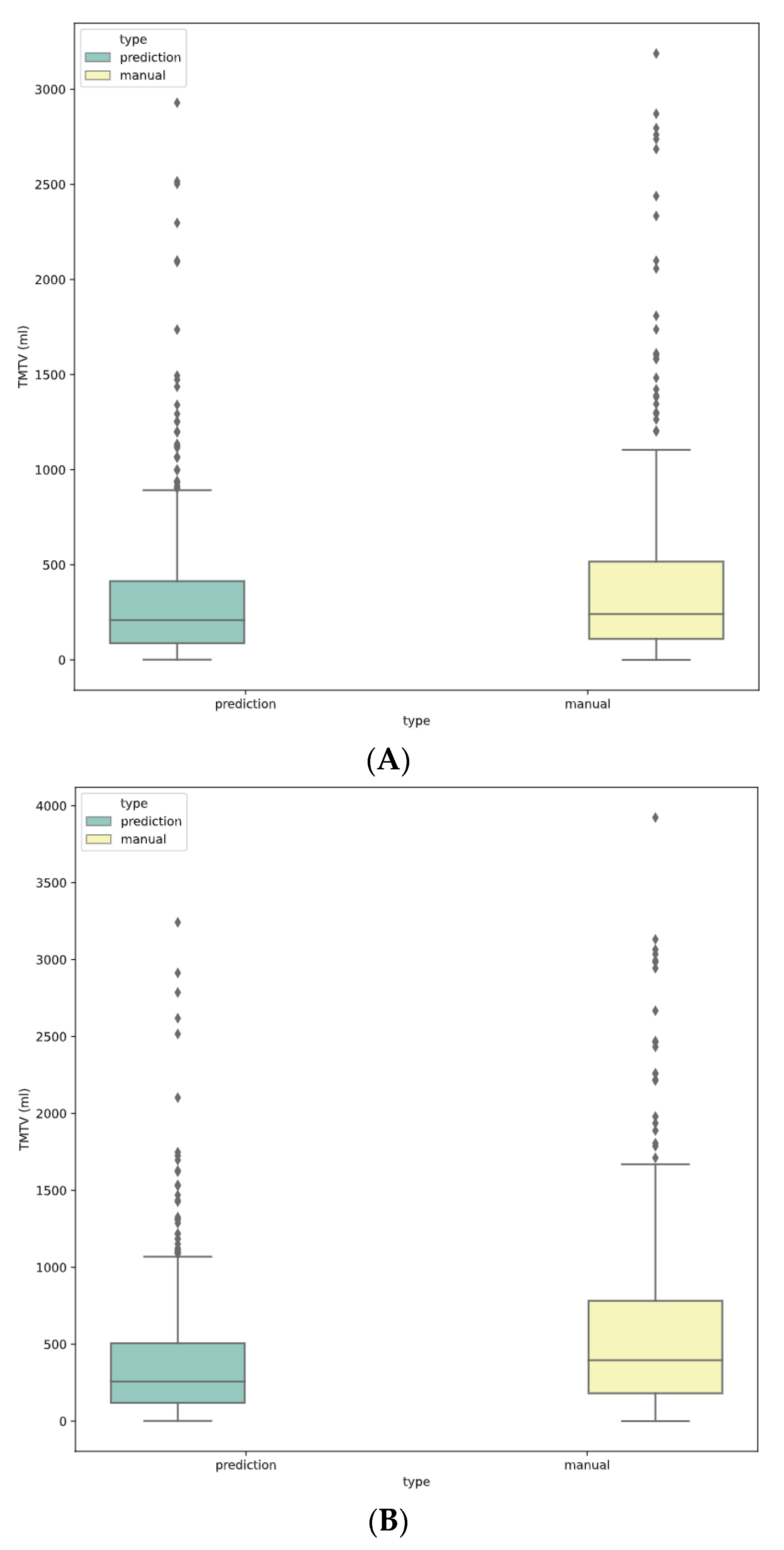
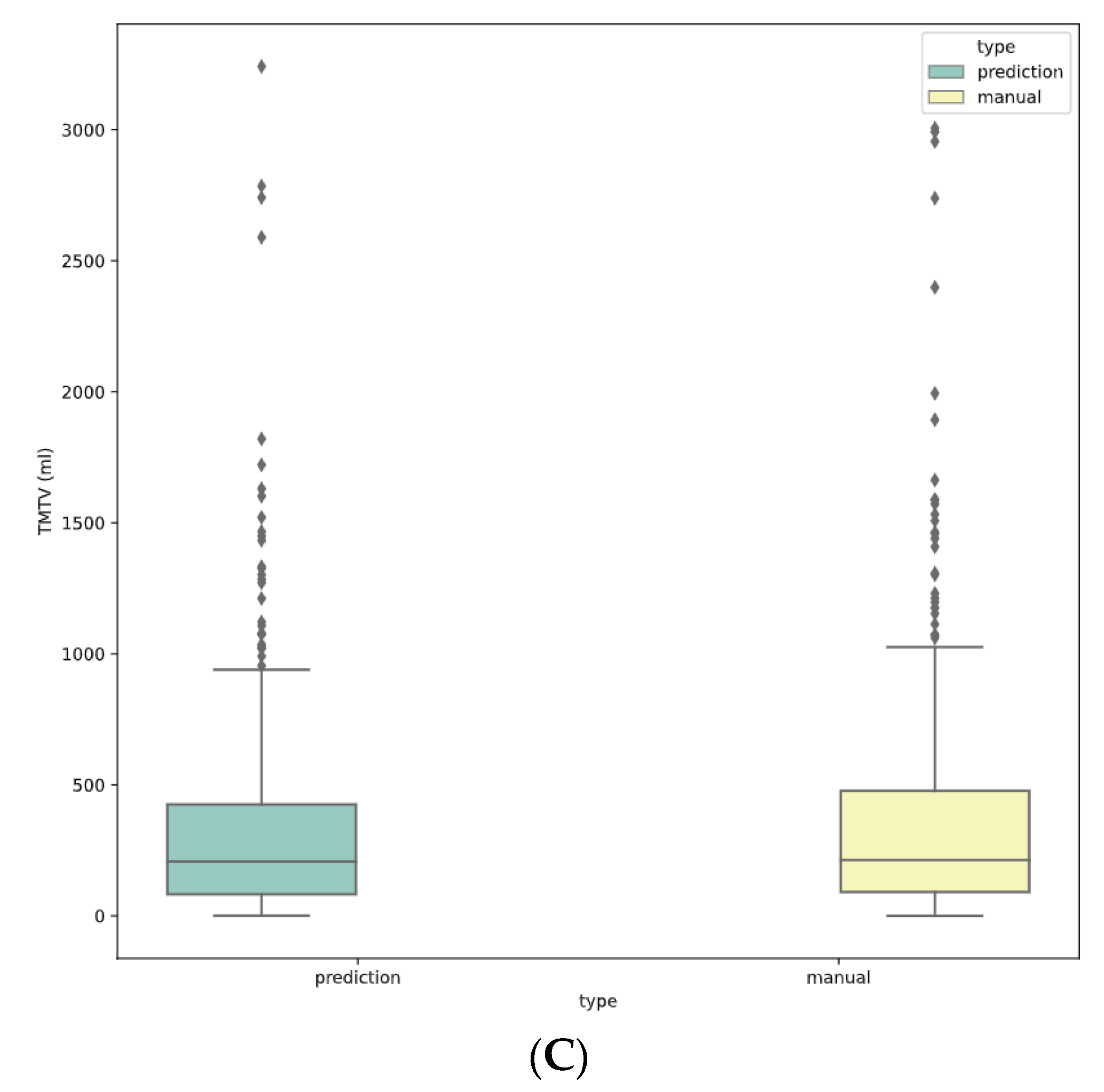
3.3. Clustering of Predicted Segmentation for 41% TMTV Calculation
3.4. Final TMTV Predicted Values Per Methodology
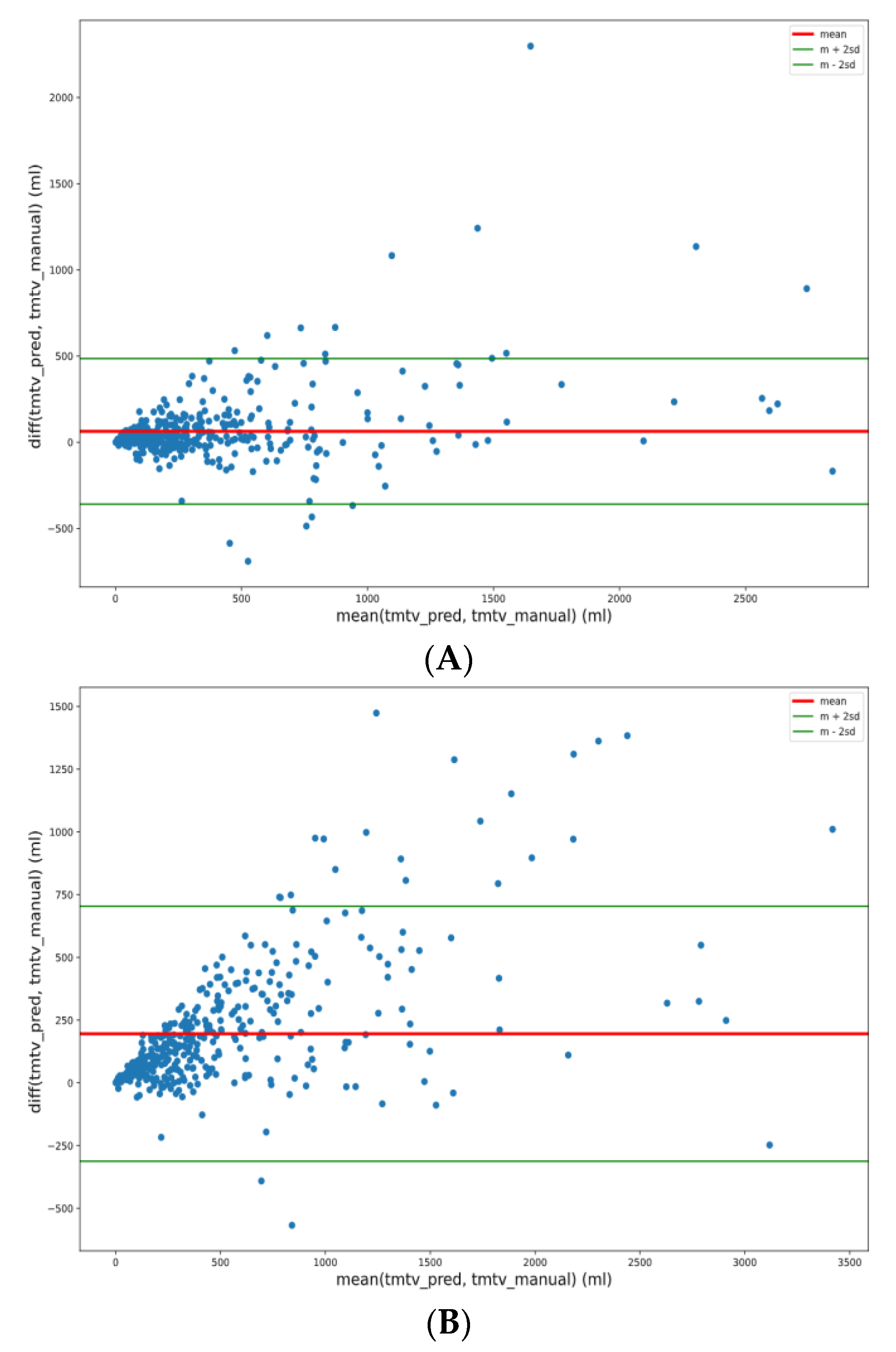
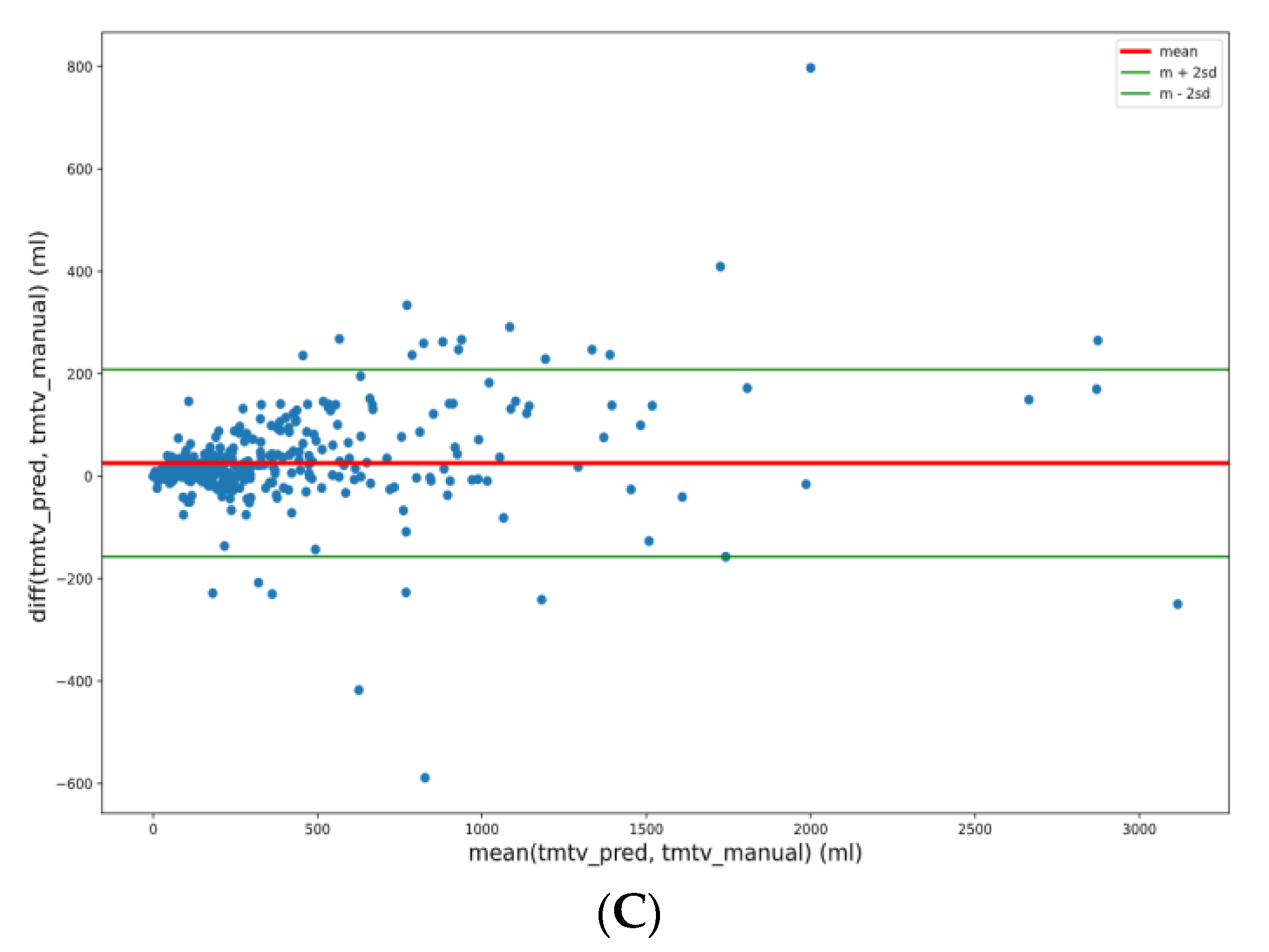
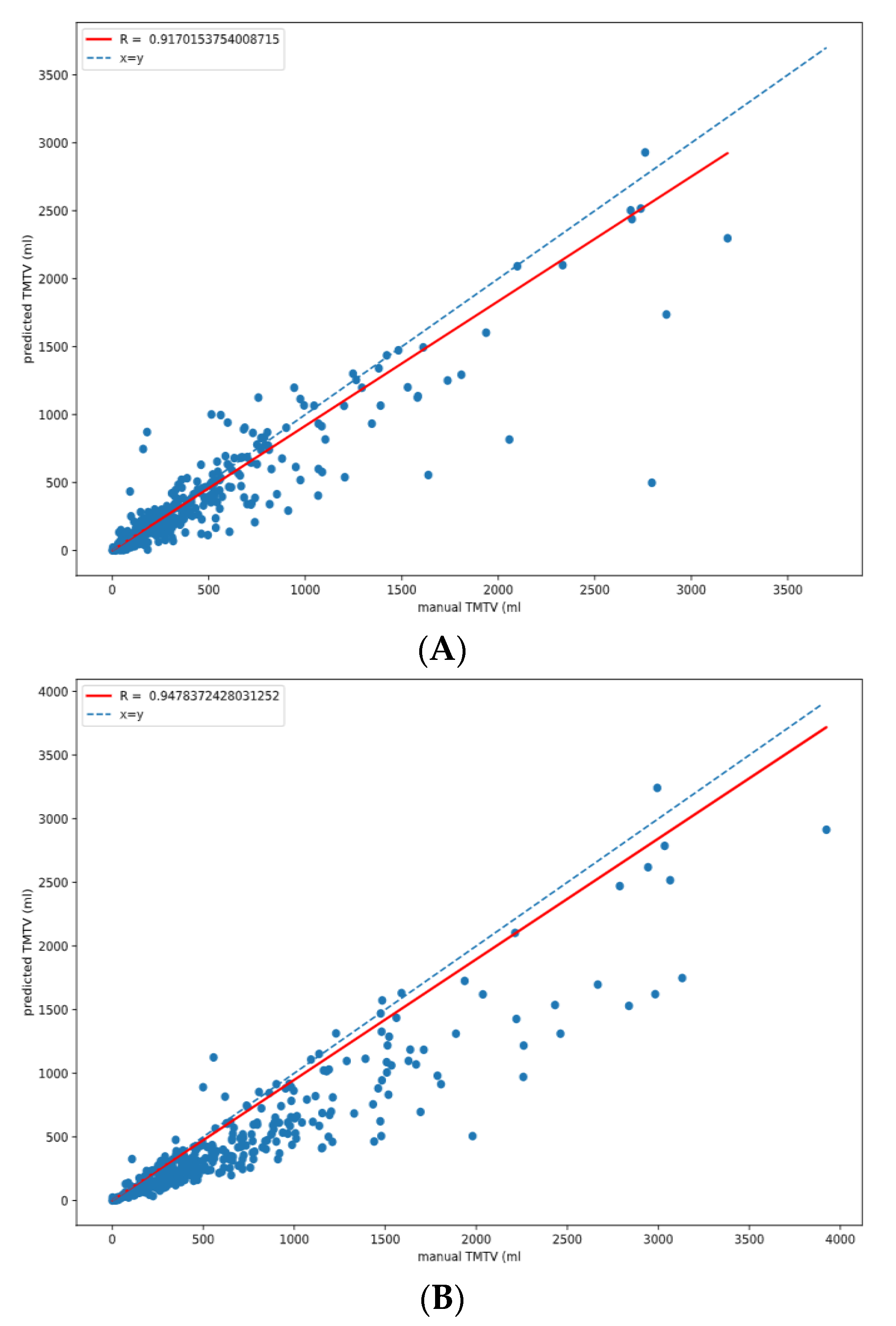
3.5. TMTV Correlation
3.6. TMTV Predicted Values per Lymphoma Subtypes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cottereau, A.-S.; Versari, A.; Loft, A.; Casasnovas, O.; Bellei, M.; Ricci, R.; Bardet, S.; Castagnoli, A.; Brice, P.; Raemaekers, J.; et al. Prognostic Value of Baseline Metabolic Tumor Volume in Early-Stage Hodgkin Lymphoma in the Standard Arm of the H10 Trial. Blood 2018, 131, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Kanoun, S.; Rossi, C.; Berriolo-Riedinger, A.; Dygai-Cochet, I.; Cochet, A.; Humbert, O.; Toubeau, M.; Ferrant, E.; Brunotte, F.; Casasnovas, R.-O. Baseline Metabolic Tumour Volume Is an Independent Prognostic Factor in Hodgkin Lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Howlett, S.; Casasnovas, R.-O.; Mounier, N.; Perrot, A.; Morschhauser, F.; Fruchart, C.; Daguindau, N.; van Eygen, K.; Obéric, L.; et al. Lenalidomide Maintenance for Diffuse Large B-Cell Lymphoma Patients Responding to R-CHOP: Quality of Life, Dosing, and Safety Results from the Randomised Controlled REMARC Study. Br. J. Haematol. 2020, 189, 84–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meignan, M.; Cottereau, A.S.; Versari, A.; Chartier, L.; Dupuis, J.; Boussetta, S.; Grassi, I.; Casasnovas, R.-O.; Haioun, C.; Tilly, H.; et al. Baseline Metabolic Tumor Volume Predicts Outcome in High–Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. J. Clin. Oncol. 2016, 34, 3618–3626. [Google Scholar] [CrossRef]
- Grossiord, E.; Passat, N.; Talbot, H.; Naegel, B.; Kanoun, S.; Tal, I.; Tervé, P.; Ken, S.; Casasnovas, O.; Meignan, M.; et al. Shaping for PET Image Analysis. Pattern Recognit. Lett. 2020, 131, 307–313. [Google Scholar] [CrossRef]
- Capobianco, N.; Meignan, M.; Cottereau, A.-S.; Vercellino, L.; Sibille, L.; Spottiswoode, B.; Zuehlsdorff, S.; Casasnovas, O.; Thieblemont, C.; Buvat, I. Deep-Learning 18F-FDG Uptake Classification Enables Total Metabolic Tumor Volume Estimation in Diffuse Large B-Cell Lymphoma. J. Nucl. Med. 2021, 62, 30–36. [Google Scholar] [CrossRef]
- Blanc-Durand, P.; Jégou, S.; Kanoun, S.; Berriolo-Riedinger, A.; Bodet-Milin, C.; Kraeber-Bodéré, F.; Carlier, T.; Le Gouill, S.; Casasnovas, R.-O.; Meignan, M.; et al. Fully Automatic Segmentation of Diffuse Large B Cell Lymphoma Lesions on 3D FDG-PET/CT for Total Metabolic Tumour Volume Prediction Using a Convolutional Neural Network. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1362–1370. [Google Scholar] [CrossRef]
- Liu, X.; Song, L.; Liu, S.; Zhang, Y. A Review of Deep-Learning-Based Medical Image Segmentation Methods. Sustainability 2021, 13, 1224. [Google Scholar] [CrossRef]
- Yang, R.; Yu, Y. Artificial Convolutional Neural Network in Object Detection and Semantic Segmentation for Medical Imaging Analysis. Front. Oncol. 2021, 11, 573. [Google Scholar] [CrossRef]
- Jemaa, S.; Fredrickson, J.; Carano, R.A.D.; Nielsen, T.; de Crespigny, A.; Bengtsson, T. Tumor Segmentation and Feature Extraction from Whole-Body FDG-PET/CT Using Cascaded 2D and 3D Convolutional Neural Networks. J. Digit. Imaging 2020, 33, 888–894. [Google Scholar] [CrossRef]
- Cottereau, A.-S.; Buvat, I.; Kanoun, S.; Versari, A.; Casasnovas, O.; Chauvie, S.; Clerc, J.; Gallamini, A.; Meignan, M. Is There an Optimal Method for Measuring Baseline Metabolic Tumor Volume in Diffuse Large B Cell Lymphoma? Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1463–1464. [Google Scholar] [CrossRef]
- Casasnovas, R.-O.; Bouabdallah, R.; Brice, P.; Lazarovici, J.; Ghesquieres, H.; Stamatoullas, A.; Dupuis, J.; Gac, A.-C.; Gastinne, T.; Joly, B.; et al. PET-Adapted Treatment for Newly Diagnosed Advanced Hodgkin Lymphoma (AHL2011): A Randomised, Multicentre, Non-Inferiority, Phase 3 Study. Lancet Oncol. 2019, 20, 202–215. [Google Scholar] [CrossRef]
- Le Gouill, S.; Ghesquières, H.; Oberic, L.; Morschhauser, F.; Tilly, H.; Ribrag, V.; Lamy, T.; Thieblemont, C.; Maisonneuve, H.; Gressin, R.; et al. Obinutuzumab vs Rituximab for Advanced DLBCL: A PET-Guided and Randomized Phase 3 Study by LYSA. Blood 2021, 137, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Fowler, N.H.; Feugier, P.; Bouabdallah, R.; Tilly, H.; Palomba, M.L.; Fruchart, C.; Libby, E.N.; Casasnovas, R.-O.; Flinn, I.W.; et al. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N. Engl. J. Med. 2018, 379, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Tosolini, M.; Gravelle, P.; Pericart, S.; Kanoun, S.; Evrard, S.; Gilhodes, J.; Franchini, D.-M.; Amara, N.; Syrykh, C.; et al. Baseline SUVmax Is Related to Tumor Cell Proliferation and Patient Outcome in Follicular Lymphoma. Haematologica 2020, 107, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Casasnovas, R.-O.; Meignan, M.; Berriolo-Riedinger, A.; Bardet, S.; Julian, A.; Thieblemont, C.; Vera, P.; Bologna, S.; Brière, J.; Jais, J.-P.; et al. SUVmax Reduction Improves Early Prognosis Value of Interim Positron Emission Tomography Scans in Diffuse Large B-Cell Lymphoma. Blood 2011, 118, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghesquieres, H.; Casasnovas, O.; Nicolas-Virelizier, E.; Gandhi Laurent, D.; Delwail, V.; Touati, M.; Le Du, K.; Morschhauser, F.; Bonnet, C.; Waultier-Rascalou, A.; et al. Prednisone, Vinblastine, Doxorubicin and Bendamustine (PVAB) Regimen in First Line Therapy for Older Patients with Advanced-Stage Classical Hodgkin Lymphoma: Results of a Prospective Multicenter Phase II Trial of the Lymphoma Study Association (LYSA). Blood 2019, 134, 2832. [Google Scholar] [CrossRef]
- Kanoun, S.; Tal, I.; Berriolo-Riedinger, A.; Rossi, C.; Riedinger, J.-M.; Vrigneaud, J.-M.; Legrand, L.; Humbert, O.; Casasnovas, O.; Brunotte, F.; et al. Influence of Software Tool and Methodological Aspects of Total Metabolic Tumor Volume Calculation on Baseline [18F]FDG PET to Predict Survival in Hodgkin Lymphoma. PLoS ONE 2015, 10, e0140830. [Google Scholar] [CrossRef] [Green Version]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Barrington, S.F.; Zwezerijnen, B.G.J.C.; de Vet, H.C.W.; Heymans, M.W.; Mikhaeel, N.G.; Burggraaff, C.N.; Eertink, J.J.; Pike, L.C.; Hoekstra, O.S.; Zijlstra, J.M.; et al. Automated Segmentation of Baseline Metabolic Total Tumor Burden in Diffuse Large B-Cell Lymphoma: Which Method Is Most Successful? A Study on Behalf of the PETRA Consortium. J. Nucl. Med. 2021, 62, 332–337. [Google Scholar] [CrossRef]
- Meignan, M.; Sasanelli, M.; Casasnovas, R.O.; Luminari, S.; Fioroni, F.; Coriani, C.; Masset, H.; Itti, E.; Gobbi, P.G.; Merli, F.; et al. Metabolic Tumour Volumes Measured at Staging in Lymphoma: Methodological Evaluation on Phantom Experiments and Patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1113–1122. [Google Scholar] [CrossRef]
- Milletari, F.; Navab, N.; Ahmadi, S.-A. V-Net: Fully Convolutional Neural Networks for Volumetric Medical Image Segmentation. arXiv 2016, arXiv160604797Cs. [Google Scholar]
- Meignan, M.; Cottereau, A.-S.; Specht, L.; Mikhaeel, N.G. Total Tumor Burden in Lymphoma–an Evolving Strong Prognostic Parameter. Br. J. Radiol. 2021, 94, 20210448. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.; Bagci, U.; Mansoor, A.; Xu, Z.; Mollura, D.J. A Review on Segmentation of Positron Emission Tomography Images. Comput. Biol. Med. 2014, 50, 76–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- wendyrvllr. Dicom-To-CNN; wendyrvllr: Toulouse, France, 2021. [Google Scholar]

| Original Dataset | Ann Arbor Stages |
| AHL2011 | IIB, III, IV |
| GAINED | I–IV |
| RELEVANCE | I–IV |
| REMARC | II–IV |
| FLIP | I–IV |
| LNH2007-3B | I–IV |
| PVAB | II–IV |
| Lymphoma Subtype | Dice Score per TMTV Segmentation Cutoff | |||
|---|---|---|---|---|
| 41% SUVmax | 2.5 SUV | 4.0 SUV | ||
| HL | Median Mean ± SD | 0.7 0.68 ± 0.16 | 0.68 0.67 ± 0.11 | 0.93 0.90 ± 0.10 |
| FL | Median Mean ± SD | 0.76 0.68 ± 0.22 | 0.68 0.64 ± 0.18 | 0.9 0.86 ± 0.17 |
| DLBCL | Median Mean ± SD | 0.85 0.79 ± 0.20 | 0.75 0.70 ± 0.19 | 0.87 0.82 ± 0.15 |
| All Patients | Median Mean ± SD | 0.77 0.73 ± 0.20 | 0.7 0.68 ± 0.16 | 0.9 0.86 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revailler, W.; Cottereau, A.S.; Rossi, C.; Noyelle, R.; Trouillard, T.; Morschhauser, F.; Casasnovas, O.; Thieblemont, C.; Gouill, S.L.; André, M.; et al. Deep Learning Approach to Automatize TMTV Calculations Regardless of Segmentation Methodology for Major FDG-Avid Lymphomas. Diagnostics 2022, 12, 417. https://doi.org/10.3390/diagnostics12020417
Revailler W, Cottereau AS, Rossi C, Noyelle R, Trouillard T, Morschhauser F, Casasnovas O, Thieblemont C, Gouill SL, André M, et al. Deep Learning Approach to Automatize TMTV Calculations Regardless of Segmentation Methodology for Major FDG-Avid Lymphomas. Diagnostics. 2022; 12(2):417. https://doi.org/10.3390/diagnostics12020417
Chicago/Turabian StyleRevailler, Wendy, Anne Ségolène Cottereau, Cedric Rossi, Rudy Noyelle, Thomas Trouillard, Franck Morschhauser, Olivier Casasnovas, Catherine Thieblemont, Steven Le Gouill, Marc André, and et al. 2022. "Deep Learning Approach to Automatize TMTV Calculations Regardless of Segmentation Methodology for Major FDG-Avid Lymphomas" Diagnostics 12, no. 2: 417. https://doi.org/10.3390/diagnostics12020417
APA StyleRevailler, W., Cottereau, A. S., Rossi, C., Noyelle, R., Trouillard, T., Morschhauser, F., Casasnovas, O., Thieblemont, C., Gouill, S. L., André, M., Ghesquieres, H., Ricci, R., Meignan, M., & Kanoun, S. (2022). Deep Learning Approach to Automatize TMTV Calculations Regardless of Segmentation Methodology for Major FDG-Avid Lymphomas. Diagnostics, 12(2), 417. https://doi.org/10.3390/diagnostics12020417







