The Interplay between Myocardial Fibrosis, Strain Imaging and Collagen Biomarkers in Adults with Repaired Tetralogy of Fallot
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Blood Samples-Biomarkers
2.3. CMR Imaging Protocol
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
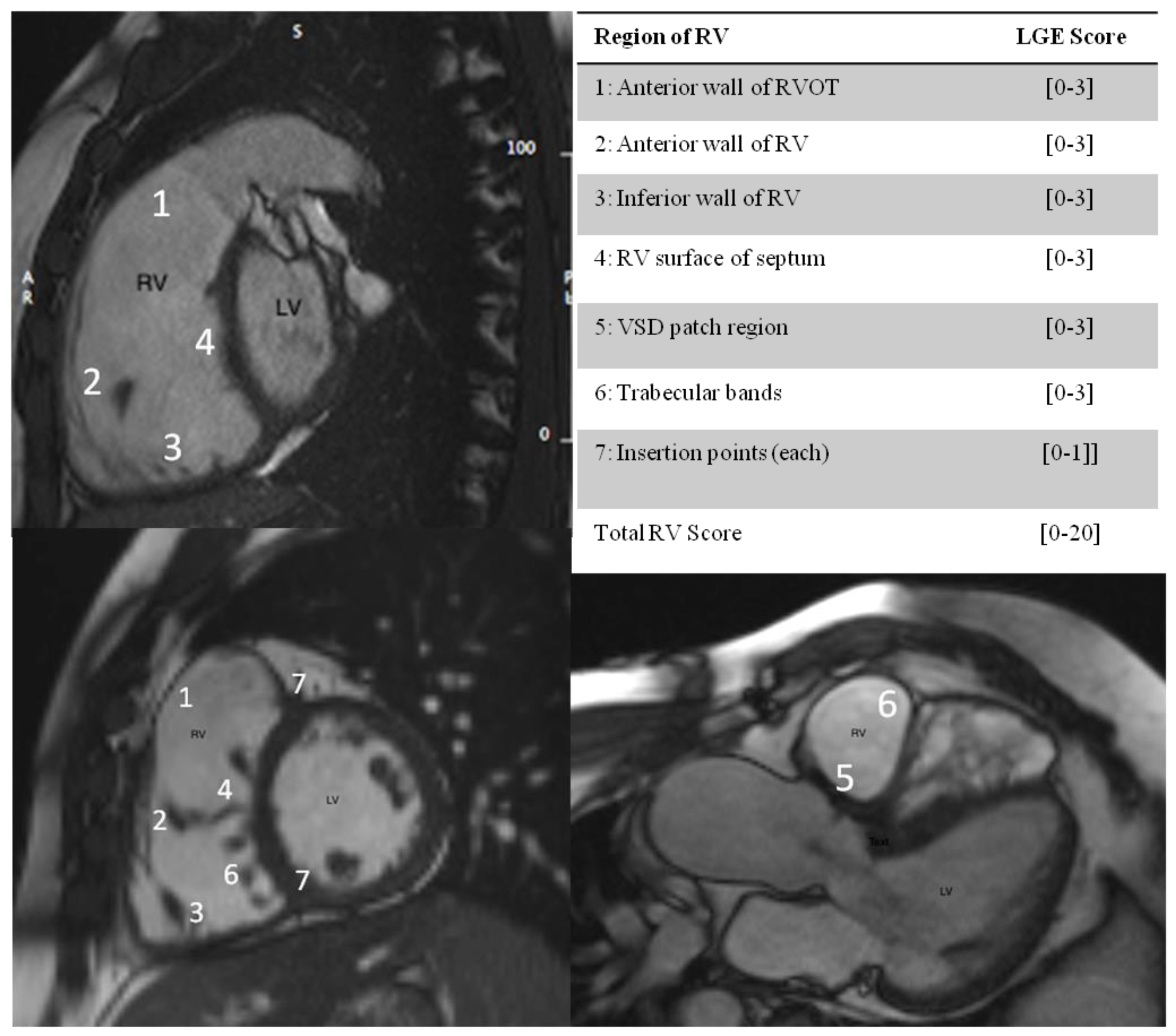
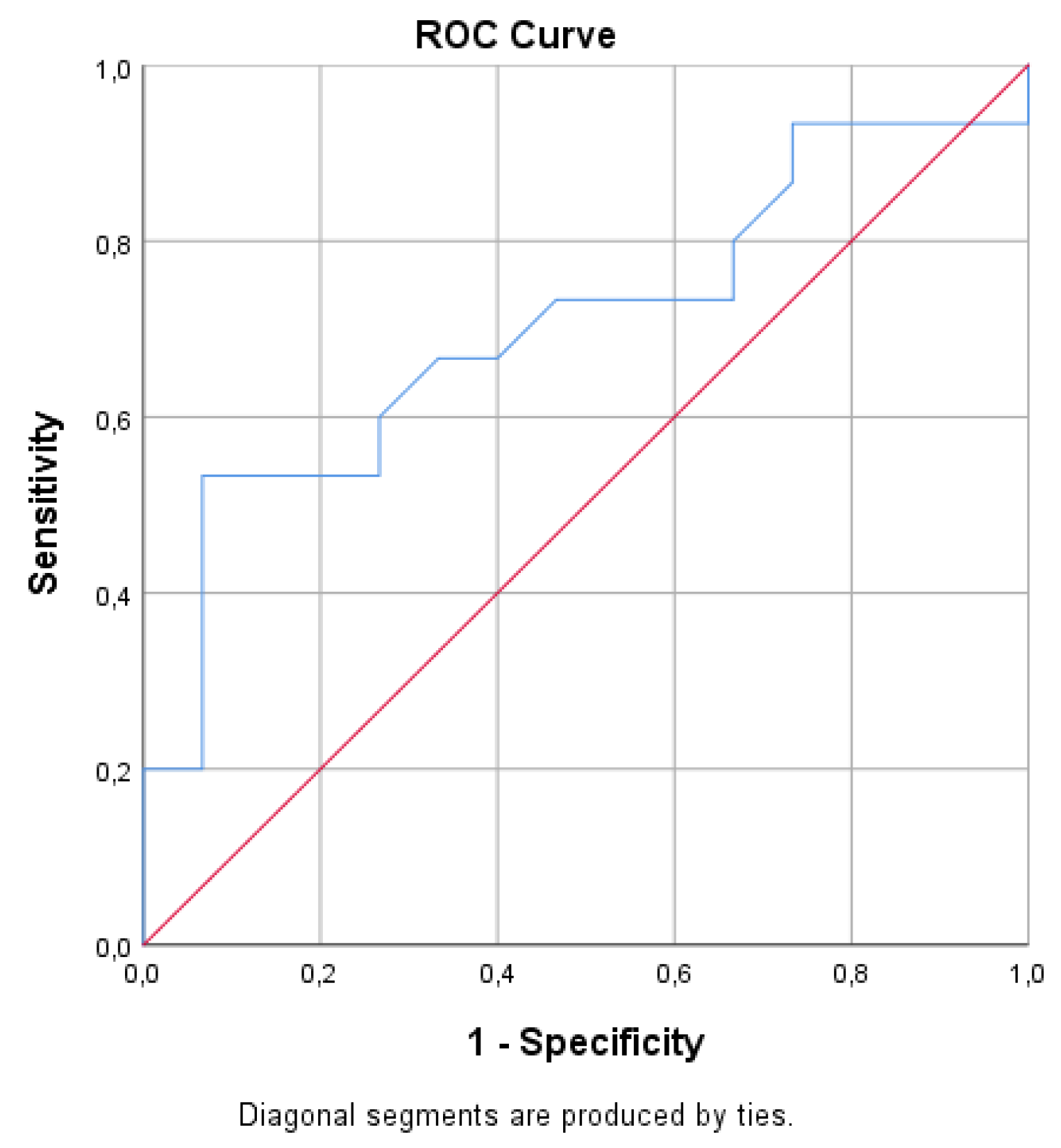
3.2. RV Fibrosis Scoring and Clinical Correlates
3.3. RV Fibrosis Scoring and Biomarker Levels in Adults with rToF
3.4. RV Fibrosis and Cardiac Magnetic Resonance Data with Feature Tracking (CMR-FT) Analysis
4. Discussion
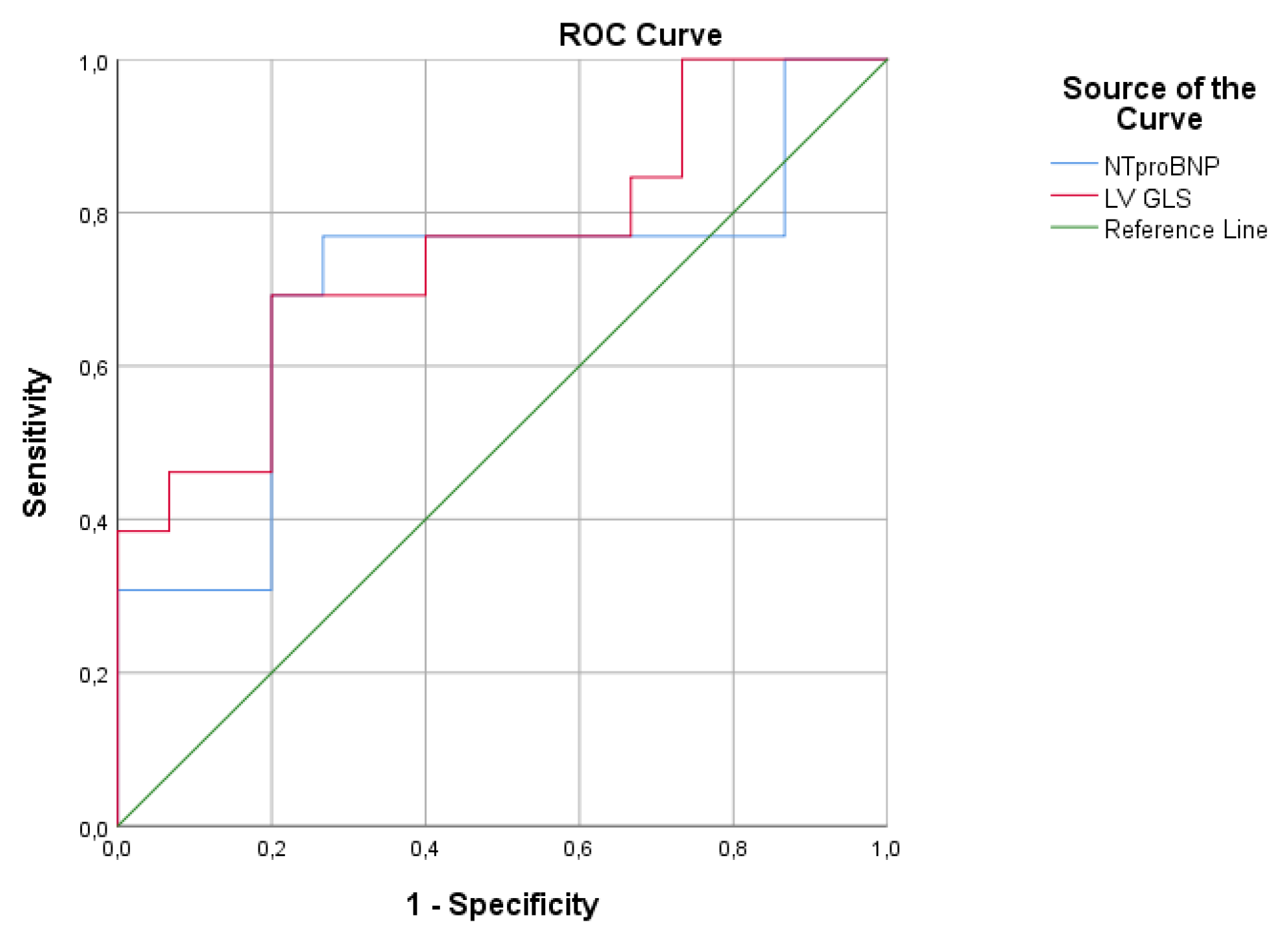
4.1. RV Fibrosis, Biomarker Levels and Prognostic Associations
4.2. Role of Feature Tracking CMR (CMR-FT) in Adults with rToF
4.3. CMR-FT Analysis and Biomarkers Levels in Adults with rToF
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marelli, A.J.; Mackie, A.S.; Ionescu-Ittu, R.; Rahme, E.; Pilote, L. Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation 2007, 115, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.S.; McDonald, D.M.; Singh, H.S.; Ginns, J.N. Heart failure in adult congenital heart disease: Tetralogy of Fallot. Heart Fail. Rev. 2020, 25, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Kantor, P.F.; Redington, A.N. Pathophysiology and Management of Heart Failure in Repaired Congenital Heart Disease. Heart Fail. Clin. 2010, 6, 497–506. [Google Scholar] [CrossRef]
- Bolger, A.P.; Sharma, R.; Li, W.; Leenarts, M.; Kalra, P.R.; Kemp, M.; Coats, A.J.; Anker, S.D.; Gatzoulis, M.A. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 2002, 106, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lok, D.J.; Van Der Meer, P.; de la Porte, P.W.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin. Res. Cardiol. 2010, 99, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samyn, M.M.; Powell, A.J.; Garg, R.; Sena, L.; Geva, T. Range of ventricular dimensions and function by steady-state free precession cine MRI in repaired tetralogy of fallot: Right ventricular outflow tract patch vs. conduit repair. J. Magn. Reson. Imaging 2007, 26, 934–940. [Google Scholar] [CrossRef]
- Stefanescu Schmidt, A.C.; DeFaria Yeh, D.; Tabtabai, S.; Kennedy, K.F.; Yeh, R.W.; Bhatt, A.B. National Trends in Hospitalizations of Adults with Tetralogy of Fallot. Am. J. Cardiol. 2016, 118, 906–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntiloudi, D.; Dimopoulos, K.; Tzifa, A.; Karvounis, H.; Giannakoulas, G. Hospitalizations in adult patients with congenital heart disease: An emerging challenge. Heart Fail. Rev. 2021, 26, 347–353. [Google Scholar] [CrossRef]
- Davlouros, P.A.; Kilner, P.J.; Hornung, T.S.; Li, W.; Francis, J.M.; Moon, J.C.; Smith, G.C.; Tat, T.; Pennell, D.J.; Gatzoulis, M.A. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: Detrimental role of right ventricular outflow aneurysms or akinesia and adverse right-to-left ventricular interaction. J. Am. Coll. Cardiol. 2002, 40, 2044–2052. [Google Scholar] [CrossRef] [Green Version]
- Mooij, C.F.; de Wit, C.J.; Graham, D.A.; Powell, A.J.; Geva, T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J. Magn. Reson. Imaging 2008, 28, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geva, T. Repaired tetralogy of Fallot: The roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J. Cardiovasc. Magn. Reson. 2011, 13, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, M.; Van, T.A.; Krug, R.; Hetts, S.W.; Wilson, M.W. Cardiac MR imaging: Current status and future direction. Cardiovasc. Diagn. Ther. 2015, 5, 290–310. [Google Scholar]
- Keller, E.J.; Fang, S.; Lin, K.; Freed, B.H.; Smith, P.M.; Spottiswoode, B.S.; Davids, R.; Carr, M.; Jolly, M.; Markl, M.; et al. The consistency of myocardial strain derived from heart deformation analysis. Int. J. Cardiovasc. Imaging 2017, 33, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Babu-Narayan, S.V.; Kilner, P.J.; Li, W.; Moon, J.C.; Goktekin, O.; Davlouros, P.A.; Khan, M.; Ho, S.Y.; Pennell, D.J.; Gatzoulis, M.A. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of Fallot and its relationship to adverse markers of clinical outcome. Circulation 2006, 113, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, A.M.; Gauvreau, K.; Assenza, G.E.; Babu-Narayan, S.V.; Schreier, J.; Gatzoulis, M.A.; Groenink, M.; Inuzuka, R.; Kilner, P.J.; Koyak, Z.; et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart 2014, 100, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, M.A.; Till, J.A.; Somerville, J.; Redington, A.N. Mechanoelectrical interaction in tetralogy of Fallot: QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation 1995, 92, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, M.A.; Balaji, S.; Webber, S.A.; Siu, S.C.; Hokanson, J.S.; Poile, C.; Rosenthal, M.; Nakazawa, M.; Moller, J.H.; Gillette, P.C.; et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: A multicentre study. Lancet 2000, 356, 975–981. [Google Scholar] [CrossRef]
- Wald, R.M.; Haber, I.; Wald, R.; Valente, A.M.; Powell, A.J.; Geva, T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of fallot. Circulation 2009, 119, 1370–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.C.; Piehler, K.; Meier, C.G.; Testa, S.M.; Klock, A.M.; Aneizi, A.A.; Shakesprere, J.; Kellman, P.; Shroff, S.G.; Schwartzman, D.S.; et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 2012, 126, 1206–1216. [Google Scholar] [CrossRef]
- Schelbert, E.B.; Piehler, K.M.; Zareba, K.M.; Moon, J.C.; Ugander, M.; Messroghli, D.R.; Valeti, U.S.; Chang, C.C.; Shroff, S.G.; Diez, J.; et al. Myocardial Fibrosis Quantified by Extracellular Volume Is Associated with Subsequent Hospitalization for Heart Failure, Death, or Both Across the Spectrum of Ejection Fraction and Heart Failure Stage. J. Am. Heart Assoc. 2015, 4, e002613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodhi, S.S.; Zhang, T.J.; McDonald, R.; al Rashida, V.; Kondapalli, N.; Barger, P.; Ludbrook, P.; Cedars, A.M. Effects of eplerenone on markers of myocardial fibrosis, 6-minute walk distance, and quality of life in adults with tetralogy of Fallot and complete transposition of the great arteries. Bayl. Univ. Med. Cent. Proc. 2018, 31, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Garcia-Ropero, A.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Vargas-Delgado, A.P.; Flores-Umanzor, E.J.; Sanz, J.; et al. Empagliflozin Ameliorates Diastolic Dysfunction and Left Ventricular Fibrosis/Stiffness in Nondiabetic Heart Failure: A Multimodality Study. JACC Cardiovasc. Imaging 2021, 14, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Requena-Ibáñez, J.A.; Santos-Gallego, C.G.; Rodriguez-Cordero, A.; Vargas-Delgado, A.P.; Mancini, D.; Sartori, S.; Atallah-Lajam, F.; Giannarelli, C.; Macaluso, F.; Lala, A. Mechanistic Insights of Empagliflozin in Nondiabetic Patients with HFrEF: From the EMPA-TROPISM Study. JACC Heart Fail. 2021, 9, 578–589. [Google Scholar] [CrossRef]
- Dardeer, A.M.; Hudsmith, L.; Wesolowski, R.; Clift, P.; Steeds, R.P. The potential role of feature tracking in adult congenital heart disease: Advantages and disadvantages in measuring myocardial deformation by cardiovascular magnetic resonance. J. Congenit. Cardiol. 2018, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Moon, T.J.; Choueiter, N.; Geva, T.; Valente, A.M.; Gauvreau, K.; Harrild, D.M. Relation of biventricular strain and dyssynchrony in repaired tetralogy of fallot measured by cardiac magnetic resonance to death and sustained ventricular tachycardia. Am. J. Cardiol. 2015, 115, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Morais, L.; Pereira-da-Silva, T.; Branco, L.; Timóteo, A.T.; Agapito, A.; de Sousa, L.; Oliveira, J.A.; Thomas, B.; Jalles-Tavares, N.; Soares, R.; et al. The value of right ventricular longitudinal strain in the evaluation of adult patients with repaired tetralogy of Fallot: A new tool for a contemporary challenge. Cardiol. Young 2017, 27, 498–506. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Lok, D.J.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011, 43, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Rector, T.S.; Kuskowski, M.; Adourian, A.; Muntendam, P.; Cohn, J.N. Baseline and serial measurements of galectin-3 in patients with heart failure: Relationship to prognosis and effect of treatment with Valsartan in the Val-HeFT. Eur. J. Heart Fail. 2013, 15, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; van der Velde, A.R.; Muller Kobold, A.C.; Dijck-Brouwer, J.; Wu, A.H.; Jaffe, A.; de Boer, R.A. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur. J. Heart Fail. 2017, 19, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Opotowsky, A.R.; Baraona, F.; Owumi, J.; Loukas, B.; Singh, M.N.; Valente, A.M.; Wu, F.; Cheng, S.; Veldtman, G.; Rimm, E.B.; et al. Galectin-3 Is Elevated and Associated with Adverse Outcomes in Patients with Single-Ventricle Fontan Circulation. J. Am. Heart Assoc. 2016, 5, e002706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geenen, L.W.; van Grootel, R.W.J.; Akman, K.; Baggen, V.J.M.; Menting, M.E.; Eindhoven, J.A.; Cuypers, J.A.A.E.; Boersma, E.; van den Bosch, A.E.; Roos-Hesselink, J.W. Exploring the Prognostic Value of Novel Markers in Adults with a Systemic Right Ventricle. J. Am. Heart Assoc. 2019, 8, e013745. [Google Scholar] [CrossRef] [PubMed]
- Frogoudaki, A.A.; Pantelakis, I.; Bistola, V.; Kroupis, C.; Birba, D.; Ikonomidis, I.; Alexopoulos, D.; Filippatos, G.; Parissis, J. Global Longitudinal Strain of the Systemic Ventricle Is Correlated with Plasma Galectin-3 and Predicts Major Cardiovascular Events in Adult Patients with Congenital Heart Disease. Medicina 2020, 56, 305. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.; Strengers, J.L.; Wielopolski, P.A.; Hop, W.C.; Meijboom, F.J.; de Rijke, Y.B.; Boomsma, F.; Bogers, A.J.; Pattynama, P.M.; Helbing, W.A. Assessment of biventricular functional reserve and NT-proBNP levels in patients with RV volume overload after repair of tetralogy of Fallot at young age. Int. J. Cardiol. 2009, 133, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Hirono, K.; Sekine, M.; Shiba, N.; Hayashi, S.; Nakaoka, H.; Ibuki, K.; Saito, K.; Watanabe, K.; Ozawa, S.; Higuma, T.; et al. N-terminal pro-brain natriuretic peptide as a predictor of reoperation in children with surgically corrected tetralogy of fallot. Circ. J. 2014, 78, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Emdin, M.; Passino, C.; Prontera, C.; Fontana, M.; Poletti, R.; Gabutti, A.; Mammini, C.; Giannoni, A.; Zyw, L.; Zucchelli, G.; et al. Comparison of brain natriuretic peptide (BNP) and amino-terminal ProBNP for early diagnosis of heart failure. Clin. Chem. 2007, 53, 1289–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, E.W.; Lam, W.W.; Chiu, C.S.; Chau, A.K.; Cheung, S.C.; Cheung, Y.F. Plasma brain natriuretic peptide levels, right ventricular volume overload and exercise capacity in adolescents after surgical repair of tetralogy of Fallot. Int. J. Cardiol. 2007, 121, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Park, J.J.; Choi, D.J.; Yoon, C.H.; Oh, I.Y.; Kang, S.M.; Yoo, B.S.; Jeon, E.S.; Kim, J.J.; Cho, M.C.; et al. Prognostic value of NT-proBNP in heart failure with preserved versus reduced EF. Heart 2015, 101, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Paolino, A.; Hussain, T.; Pavon, A.; Velasco, M.N.; Uribe, S.; Ordoñez, A.; Valverde, I. NT-proBNP as Marker of Ventricular Dilatation and Pulmonary Regurgitation After Surgical Correction of Tetralogy of Fallot: A MRI Validation Study. Pediatr. Cardiol. 2017, 38, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.T.; Hørslev-Petersen, K.; Toft, P.; Bentsen, K.D.; Grande, P.; Simonsen, E.E.; Lorenzen, I. Serum aminoterminal type III procollagen peptide reflects repair after acute myocardial infarction. Circulation 1990, 81, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.T.; Chan, K.W.; Wong, S.J.; Chow, P.C.; Cheung, Y.F. Circulating levels of biomarkers of collagen synthesis and ventricular function and dyssynchrony in adolescents and young adults after repair of tetralogy of Fallot. Am. Heart J. 2011, 162, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Muser, D.; Castro, S.A.; Santangeli, P.; Nucifora, G. Clinical applications of feature-tracking cardiac magnetic resonance imaging. World J. Cardiol. 2018, 10, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Kavurt, A.V.; Paç, F.A.; Koca, S.; Mutlu Mıhçıoğlu, A.; Yiğit, H. The evaluation of right ventricular systolic function in patients with repaired Tetralogy of Fallot by conventional echocardiographic methods and speckle tracking echocardiography: Compared with the gold standard cardiac mangenetic resonance. Echocardiography 2019, 36, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; Triedman, J.K.; Geva, T.; Harrild, D.M. Relation of Left ventricular dyssynchrony measured by cardiac magnetic resonance tissue tracking in repaired tetralogy of fallot to ventricular tachycardia and death. Am. J. Cardiol. 2011, 107, 1535–1540. [Google Scholar] [CrossRef]
- Bernard, Y.; Morel, M.; Descotes-Genon, V.; Jehl, J.; Meneveau, N.; Schiele, F. Value of speckle tracking for the assessment of right ventricular function in patients operated on for tetralogy of Fallot. Comparison with magnetic resonance imaging. Echocardiography 2014, 31, 474–482. [Google Scholar] [CrossRef]
- Orwat, S.; Diller, G.P.; Kempny, A.; Radke, R.; Peters, B.; Kühne, T.; Boethig, D.; Gutberlet, M.; Dubowy, K.O.; Beerbaum, P.; et al. Myocardial deformation parameters predict outcome in patients with repaired tetralogy of Fallot. Heart 2016, 102, 209–215. [Google Scholar] [CrossRef]
- Van Grootel, R.W.J.; van den Bosch, A.E.; Baggen, V.J.M.; Menting, M.E.; Baart, S.J.; Cuypers, J.A.A.E.; Witsenburg, M.; Roos-Hesselink, J.W. The Prognostic Value of Myocardial Deformation in Adult Patients with Corrected Tetralogy of Fallot. J. Am. Soc. Echocardiogr. 2019, 32, 866–875.e2. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Wehner, G.J.; Suever, J.D.; Charnigo, R.J.; Alhadad, S.; Stearns, E.; Mojsejenko, D.; Haggerty, C.M.; Hickey, K.; Valente, A.M.; et al. Left and right ventricular dyssynchrony and strains from cardiovascular magnetic resonance feature tracking do not predict deterioration of ventricular function in patients with repaired tetralogy of Fallot. J. Cardiovasc. Magn. Reson. 2016, 18, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz-de la Garza, M.; Giraldeau, G.; Marin, J.; Imre Sarvari, S.; Guasch, E.; Gabrielli, L.; Brambila, C.; Bijnens, B.; Sitges, M. Should the septum be included in the assessment of right ventricular longitudinal strain? An ultrasound two-dimensional speckle-tracking stress study. Int. J. Cardiovasc. Imaging 2019, 35, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.P.; Kempny, A.; Liodakis, E.; Alonso-Gonzalez, R.; Inuzuka, R.; Uebing, A.; Orwat, S.; Dimopoulos, K.; Swan, L.; Li, W.; et al. Left ventricular longitudinal function predicts life-threatening ventricular arrhythmia and death in adults with repaired tetralogy of fallot. Circulation 2012, 125, 2440–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagdorn, Q.A.J.; Vos, J.D.L.; Beurskens, N.E.G.; Gorter, T.M.; Meyer, S.L.; van Melle, J.P.; Berger, R.M.F.; Willems, T.P. CMR feature tracking left ventricular strain-rate predicts ventricular tachyarrhythmia, but not deterioration of ventricular function in patients with repaired tetralogy of Fallot. Int. J. Cardiol. 2019, 295, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Alba, C.G.; Khan, A.; Woods, P.; Broberg, C.S. Left ventricular strain and fibrosis in adults with repaired tetralogy of Fallot: A case-control study. Int. J. Cardiol. 2021, 323, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, M.A.; Clark, A.L.; Cullen, S.; Newman, C.G.; Redington, A.N. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot. Restrictive physiology predicts superior exercise performance. Circulation 1995, 91, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Geva, T.; Mulder, B.; Gauvreau, K.; Babu-Narayan, S.V.; Wald, R.M.; Hickey, K.; Powell, A.J.; Gatzoulis, M.A.; Valente, A.M. Preoperative Predictors of Death and Sustained Ventricular Tachycardia after Pulmonary Valve Replacement in Patients with Repaired Tetralogy of Fallot Enrolled in the INDICATOR Cohort. Circulation 2018, 138, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
| Median (SD) or N (%) | |
|---|---|
| Demographics | |
| Age (years) | 31.48 (10.8) |
| Age at ToF Repair (years) | 1.8 (3.04) |
| Gender (male) | 14 (40%) |
| Body surface area, (m2) | 1.80 (0.20) |
| Height (cm) | 168.6 (9.5) |
| Weight (kg) | 71.34 (16.2) |
| Surgical History | |
| ToF repair | 15 (57.7%) |
| ToF repair + PVR | 11 (42.3%) |
| B-Tshunt + ToF repair | 3 (33.3%) |
| B-T shunt + ToF repair + PVR | 6 (66.7%) |
| Maximum number of surgeries (Nr ≥ 3) | 6 (17.1%) |
| Clinical Data | |
| New York Heart Association class > II | 25 (71.5%) |
| Rest Oxygen saturation > 96% | 31 (88.5%) |
| QRS duration, (ms) | 130.8 (18.8) |
| CMR | |
| RV EDVi, mL/m2 | 115.4 (35.5) |
| RV ESVi, mL/m2 | 55.6 (26.4) |
| RV EF, (%) | 49.4 (8.2) |
| RV GLS, (%) | −20.8 (2.4) |
| LV EF, (%) | 58.7 (6.0) |
| LV GLS, (%) | −17.04 (2.6) |
| Pulmonary regurgitation fraction, (%) | 21.1 (17.1) |
| PR Fraction > 20% | 16 (45.7%) |
| Biomarkers | |
| Galectin 3, (ng/mL) | 6.4 (1.57) |
| Procollagen III, (ng/mL) | 43.8 (11.1) |
| NTproBNP, (pg/mL) | 181.28 (178.4) |
| Low RV Score LGE RV < 8 Points (n = 15) | High RV Score LGE RV ≥ 8 Points (n = 20) | p Value | Total RV Score Spearman Correlation Coefficient, (p) | |
|---|---|---|---|---|
| Age, (years) | 30.4 (11.6) | 33.8 (10.7) | 0.40 | 0.24 (0.170) |
| Age at repair, (years) | 0.43 (0.37) | 0.35 (0.30) | 0.54 | −0.02 (0.916) |
| Shunt to repair time (years) | 1.9 (3.9) | 1.3 (2.6) | 0.67 | 0.15 (0.416) |
| Follow-up since repair, (years) | 25.1 (10.9) | 27.5(8.4) | 0.52 | 0.27 (0.152) |
| Clinical | ||||
| NYHA Class ≥ II, [N, (%)] | 9 (60%) | 12 (60%) | 0.21 | ----- |
| Rest SatO2, (%) | 97.6 (1.1) | 96.5 (1.8) | 0.05 | −0.46 (0.012) |
| QRS duration, (ms) | 134.1 (17.6) | 130.0 (20.3) | 0.56 | −0.18 (0.328) |
| Cardiac Magnetic Resonance | ||||
| RV EDVi, mL/m2 | 101.39 (19.8) | 134.61 (43.1) | 0.017 | 0.44 (0.015) |
| RV ESVi, mL/m2 | 46.0 (12.4) | 73.4 (27.0) | 0.003 | 0.66 (<0.001) |
| RV EF, (%) | 53.4 (4.4) | 43.3 (8.1) | 0.001 | −0.69 (<0.001) |
| RV GLS, (%) | −20.75 (2.3) | −20.75 (2.7) | 0.99 | −0.03 (0.844) |
| LV EF, (%) | 61.1 (5.3) | 56.5 (6.3) | 0.04 | −0.46 (0.011) |
| LV GLS, (%) | −18.0 (2.6) | −15.9 (2.1) | 0.03 | 0.49 (0.007) |
| PR Fraction, (%) | 16.2 (16.0) | 24.6 (18.0) | 0.20 | 0.44 (0.017) |
| Biomarker Levels | ||||
| Galectin 3, (ng/mL) | 5.9 (1.33) | 6.8 (1.64) | 0.10 | 0.23 (0.211) |
| Procollagen III, (ng/mL) | 42.5 (8.06) | 43.5 (13.0) | 0.78 | −0.28 (0.123) |
| NTproBNP, (pg/mL) | 121.6 (70.3) | 196.4 (99.9) | 0.02 | 0.29 (0.110) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karali, K.; Makedou, K.; Kallifatidis, A.; Didagelos, M.; Giannakoulas, G.; Davos, C.H.; Karamitsos, T.D.; Ziakas, A.; Karvounis, H.; Hadjimiltiades, S. The Interplay between Myocardial Fibrosis, Strain Imaging and Collagen Biomarkers in Adults with Repaired Tetralogy of Fallot. Diagnostics 2021, 11, 2101. https://doi.org/10.3390/diagnostics11112101
Karali K, Makedou K, Kallifatidis A, Didagelos M, Giannakoulas G, Davos CH, Karamitsos TD, Ziakas A, Karvounis H, Hadjimiltiades S. The Interplay between Myocardial Fibrosis, Strain Imaging and Collagen Biomarkers in Adults with Repaired Tetralogy of Fallot. Diagnostics. 2021; 11(11):2101. https://doi.org/10.3390/diagnostics11112101
Chicago/Turabian StyleKarali, Konstantina, Kali Makedou, Alexandros Kallifatidis, Matthaios Didagelos, George Giannakoulas, Constantinos H. Davos, Theodoros D. Karamitsos, Antonios Ziakas, Haralambos Karvounis, and Stavros Hadjimiltiades. 2021. "The Interplay between Myocardial Fibrosis, Strain Imaging and Collagen Biomarkers in Adults with Repaired Tetralogy of Fallot" Diagnostics 11, no. 11: 2101. https://doi.org/10.3390/diagnostics11112101
APA StyleKarali, K., Makedou, K., Kallifatidis, A., Didagelos, M., Giannakoulas, G., Davos, C. H., Karamitsos, T. D., Ziakas, A., Karvounis, H., & Hadjimiltiades, S. (2021). The Interplay between Myocardial Fibrosis, Strain Imaging and Collagen Biomarkers in Adults with Repaired Tetralogy of Fallot. Diagnostics, 11(11), 2101. https://doi.org/10.3390/diagnostics11112101








