Added Value of Computed Tomography Virtual Intravascular Endoscopy in the Evaluation of Coronary Arteries with Stents or Plaques
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Techniques for Coronary Calcium Scoring and CCTA
2.3. Invasive Coronary Angiography
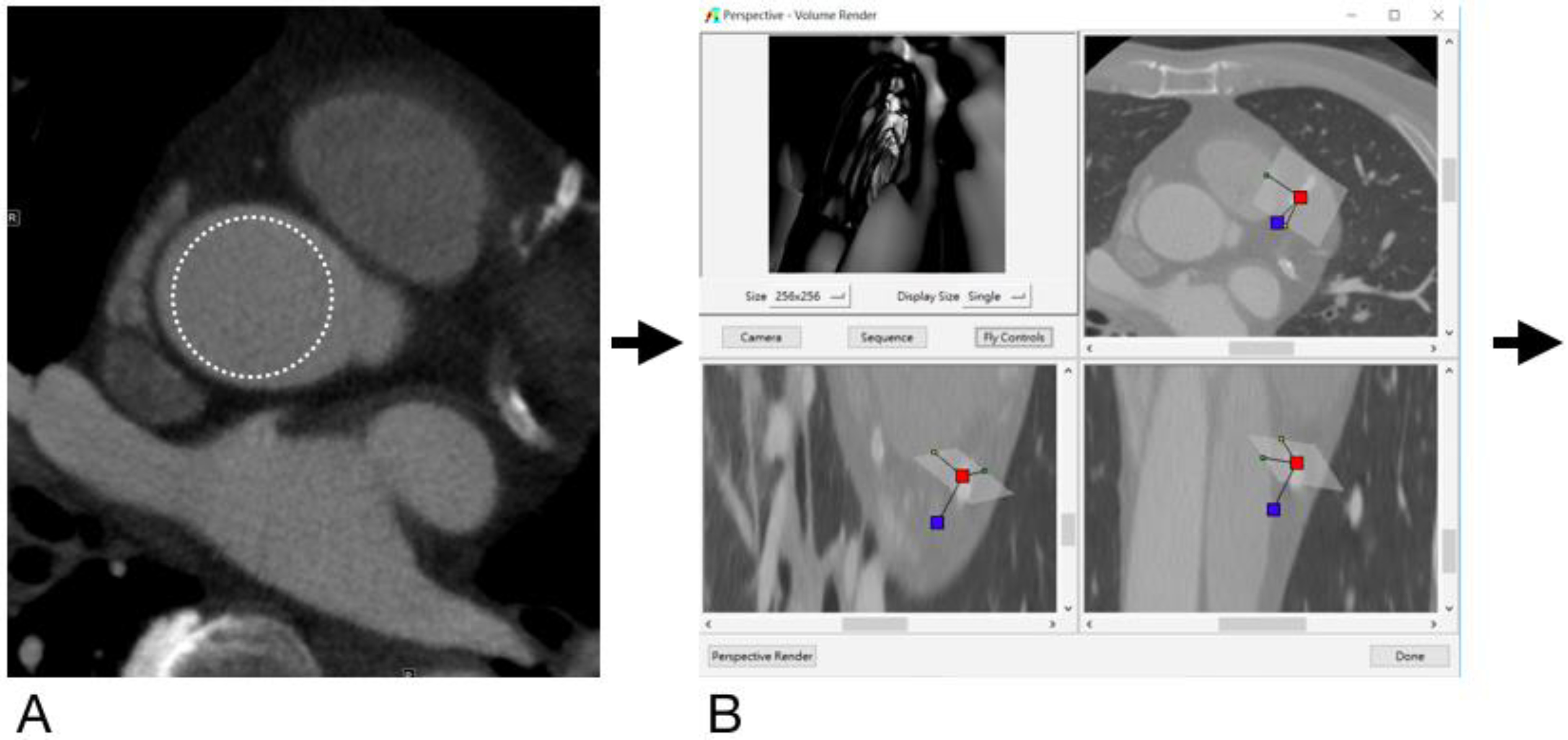
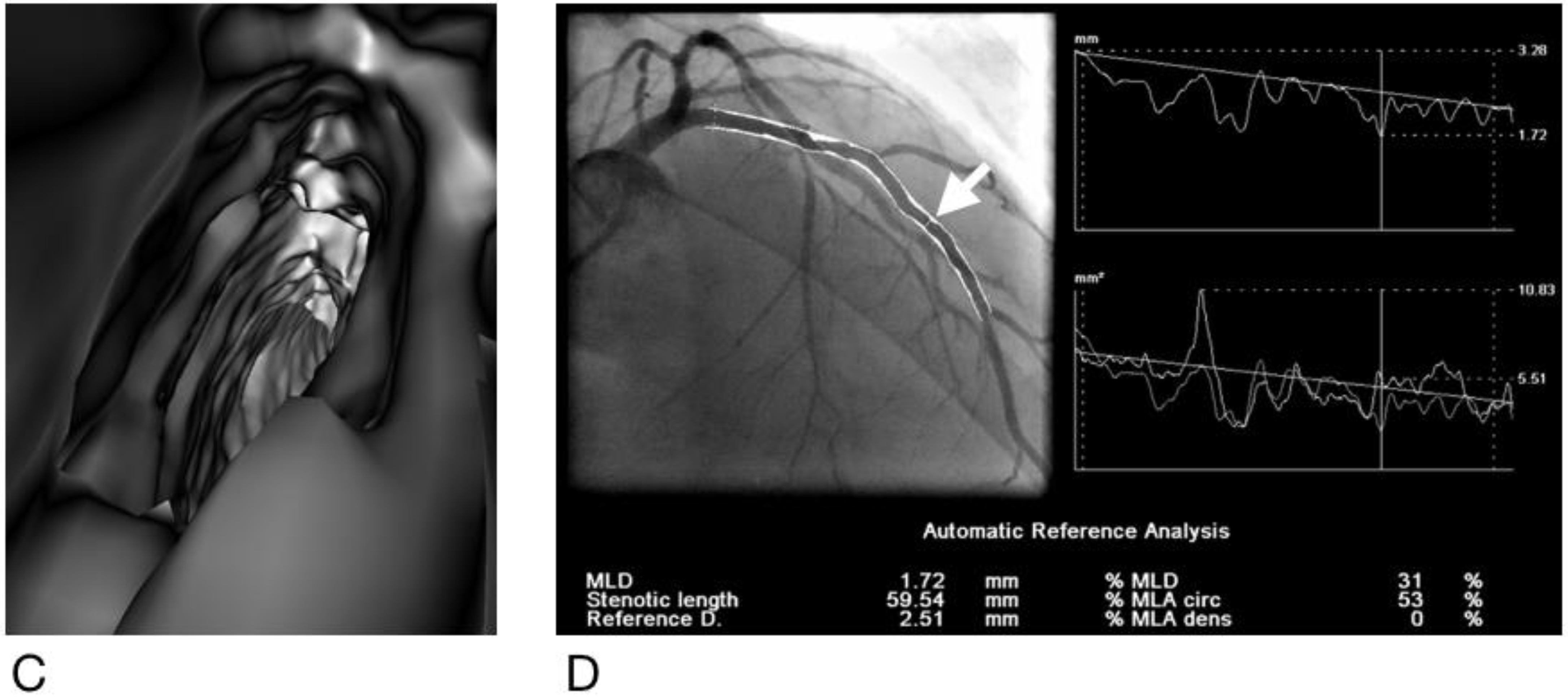
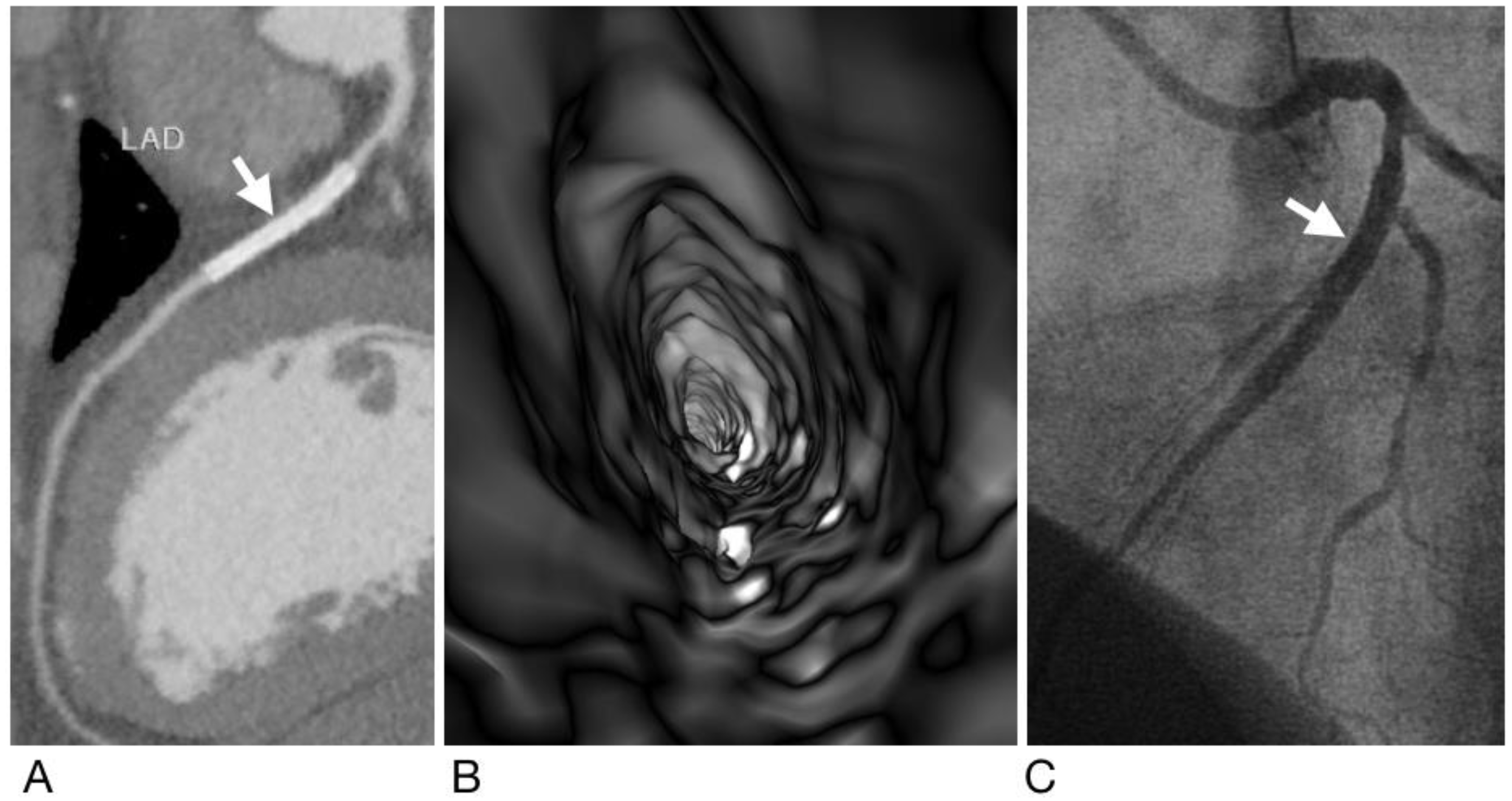
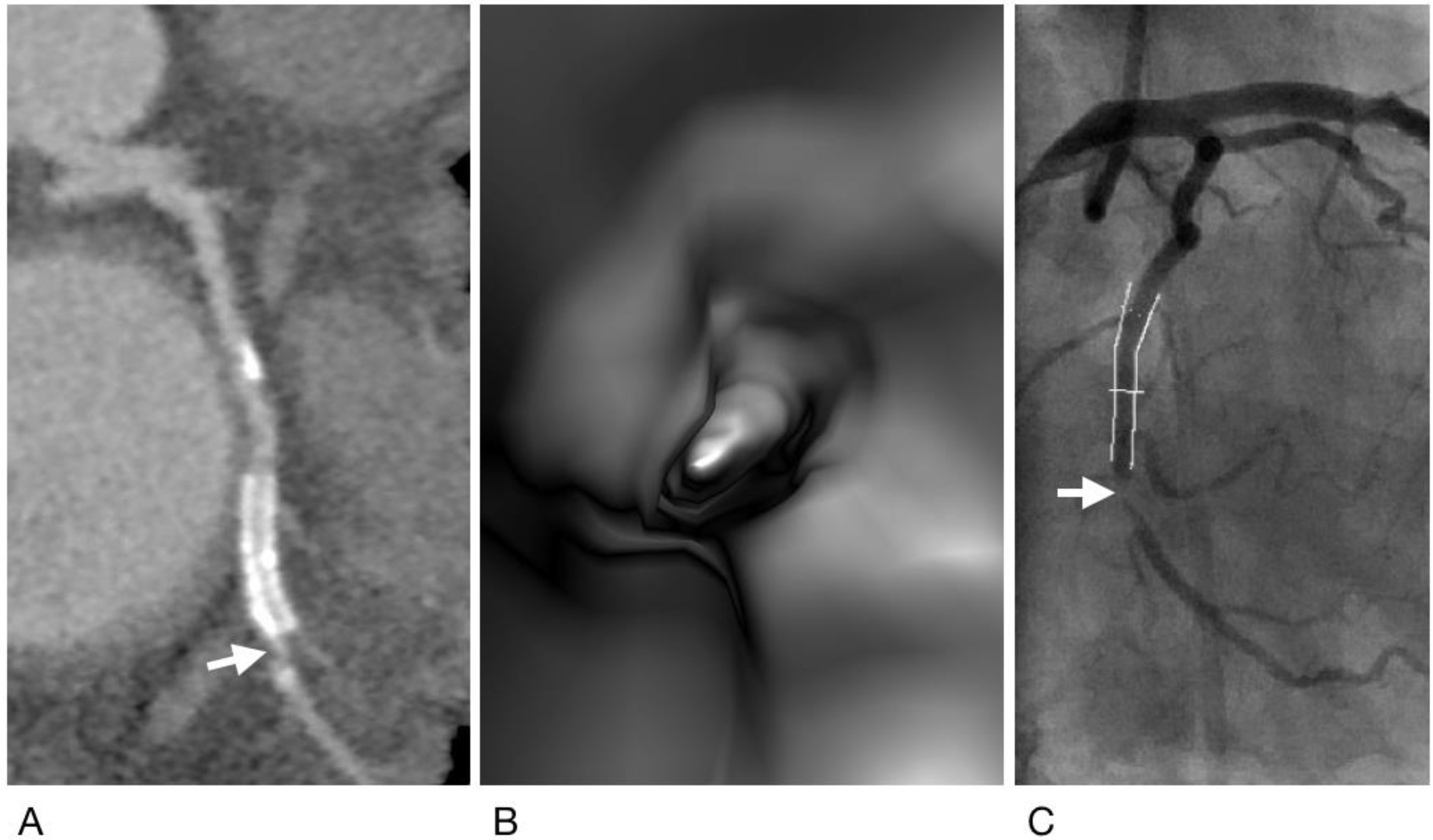
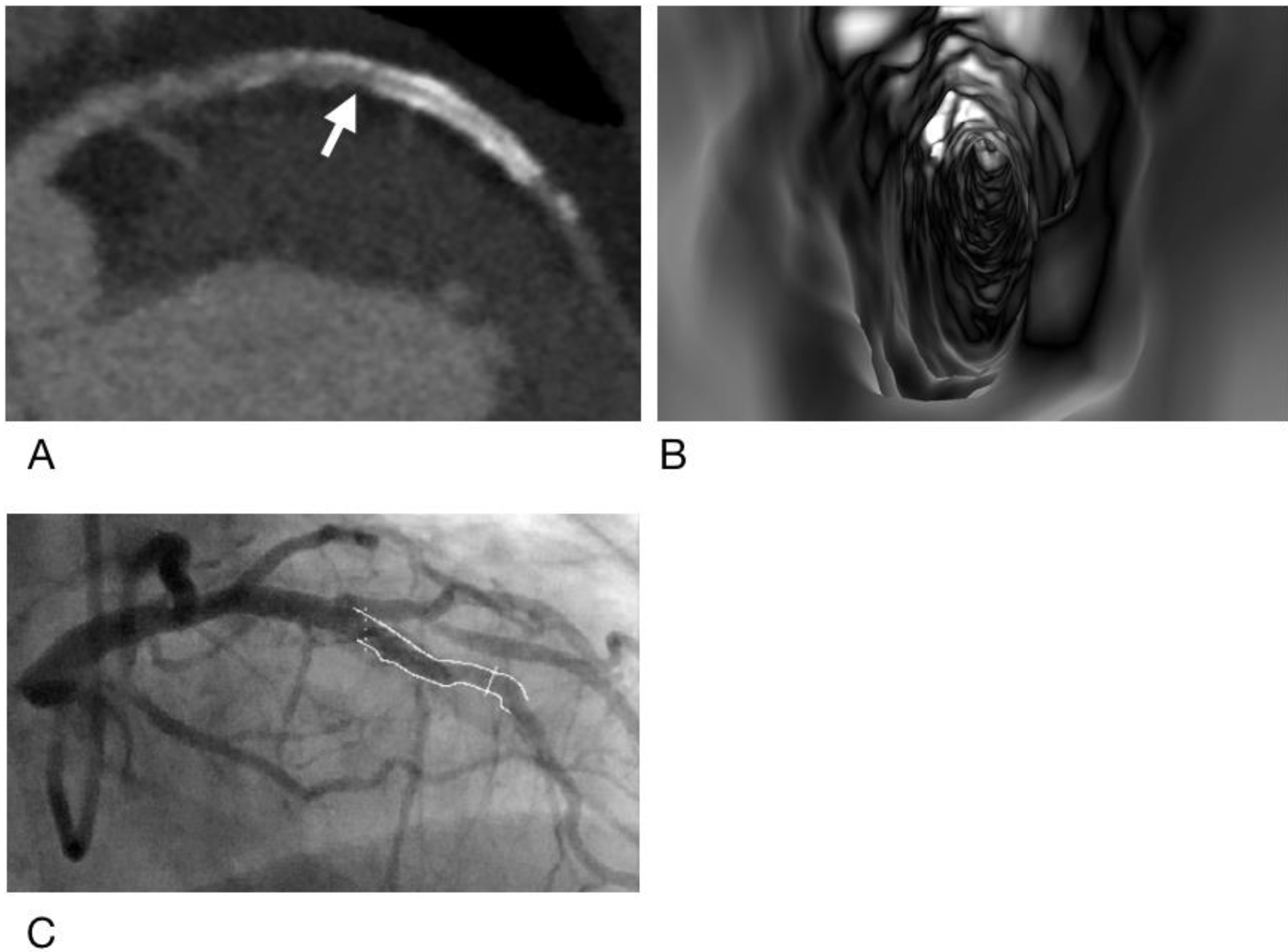
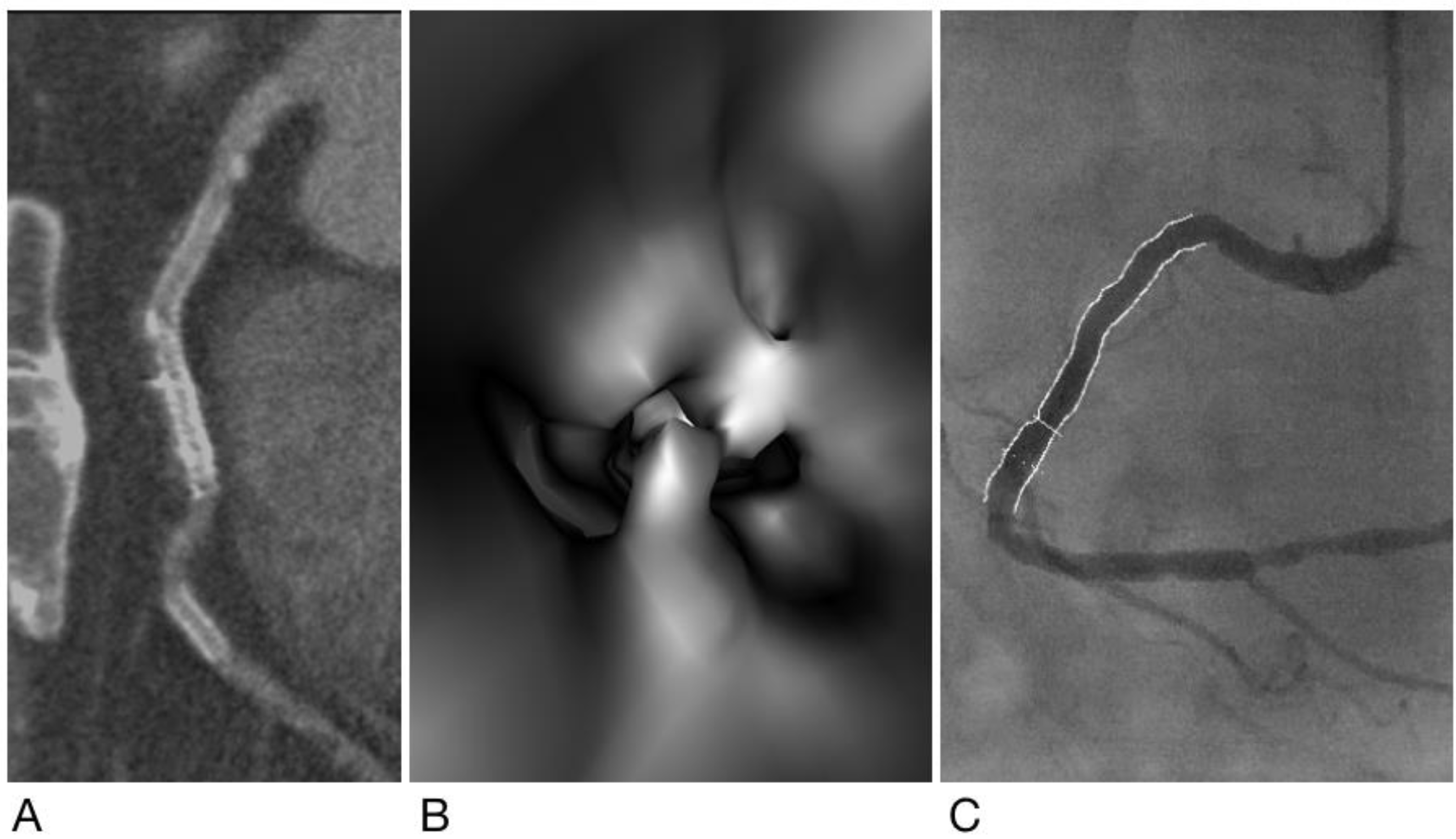
2.4. Generation of Virtual Intravascular Endoscopy Images
2.5. Analysis and Interpretation of CCTA and VIE Images
2.6. Statistical Analysis
3. Results
3.1. Inter- and Intra-Observer Variability
3.2. Diagnostic Performance of CCTA and VIE
3.3. Effect of Stents on the Diagnostic Performance of CCTA and VIE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.; Wan, Y.L.; Hsieh, I.C.; Liu, Y.C.; Wen, M.S. Coronary CT angiography in the diagnosis of coronary artery disease. Curr. Med. Imaging Rev. 2013, 9, 184–193. [Google Scholar] [CrossRef]
- Sun, Z.; Choo, G.H.; Ng, K.H. Coronary CT angiography: Current status and continuing challenges. Br. J. Radiol. 2012, 85, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Jeng, C.M.; Wu, M.T.; Wang, P.C.; Chan, T.; Wan, Y.L. A survey of the current status of coronary CT angiography using 64-slice multidetector CT in Taiwan. J. Formos. Med. Assoc. 2014, 113, 124–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vanhoenacker, P.K.; Heijenbrok-Kal, M.H.; Van Heste, R.; Decramer, I.; Van Hoe, L.R.; Wijns, W.; Hunink, M.M. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: Meta-analysis. Radiology 2007, 244, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lin, C.; Davidson, R.; Dong, C.; Liao, Y. Diagnostic value of 64-slice CT angiography in coronary artery disease: A systematic review. Eur. J. Radiol. 2008, 67, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, J.; Abildstrom, S.Z.; Gotzsche, O.; Christensen, E.; Kober, L.; Torp-Pedersen, C. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: A systematic review and meta-analysis. Eur. Heart J. 2007, 28, 3042–3050. [Google Scholar] [CrossRef]
- Mowatt, G.; Cook, J.A.; Hillis, G.S.; Walker, S.; Fraser, C.; Jia, X.; Waugh, N. 64-slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: Systematic review and meta-analysis. Heart 2008, 94, 1386–1393. [Google Scholar] [CrossRef]
- Cury, R.C.; Abbara, S.; Achenbach, S.; Agatson, A.; Berman, D.S.; Budoff, M.J.; Dill, K.E.; Jacobs, J.E.; Maroules, C.D.; Rubin, G.D.; et al. Coronary artery disease-reporting and data system (CAD-RADS): An expert consensus document of SCCT, ACR and NASCI: Endorsed by the ACC. J. Am. Coll. Cardiol. Imaging 2016, 9, 1099–1113. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, C.C.; Hsieh, I.C.; Liu, C.Y.; Chan, T.; Wen, M.S.; Wan, Y.L. The effect of calcium score on the diagnostic accuracy of coronary computed tomography angiography. Int. J. Cardiovasc. Imaging 2011, 27 (Suppl. 1), 37–42. [Google Scholar] [CrossRef]
- Diederichsen, A.C.; Petersen, H.; Jensen, L.O.; Thayeesn, P.; Gerke, O.; Sandgaard, N.C.F.; Carlsen, P.F.H.; Mickley, H. Diagnostic value of cardiac 64-slice computed tomography: Importance of coronary calcium. Scand. Cardiovasc. J. 2009, 43, 337–344. [Google Scholar] [CrossRef]
- Kuettner, A.; Kopp, A.F.; Schroeder, S.; Rieger, T.; Brunn, J.; Meisner, C.; Heuschmid, M.; Trabold, T.; Burgstahler, C.; Martensen, J.; et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with angiographically proven coronary artery disease. J. Am. Coll. Cardiol. 2004, 43, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Abbara, S.; Blanke, P.; Maroules, C.D.; Cheezum, M.; Choi, A.D.; Han, B.K.; Marwan, M.; Naoum, C.; Norgaard, B.L.; Rubinshtein, R.; et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of cardiovascular computed tomography guidelines committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2016, 10, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, N.; Schuijf, J.D.; de Graaf, F.R.; Parodi, G.; Maffei, E.; Valenti, R.; Palumbo, A.; Wenustink, A.C.; Mollet, N.R.; Accetta, G.; et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography for the detection of in-stent restenosis: A meta-analysis. J. Nucl. Cardiol. 2010, 17, 470–478. [Google Scholar] [CrossRef]
- Kumbhani, D.J.; Ingelmo, C.P.; Schoenhagen, P.; Curtin, R.J.; Flamm, S.D.; Desai, M.Y. Meta-analysis of diagnostic efficacy of 64-slice computed tomography in the evaluation of coronary in-stent restenosis. Am. J. Cardiol. 2009, 103, 1675–1681. [Google Scholar] [CrossRef]
- Sun, Z.; Almutairi, A.M. Diagnostic accuracy of 64 multislice CT angiography in the assessment of coronary in-stent restenosis: A meta-analysis. Eur. J. Radiol. 2010, 73, 266–273. [Google Scholar] [CrossRef]
- de Graaf, F.R.; Schuijf, J.D.; van Velzen, J.E.; Boogers, M.J.; Kroft, L.J.; de Roos, A.; Reiber, J.H.C.; Slieders, A.; Spanó, F.; Jukema, J.W.; et al. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography to noninvasively assess in-stent restenosis. Investig. Radiol. 2010, 45, 331–340. [Google Scholar] [CrossRef]
- Wan, Y.L.; Tsay, P.K.; Chen, C.C.; Juan, Y.H.; Huang, Y.C.; Chan, W.H.; Wen, M.S.; Hsieh, I.C. Coronary in-stent restenosis: Predisposing clinical and stent-related factors, diagnostic performance and analyses of inaccuracies in 320-row computed tomography angiography. Int. J. Cardiovasc. Imaging 2016, 32 (Suppl. 1), 105–115. [Google Scholar] [CrossRef]
- Sun, Z.; Winder, R.J.; Kelly, B.E.; Ellis, P.K.; Kennedy, P.T.; Hirst, D.G. Diagnostic value of CT virtual intravascular endoscopy in aortic stent-grafting. J. Endovasc. Ther. 2004, 11, 13–25. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, H. Effect of suprarenal stent struts on the renal artery with ostial calcification observed on CT virtual intravascular endoscopy. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 534–542. [Google Scholar] [CrossRef]
- Sun, Z.; Ferris, C. Optimal scanning protocol of multislice CT virtual intravascular endoscopy in pre-aortic stent grafting: In vitro phantom study. Eur. J. Radiol. 2006, 58, 310–316. [Google Scholar] [CrossRef][Green Version]
- Sun, Z.; Mwipatayi, B.P.; Allen, Y.B.; Hartley, D.E.; Lawrence-Brown, M.M. Computed tomography virtual intravascular endoscopy in the evaluation of fenestrated stent graft repair of abdominal aortic aneurysms. ANZ J. Surg. 2009, 79, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Louis, N.; Bruguiere, E.; Kobeiter, H.; Desgranges, P.; Allaire, E.; Kirsch, M.; Becquemin, J.P. Virtual angioscopy and 3D navigation: A new technique for analysis of the aortic arch after vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 340–347. [Google Scholar] [CrossRef][Green Version]
- Qi, Y.; Ma, X.; Li, G.; Ma, X.; Wang, Q.; Yu, D. Three-dimensional visualization and imaging of the entry tear and intimal flap of aortic dissection using CT virtual intravascular endoscopy. PLoS ONE 2016, 11, e0164750. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Dimpudus, F.J.; Nugroho, J.; Adipranoto, J.D. CT virtual intravascular endoscopy assessment of coronary artery plaques: A preliminary study. Eur. J. Radiol. 2010, 75, e112–e119. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, Z. Virtual intravascular endoscopy visualization of calcified coronary plaques: A novel approach of identifying plaque features for more accurate assessment of coronary lumen stenosis. Medicine 2015, 94, e805. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xu, L. CT virtual intravascular endoscopy in the visualization of coronary plaques: A pictorial essay. Curr. Med. Imaging Rev. 2017, 13, 154–161. [Google Scholar] [CrossRef][Green Version]
- Sun, Z. Coronary CT angiography in coronary artery disease: Correlation between virtual intravascular endoscopic appearances and left bifurcation angulation and coronary plaques. Biomed. Res. Int. 2013, 2013, 732059. [Google Scholar] [CrossRef]
- Juan, Y.H.; Huang, Y.C.; Sun, Z.; Hsieh, I.C.; Chan, W.H.; Chen, C.C.; Hung, K.C.; Wen, M.S.; Wan, Y.L. The evolution and investigation of native coronary arteries in patients after coronary stent implantation: A study by 320-detector CT angiography. Int. J. Cardiovasc. Imaging 2014, 30 (Suppl. 1), 13–24. [Google Scholar] [CrossRef]
- Dewey, M.; Zimmermann, E.; Deissenrieder, F.; Laule, M.; Dübel, H.P.; Schlattmann, P.; Knebel, F.; Rutsch, W.; Bernd, H. Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: Comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation 2009, 120, 867–875. [Google Scholar] [CrossRef]
- Alfonso, F.; Zueco, J.; Cequier, A.; Mantilla, R.; Bethencourt, A.; López-Minguez, J.R.; Angel, J.; Augé, J.M.; Gómez-Recio, M.; Morís, C.; et al. A randomized comparison of repeat stenting with balloon angioplasty in patients with in-stent restenosis. J. Am. Coll. Cardiol. 2003, 42, 796–805. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, C. Diagnostic value of 320-slice coronary CT angiography in coronary artery disease: A systematic review and meta-analysis. Curr. Med. Imaging Rev. 2015, 10, 272–280. [Google Scholar] [CrossRef]
- Li, Y.; Yu, M.; Li, W.; Lu, Z.; Wei, M.; Zhang, J. Third generation dual-source CT enables accurate diagnosis of coronary restenosis in all size stents with low radiation dose and preserved image quality. Eur. Radiol. 2018, 28, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Bhatt, D. Coronary intravascular ultrasound. Circulation 2013, 127, e868–e874. [Google Scholar] [CrossRef]
- Jang, I.-K.; Bouma, B.E.; Kang, D.H.; Park, S.J.; Park, S.W.; Seung, K.B.; Choi, K.B.; Shishkov, M.; Schlendorf, K.; Pomerantsev, E.; et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: Comparison with intravascular ultrasound. J. Am. Coll. Cardiol. 2002, 39, 604–609. [Google Scholar] [CrossRef]
| CCTA | VIE | p Value | |
|---|---|---|---|
| Sensitivity | 77/82 = 93.9% (86.3–98.0%) | 74/82 = 90.2% (81.7–95.7%) | 0.563 |
| Specificity | 789/820 = 96.2% (94.7–97.4%) | 805/820 = 98.2% (97.0–98.9%) | 0.025 |
| Accuracy | 866/902 = 96.0% (94.5–97.2%) | 879/902 = 97.7% (96.2–98.4%) | 0.046 |
| Positive predictive value | 77/110 = 70.0% (60.5–78.4%) | 74/89 = 83.1% (73.7–90.4%) | 0.047 |
| Negative predictive value | 787/792 = 99.4% (98.5–99.8%) | 805/813 = 99.0% (98.1–99.6%) | 0.610 |
| kappa value | 0.7790.713–0.847 | 0.8510.791–0.911 | 0.112 |
| CCTA | VIE | p Value | |
|---|---|---|---|
| Sensitivity | 22/23 = 95.7% (78.1–99.9%) | 20/23 = 87.0% (66.4–97.2%) | 0.608 |
| Specificity | 159/170 = 93.5% (88.7–96.7%) | 166/170 = 97.6% (94.1–99.4%) | 0.113 |
| Accuracy | 181/193 = 93.8% (89.4–96.8%) | 186/193 = 96.9% (92.7–98.5%) | 0.347 |
| Positive predictive value | 22/33 = 66.7% (48.2–82.0%) | 20/24 = 83.3% (62.6–95.3%) | 0.269 |
| Negative predictive value | 159/160 = 99.4% (96.6–99.9%) | 166/169 = 98.2% (94.9–99.4%) | 0.623 |
| kappa value | 0.7510.622–0.881 | 0.8300.709–0.941 | 0.391 |
| CCTA | VIE | p Value | |
|---|---|---|---|
| Sensitivity | 3/4 = 75.0% (19.4–99.4%) | 2/4 = 50.0% (6.8–93.2%) | 0.999 |
| Specificity | 98/111 = 88.3% (80.8–93.6%) | 106/111 = 95.5% (89.8–98.5%) | 0.085 |
| Accuracy | 101/115 = 87.8% (80.4–93.2%) | 108/115 = 93.9% (87.9–97.5%) | 0.170 |
| Positive predictive value | 3/16 = 18.8% (4.1–45.7%) | 2/7 = 28.6% (3.7–71.0%) | 0.621 |
| Negative predictive value | 98/99 = 99.0% (94.5–99.9%) | 106/108 = 98.1% (93.5–99.8%) | 0.999 |
| kappa value | 0.2590.109–0.498 | 0.3340.094–0.573 | 0.743 |
| CCTA | VIE | p Value | |
|---|---|---|---|
| Sensitivity | 31/34 = 91.2% (76.3–98.1%) | 31/34 = 91.2% (76.3–98.1%) | 0.999 |
| Specificity | 45/51 = 88.2% (76.1–95.6%) | 47/51 = 92.2% (81.1–97.8%) | 0.739 |
| Accuracy | 76/85 = 89.4% (80.9–95.0%) | 78/85 = 91.8% (83.8–96.6%) | 0.793 |
| Positive predictive value | 31/37 = 83.8% (68.0–93.8%) | 31/35 = 88.6% (73.3–96.8%) | 0.806 |
| Negative predictive value | 45/48 = 93.8% (82.8–98.7%) | 47/50 = 94.0% (83.5–98.8%) | 0.999 |
| kappa value | 0.7830.662–0.903 | 0.8290.708–0.949 | 0.617 |
| CCTA | VIE | p Value | |
|---|---|---|---|
| Sensitivity | 21/21 = 100.0% (83.9–100.0%) | 21/21 = 100.0% (83.9–100.0%) | 0.999 |
| Specificity | 7/10 = 70.0% (34.8–93.3%) | 8/10 = 80.0% (44.4–97.5%) | 0.999 |
| Accuracy | 28/31 = 94.3% (74.3–98.0%) | 29/31 = 93.6% (78.6–99.2%) | 0.999 |
| Positive predictive value | 21/24 = 87.5% (67.6–97.3%) | 21/23 = 91.3% (72.0–98.9%) | 0.999 |
| Negative predictive value | 7/7 = 100.0% (59.0–100.0%) | 8/8 = 100.0% (63.1–100.0%) | 0.999 |
| kappa value | 0.7600.641–0.880 | 0.8440.710–0.966 | 0.612 |
| CCTA | VIE | p Value | |
|---|---|---|---|
| Sensitivity | 77/82 = 93.9% (86.3–98.0%) | 74/82 = 90.2% (81.7–95.7%) | 0.563 |
| Specificity | 309/342 = 90.4% (86.7–93.3%) | 327/342 = 95.6% (93.9–97.5%) | 0.011 |
| Accuracy | 386/424 = 91.0% (87.9–93.6%) | 401/424 = 94.6% (92.0–96.5%) | 0.063 |
| Positive predictive value | 77/110 = 70.0% (60.5–78.4%) | 74/89 = 83.1% (73.7–90.3%) | 0.047 |
| Negative predictive value | 309/314 = 98.4% (96.3–99.5%) | 327/335 = 97.6% (95.4–99.0%) | 0.658 |
| kappa value | 0.7460.675–0.826 | 0.8320.764–0.901 | 0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.W.; Tsay, P.-K.; Sun, Z.; Peng, S.-J.; Lee, C.-Y.; Hsu, M.-Y.; Ko, Y.-S.; Hsieh, I.-C.; Wen, M.-S.; Wan, Y.-L. Added Value of Computed Tomography Virtual Intravascular Endoscopy in the Evaluation of Coronary Arteries with Stents or Plaques. Diagnostics 2022, 12, 390. https://doi.org/10.3390/diagnostics12020390
Wu PW, Tsay P-K, Sun Z, Peng S-J, Lee C-Y, Hsu M-Y, Ko Y-S, Hsieh I-C, Wen M-S, Wan Y-L. Added Value of Computed Tomography Virtual Intravascular Endoscopy in the Evaluation of Coronary Arteries with Stents or Plaques. Diagnostics. 2022; 12(2):390. https://doi.org/10.3390/diagnostics12020390
Chicago/Turabian StyleWu, Patricia Wanping, Pei-Kwei Tsay, Zhonghua Sun, Syu-Jyun Peng, Chia-Yen Lee, Ming-Yi Hsu, Yu-Shien Ko, I-Chang Hsieh, Ming-Shien Wen, and Yung-Liang Wan. 2022. "Added Value of Computed Tomography Virtual Intravascular Endoscopy in the Evaluation of Coronary Arteries with Stents or Plaques" Diagnostics 12, no. 2: 390. https://doi.org/10.3390/diagnostics12020390
APA StyleWu, P. W., Tsay, P.-K., Sun, Z., Peng, S.-J., Lee, C.-Y., Hsu, M.-Y., Ko, Y.-S., Hsieh, I.-C., Wen, M.-S., & Wan, Y.-L. (2022). Added Value of Computed Tomography Virtual Intravascular Endoscopy in the Evaluation of Coronary Arteries with Stents or Plaques. Diagnostics, 12(2), 390. https://doi.org/10.3390/diagnostics12020390







