Antibodies against 4 Atypical Post-Translational Protein Modifications in Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. In Vitro Production of the Four PTMs
2.3. Selection of Ten Nitrated Peptides
2.4. Determination of Autoantibodies
2.5. Statistical Analysis
3. Results
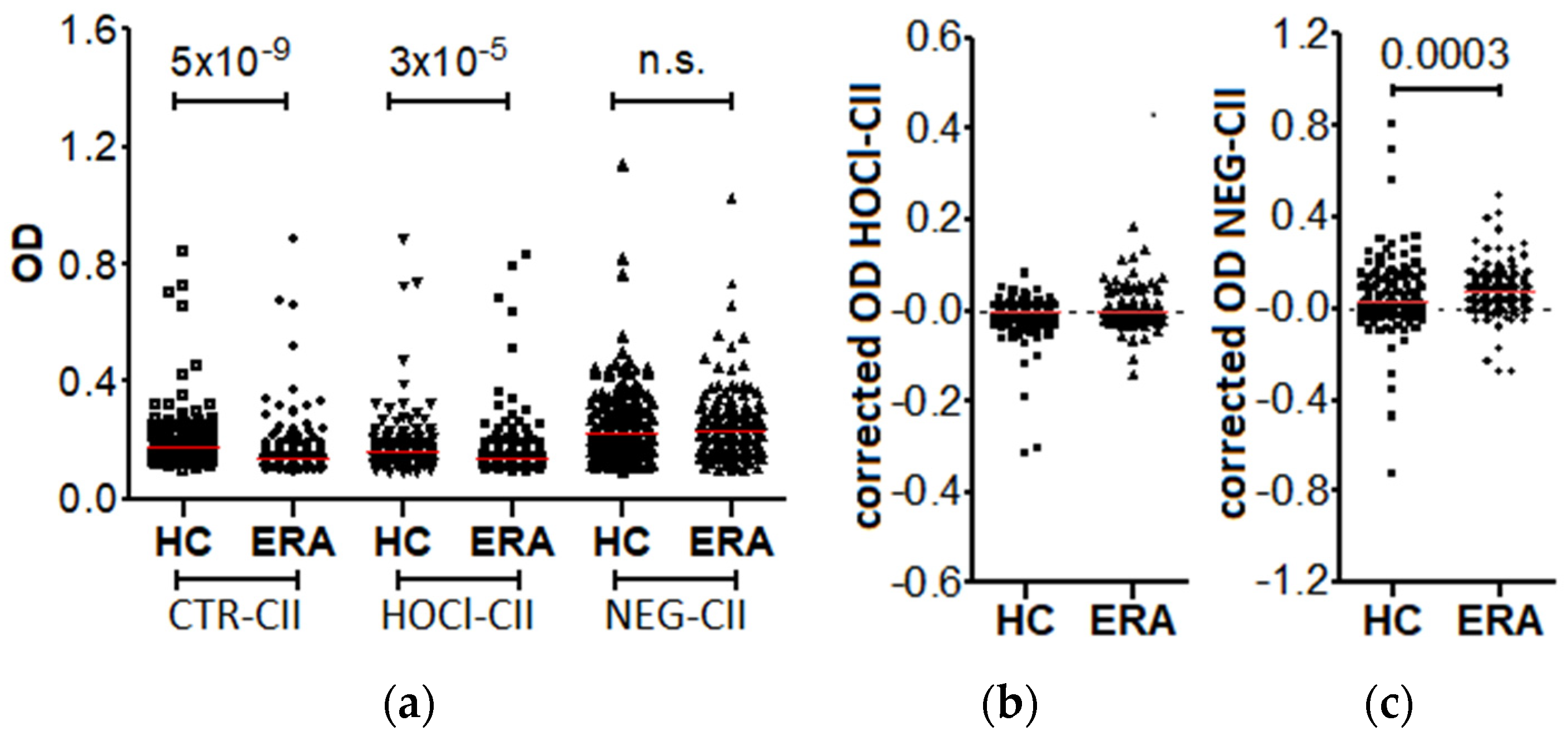
3.1. Prevalence of the Anti-HOCl-CII and Anti-NEG-CII Antibodies in the Patients with Early RA
3.2. Antibodies against NO2-SynP and 3-NT Peptides in RA Patients
3.3. Prevalence of Anti-Hcy-SynP Antibodies in Patients with RA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Nature of the PTM Induced by HOCl, Ribose and Peroxynitrite
Appendix A.2. Verification of the PTM Induced by HOCl and Ribose
| Antigen | Assay | Information |
|---|---|---|
| HOCl-CII & NEG-CII | SDS-PAGE | Integrity, apparent MW, aggregates |
| Fluorescence | Tryptophan, PLP, other AGE | |
| DNPH | Carbonyls | |
| NO2-SynP | Absorbance | 3-NT (low specificity) |
| ELISA | 3-NT (high specificity) | |
| Fluorescence | Tyrosine (unmodified) | |
| Hcy-SynP | Competitive ELISA | Total homocysteine |
| 3-NT-pep 1 | HPLC | Purity, elution time |
| MS | Purity and peptide mass |
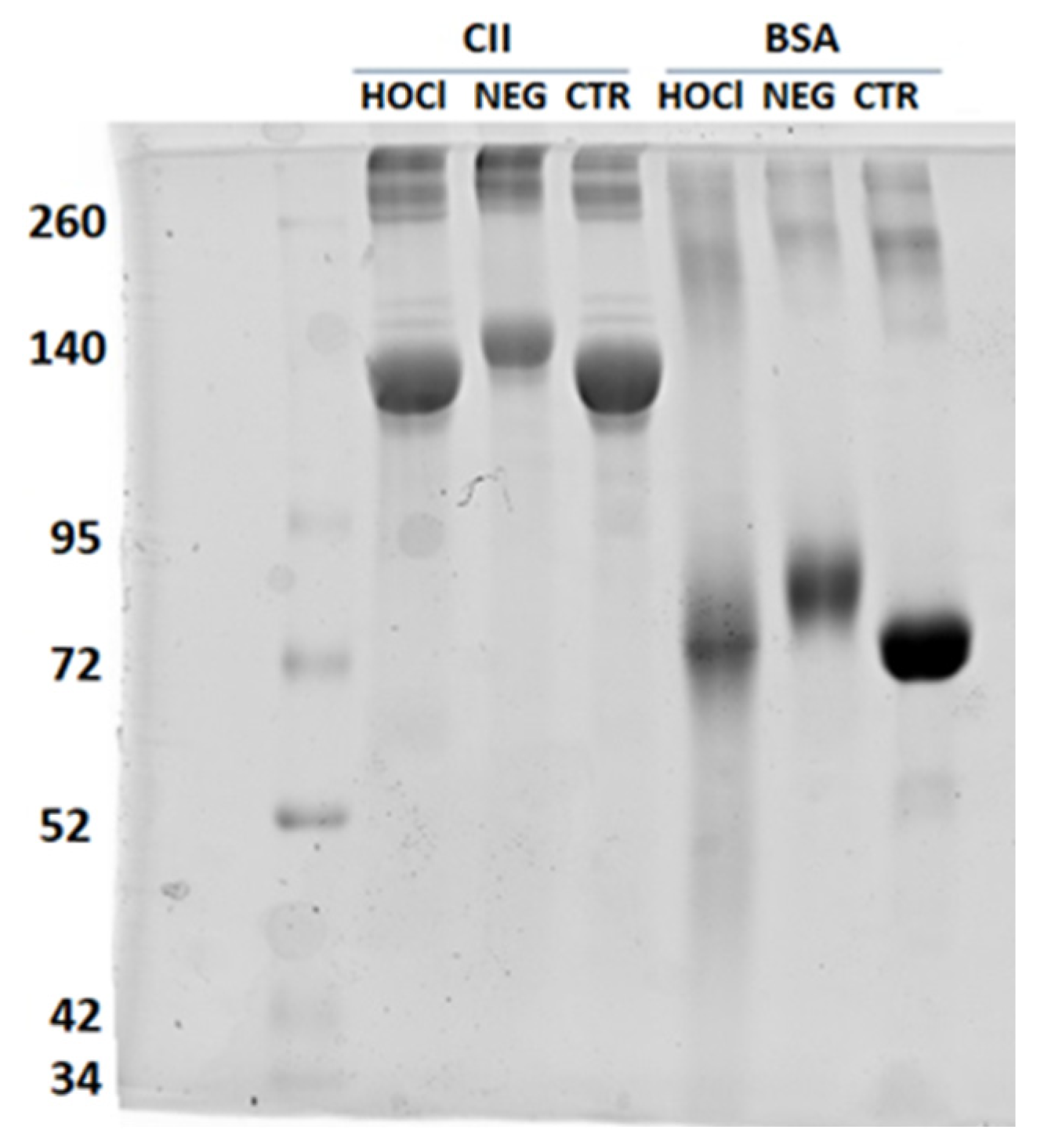
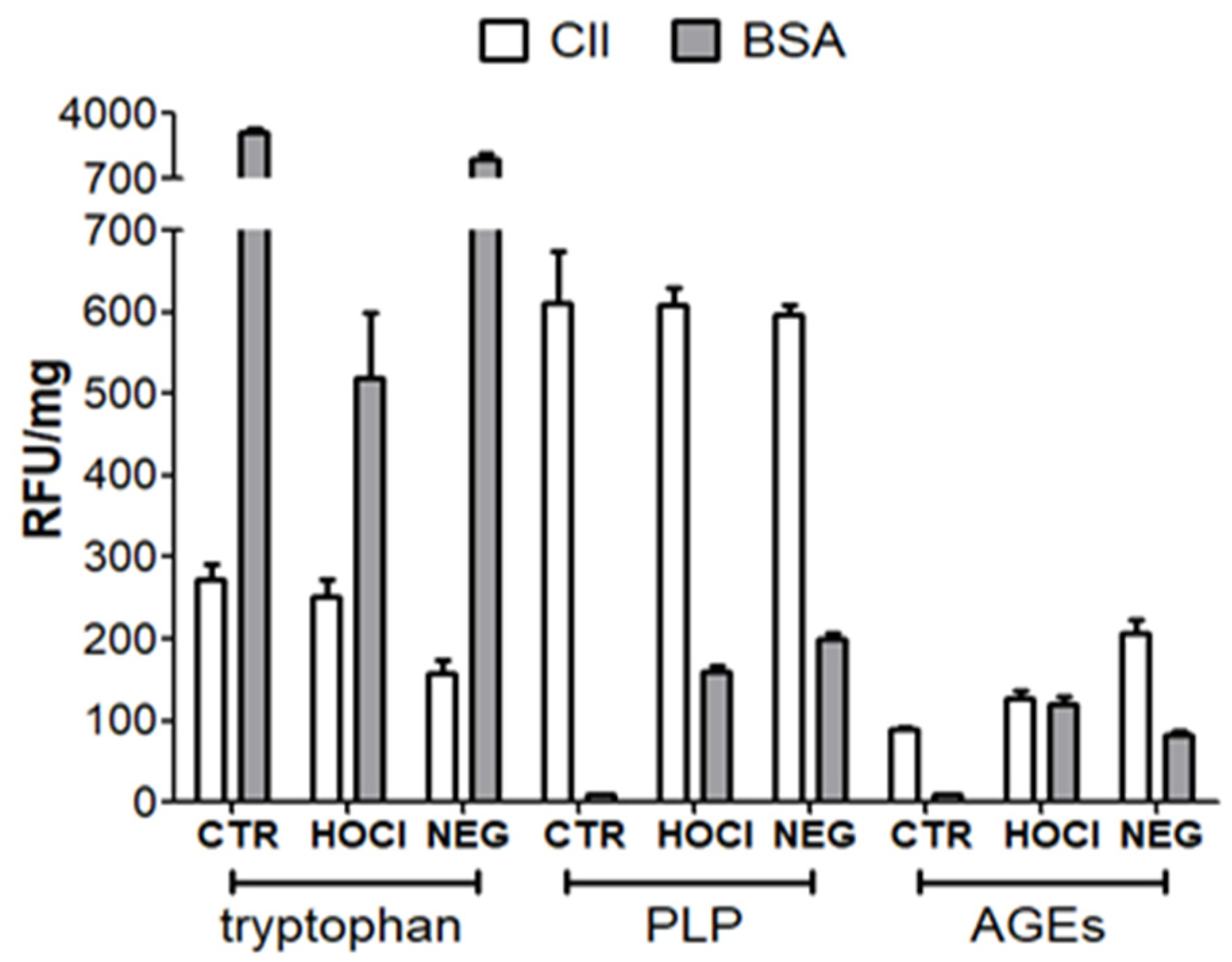
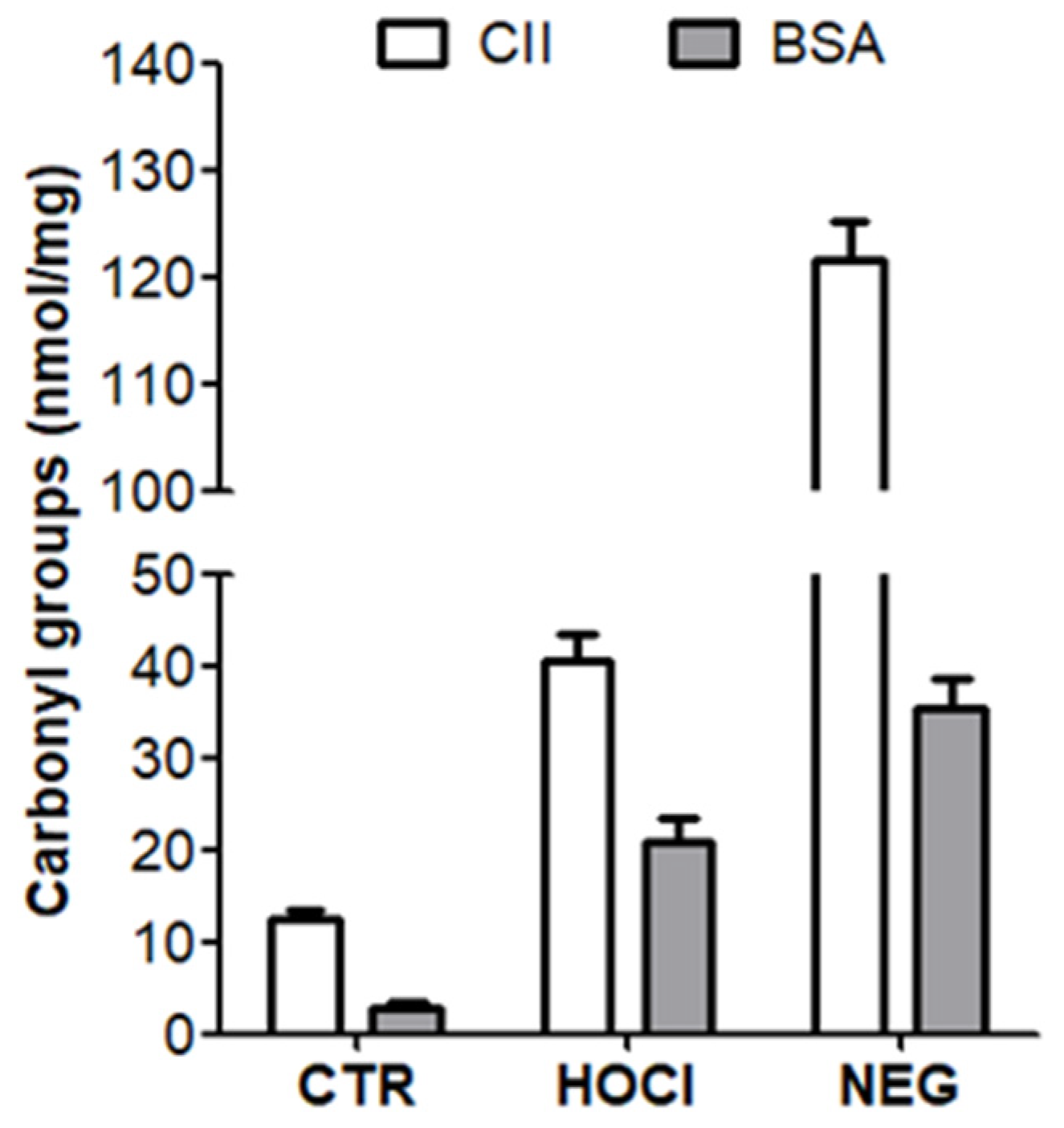
Appendix A.3. Verification of the Nitration of Proteins with Peroxynitrite
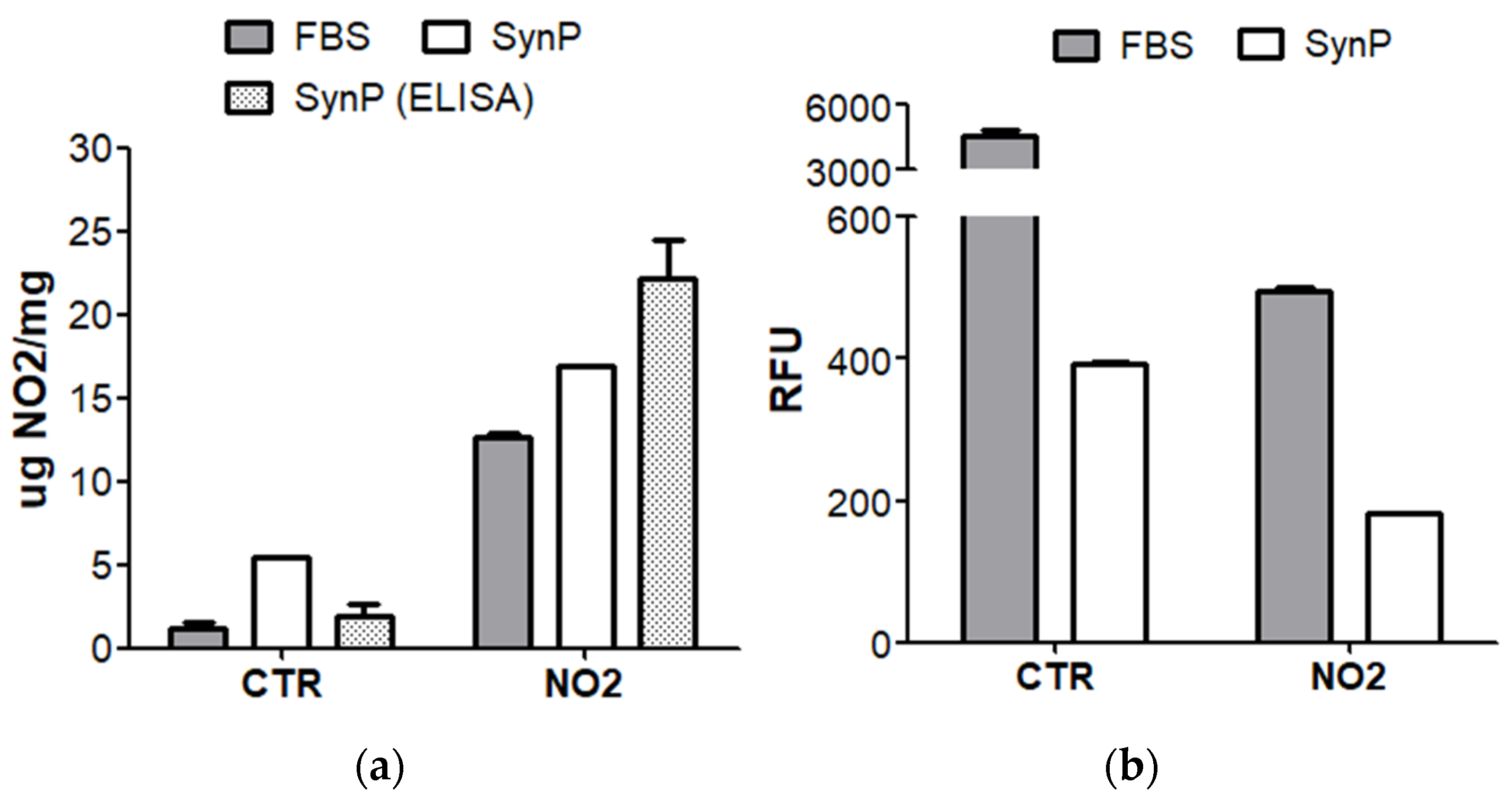
Appendix A.4. Nature and Verification of Homocysteinylation
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthri-tis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Catrina, A.; Krishnamurthy, A.; Rethi, B. Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis. RMD Open 2021, 7, e001228. [Google Scholar] [CrossRef] [PubMed]
- Trouw, L.A.; Rispens, T.; Toes, R.E.M. Beyond citrullination: Other post-translational protein modifications in rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Grönwall, C.; Liljefors, L.; Bang, H.; Hensvold, A.H.; Hansson, M.; Mathsson-Alm, L.; Israelsson, L.; Joshua, V.; Svärd, A.; Stålesen, R.; et al. A Comprehensive Evaluation of the Relationship Between Different IgG and IgA Anti-Modified Protein Autoantibodies in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 627986. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Duryee, M.J.; England, B.R.; Anderson, D.R.; Hearth-Holmes, M.; Su, K.; Michaud, K.; Payne, J.B.; Sayles, H.; Hunter, C.; et al. Malondialdehyde-acetaldehyde antibody concentrations in rheumatoid arthritis and other rheumatic conditions. Int. Immunopharmacol. 2018, 56, 113–118. [Google Scholar] [CrossRef]
- Steen, J.; Forsström, B.; Sahlström, P.; Odowd, V.; Israelsson, L.; Krishnamurthy, A.; Badreh, S.; Mathsson Alm, L.; Compson, J.; Ramsköld, D.; et al. Recognition of Amino Acid Motifs, Rather Than Specific Proteins, by Human Plasma Cell-Derived Monoclonal Antibodies to Posttranslationally Modified Proteins in Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 196–209. [Google Scholar] [CrossRef]
- Sahlström, P.; Hansson, M.; Steen, J.; Amara, K.; Titcombe, P.J.; Forsström, B.; Stålesen, R.; Israelsson, L.; Piccoli, L.; Lundberg, K.; et al. Different Hierarchies of Anti-Modified Protein Autoantibody Reactivities in Rheumatoid Arthritis. Arthritis Rheumatol. 2020, 72, 1643–1657. [Google Scholar] [CrossRef]
- Kissel, T.; Reijm, S.; Slot, L.M.; Cavallari, M.; Wortel, C.M.; Vergroesen, R.D.; Stoeken-Rijsbergen, G.; Kwekkeboom, J.C.; Kampstra, A.; Levarht, E.; et al. Antibodies and B cells recognising citrullinated proteins display a broad cross-reactivity towards other post-translational modifications. Ann. Rheum. Dis. 2020, 79, 472–480. [Google Scholar] [CrossRef]
- Colasanti, T.; Sabatinelli, D.; Mancone, C.; Giorgi, A.; Pecani, A.; Spinelli, F.R.; Di Giamberardino, A.; Navarini, L.; Speziali, M.; Vomero, M.; et al. Homocysteinylated alpha 1 antitrypsin as an antigenic target of autoantibodies in seronegative rheumatoid arthritis patients. J. Autoimmun. 2020, 113, 102470. [Google Scholar] [CrossRef]
- Khan, F.; Siddiqui, A.A. Prevalence of anti-3-nitrotyrosine antibodies in the joint synovial fluid of patients with rheumatoid arthritis, osteoarthritis and systemic lupus erythematosus. Clin. Chim. Acta 2006, 370, 100–107. [Google Scholar] [CrossRef]
- Nissim, A.; Winyard, P.G.; Corrigall, V.; Fatah, R.; Perrett, D.; Panayi, G.; Chernajovsky, Y. Generation of neoantigenic epitopes after posttranslational modification of type II collagen by factors present within the inflamed joint. Arthritis Rheum. 2005, 52, 3829–3838. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska-Plaza, A.; Potaczek, D.P.; Gluszko, P.; Undas, A. Antibodies to N-homocysteinylated albumin and haemoglobin in patients with rheumatoid arthritis: A potential new marker of disease severity. Scand. J. Rheumatol. 2014, 43, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Strollo, R.; Ponchel, F.; Malmström, V.; Rizzo, P.; Bombardieri, M.; Wenham, C.Y.; Landy, R.; Perret, D.; Watt, F.; Corrigall, V.M.; et al. Autoantibodies to posttranslationally modified type II collagen as potential biomarkers for rheumatoid arthritis. Arthritis Rheum. 2013, 65, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; van Delft, M.A.M.; Shuweihdi, F.; Kingsbury, S.R.; Trouw, L.A.; Doody, G.M.; Conaghan, P.G.; Ponchel, F. Auto-antibodies to post-translationally modified proteins in osteoarthritis. Osteoarthr. Cartil. 2021, 29, 924–933. [Google Scholar] [CrossRef]
- Regueiro, C.; Rodríguez-Martínez, L.; Nuño, L.; Ortiz, A.M.; Villalba, A.; Pascual-Salcedo, D.; Martínez-Feito, A.; González-Alvaro, I.; Balsa, A.; Gonzalez, A. Improved RA classification among early arthritis patients with the concordant presence of three RA autoantibodies: Analysis in two early arthritis clinics. Arthritis Res. Ther. 2019, 21, 280. [Google Scholar] [CrossRef]
- Rodriguez-Martínez, L.; Bang, H.; Regueiro, C.; Nuño, L.; Triguero-Martinez, A.; Peiteado, D.; Ortiz, A.M.; Villalba, A.; Martinez-Feito, A.; Balsa, A.; et al. Improved classification of rheumatoid arthritis with a score including anti-acetylated ornithine antibodies. Sci. Rep. 2020, 10, 19263. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef]
- Santos, A.L.; Lindner, A.B. Protein Posttranslational Modifications: Roles in Aging and Age-Related Disease. Oxid. Med. Cell. Longev. 2017, 2017, 5716409. [Google Scholar] [CrossRef]
- Hawkins, C.L. Hypochlorous acid-mediated modification of proteins and its consequences. Essays Biochem. 2020, 64, 75–86. [Google Scholar] [CrossRef]
- Radi, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef]
- Jakubowski, H. Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Anwar, A.; Savage, R.S.; Thornalley, P.J.; Rabbani, N. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res. Ther. 2016, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Undas, A.; Kolarz, M.; Kopec, G.; Glowacki, R.; Placzkiewicz-Jankowska, E.; Tracz, W. Autoantibodies against N-homocysteinylated proteins in patients on long-term haemodialysis. Nephrol. Dial. Transplant 2007, 22, 1685–1689. [Google Scholar] [CrossRef][Green Version]
- Sandhu, J.K.; Robertson, S.; Birnboim, H.C.; Goldstein, R. Distribution of protein nitrotyrosine in synovial tissues of patients with rheumatoid arthritis and osteoarthritis. J. Rheumatol. 2003, 30, 1173–1181. [Google Scholar]
- Xu, Y.; Wen, X.; Wen, L.-S.; Wu, L.-Y.; Deng, N.-Y.; Chou, K.-C. iNitro-Tyr: Prediction of nitrotyrosine sites in proteins with general pseudo amino acid composition. PLoS ONE 2014, 9, e105018. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, J.; Ma, Q.; Gao, X.; Ren, J.; Xue, Y. GPS-YNO2: Computational prediction of tyrosine nitration sites in proteins. Mol. Biosyst. 2011, 7, 1197–1204. [Google Scholar] [CrossRef]
- El-Manzalawy, Y.; Dobbs, D.; Honavar, V. Predicting linear B-cell epitopes using string kernels. J. Mol. Recognit. 2008, 21, 243–255. [Google Scholar] [CrossRef]
- Montes, A.; Regueiro, C.; Perez-Pampin, E.; Boveda, M.D.; Gomez-Reino, J.J.; Gonzalez, A. Anti-Carbamylated Protein Antibodies as a Reproducible Independent Type of Rheumatoid Arthritis Autoantibodies. PLoS ONE 2016, 11, e0161141. [Google Scholar] [CrossRef]
- Clague, R.B. Autoantibodies to cartilage collagens in rheumatoid arthritis. Do they perpetuate the disease or are they irrelevant? Br. J. Rheumatol. 1989, 28, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morgan, K. What do anti-collagen antibodies mean? Ann. Rheum. Dis. 1990, 49, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Kirk, A.P.; O’Hara, B.P.; Mageed, R.A.; McMahon, M.S.; McCarthy, D.; Menashi, S.; Archer, J.R.; Currey, H.L. Pepsinogen—An immunoglobulin binding artefact in ‘collagen’ preparations. Clin. Exp. Immunol. 1986, 65, 671–678. [Google Scholar] [PubMed]
- Williams, R.O.; Williams, D.G.; Maini, R.N. Anti-type II collagen ELISA. Increased disease specificity following removal of anionic contaminants from salt-fractionated type II collagen. J. Immunol. Methods 1992, 147, 93–100. [Google Scholar]
- Manivel, V.A.; Mullazehi, M.; Padyukov, L.; Westerlind, H.; Klareskog, L.; Alfredsson, L.; Saevarsdottir, S.; Rönnelid, J. Anticollagen type II antibodies are associated with an acute onset rheumatoid arthritis phenotype and prognosticate lower degree of inflammation during 5 years follow-up. Ann. Rheum. Dis. 2017, 76, 1529–1536. [Google Scholar] [CrossRef]
- van Wesemael, T.J.; Ajeganova, S.; Humphreys, J.; Terao, C.; Muhammad, A.; Symmons, D.P.M.; MacGregor, A.J.; Hafström, I.; Trouw, L.A.; van der Helm-van Mil, A.H.M.; et al. Smoking is associated with the concurrent presence of multiple autoantibodies in rheumatoid arthritis rather than with anti-citrullinated protein antibodies per se: A multicenter cohort study. Arthritis Res. Ther. 2016, 18, 285. [Google Scholar] [CrossRef]
- Regueiro, C.; Rodriguez-Rodriguez, L.; Lopez-Mejias, R.; Nuño, L.; Triguero-Martinez, A.; Perez-Pampin, E.; Corrales, A.; Villalba, A.; Lopez-Golan, Y.; Abasolo, L.; et al. A predominant involvement of the triple seropositive patients and others with rheumatoid factor in the association of smoking with rheumatoid arthritis. Sci. Rep. 2020, 10, 3355. [Google Scholar] [CrossRef]
- Barelli, S.; Canellini, G.; Thadikkaran, L.; Crettaz, D.; Quadroni, M.; Rossier, J.S.; Tissot, J.-D.; Lion, N. Oxidation of proteins: Basic principles and perspectives for blood proteomics. Proteom. Clin. Appl. 2008, 2, 142–157. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; al-Mehdi, A.B. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995, 364, 279–282. [Google Scholar] [CrossRef]
- Andreea, S.I.; Marieta, C.; Anca, D. AGEs and glucose levels modulate type I and III procollagen mRNA synthesis in dermal fibroblasts cells culture. Exp. Diabetes Res. 2008, 2008, 473603. [Google Scholar] [CrossRef]
- Khan, M.A.; Arif, Z.; Moinuddin; Alam, K. Characterization of methylglyoxal-modified human IgG by physicochemical methods. J. Biomol. Struct. Dyn. 2018, 36, 3172–3183. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K.; Makita, Z.; Shiroshita, K.; Ueda, T.; Fusegawa, T.; Kuwajima, S.; Takeuchi, M.; Koike, T. Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabolism 1998, 47, 1348–1353. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Soglia, F.; Petracci, M.; Ertbjerg, P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chem. 2016, 197, 670–675. [Google Scholar] [CrossRef]
- Uversky, V.N.; Yamin, G.; Munishkina, L.A.; Karymov, M.A.; Millett, I.S.; Doniach, S.; Lyubchenko, Y.L.; Fink, A.L. Effects of nitration on the structure and aggregation of alpha-synuclein. Mol. Brain Res. 2005, 134, 84–102. [Google Scholar] [CrossRef]


| Early RA 2 n = 182 | Established RA 2 n = 143 | |
|---|---|---|
| Age, median (IQR) 3 years | 58 (48–71) | 61 (50–72) |
| Age of diagnosis, median (IQR) years | 58 (48–71) | 47 (35–56) |
| Symptom duration, median (IQR) years | 0 (0–1) | 11 (5–21) |
| Women, n (%) | 112 (62) | 112 (78) |
| Ever smoked, n (%) | 54 (30) | 23 (18) |
| Erosions, n (%) | 17 (14) | 92 (66) |
| RF positive, n (%) | 94 (56) | 84 (61) |
| Anti-CCP positive, n (%) | 119 (65) | 97 (68) |
| Anti-CarP positive, n (%) | 63 (41) | 48 (34) |
| DMARD naïve, n (%) | 109 (82) | - |
| MTX, n (%) | 6/133 (5) | - |
| Other csDMARD, n (%) | 10/129 (8) | - |
| bDMARD, n (%) | 8/133 (6) | - |
| Glucocorticoids, n (%) | 115/122 (94) | - |
| NSAIDs, n (%) | 83/113 (73) | - |
| DAS28, median (IQR) | 4.94 (3.53–5.97) | - |
| IgG [mg/dL], median (IQR) | 1195 (1010–1440) | - |
| PTM | Protein | Modifying Agent | Abbreviation |
|---|---|---|---|
| chlorination | collagen type II | hipochlorous acid | HOCl-CII |
| glycation | collagen type II | ribose | NEG-CII |
| nitration | synovial proteins | sodium peroxinitrite | NO2-SynP |
| nitration | 8 peptides | synthesis | 3-NT-pep |
| homocysteinylation | synovial proteins | L-homocysteine-thiolactone | Hcy-SynP |
| Protein | Sequence | Δ 1 | Score 2 |
|---|---|---|---|
| Enolase 1247–267 | AASEFFRSGK(3-NO2-Y)DLDFKSPDDP | 0.999 | 2.881 |
| Enolase 234–54 | AVPSGASTGI(3-NO2-Y)EALELRDNDK | 0.584 | 1.498 |
| Fibrinogen312–332 | GKNYCGLPGE(3-NO2-Y)WLGNDKISQL | 0.903 | 1.611 |
| Vimentin20–40 | TASRPSSSRS(3-NO2-Y)VTTSTRTYSL | 0.963 | 2.693 |
| 14-3-3 η206–226 | AELDTLNEDS(3-NO2-Y)KDSTLIMQLL | 1.061 | 1.695 |
| BiP165–185 3 | VTHAVVTVPA(3-NO2-Y)FNDAQRQATK | 3.237 | 2.824 |
| Tenascin1676–1696 3 | DITGLREATE(3-NO2-Y)EIELYGISKG | 0.457 | 0.912 |
| Histone H332–52 | ATGGVKKPHR(3-NO2-Y)RPGTVALREI | 1.319 | 1.679 |
| Histone H4 163–83 | LENVIRDAVT(3-NO2-Y)TEHAKRKTVT | 0.731 | 1.866 |
| Histone H4 242–62 | GGVKRISGLI(3-NO2-Y)YEETRGVLKVF | 0.546 | 1.448 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Martínez, L.; Regueiro, C.; Amhaz-Escanlar, S.; Pena, C.; Herbello-Hermelo, P.; Moreda-Piñeiro, A.; Rodriguez-Garcia, J.; Mera-Varela, A.; Pérez-Pampín, E.; González, A. Antibodies against 4 Atypical Post-Translational Protein Modifications in Patients with Rheumatoid Arthritis. Diagnostics 2022, 12, 352. https://doi.org/10.3390/diagnostics12020352
Rodríguez-Martínez L, Regueiro C, Amhaz-Escanlar S, Pena C, Herbello-Hermelo P, Moreda-Piñeiro A, Rodriguez-Garcia J, Mera-Varela A, Pérez-Pampín E, González A. Antibodies against 4 Atypical Post-Translational Protein Modifications in Patients with Rheumatoid Arthritis. Diagnostics. 2022; 12(2):352. https://doi.org/10.3390/diagnostics12020352
Chicago/Turabian StyleRodríguez-Martínez, Lorena, Cristina Regueiro, Sámer Amhaz-Escanlar, Carmen Pena, Paloma Herbello-Hermelo, Antonio Moreda-Piñeiro, Javier Rodriguez-Garcia, Antonio Mera-Varela, Eva Pérez-Pampín, and Antonio González. 2022. "Antibodies against 4 Atypical Post-Translational Protein Modifications in Patients with Rheumatoid Arthritis" Diagnostics 12, no. 2: 352. https://doi.org/10.3390/diagnostics12020352
APA StyleRodríguez-Martínez, L., Regueiro, C., Amhaz-Escanlar, S., Pena, C., Herbello-Hermelo, P., Moreda-Piñeiro, A., Rodriguez-Garcia, J., Mera-Varela, A., Pérez-Pampín, E., & González, A. (2022). Antibodies against 4 Atypical Post-Translational Protein Modifications in Patients with Rheumatoid Arthritis. Diagnostics, 12(2), 352. https://doi.org/10.3390/diagnostics12020352






