The Mechanisms Underlying Vertical Artifacts in Lung Ultrasound and Their Proper Utilization for the Evaluation of Cardiogenic Pulmonary Edema
Abstract
:1. Introduction
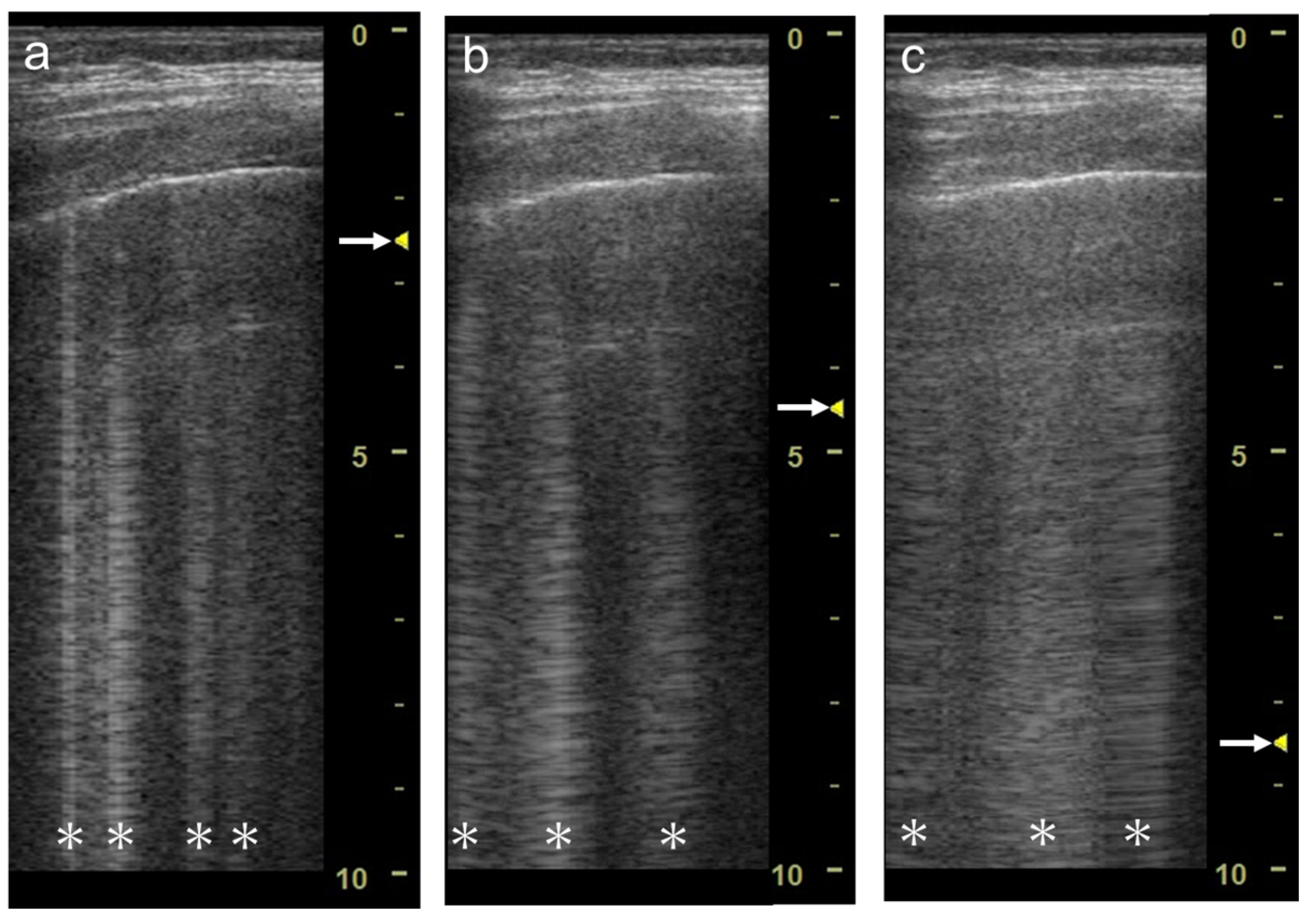
2. Generation of Vertical Artifacts
2.1. Clinical Implications
2.2. The Theory of Acoustic Trap
2.3. Our Basic Research Supporting the Theory
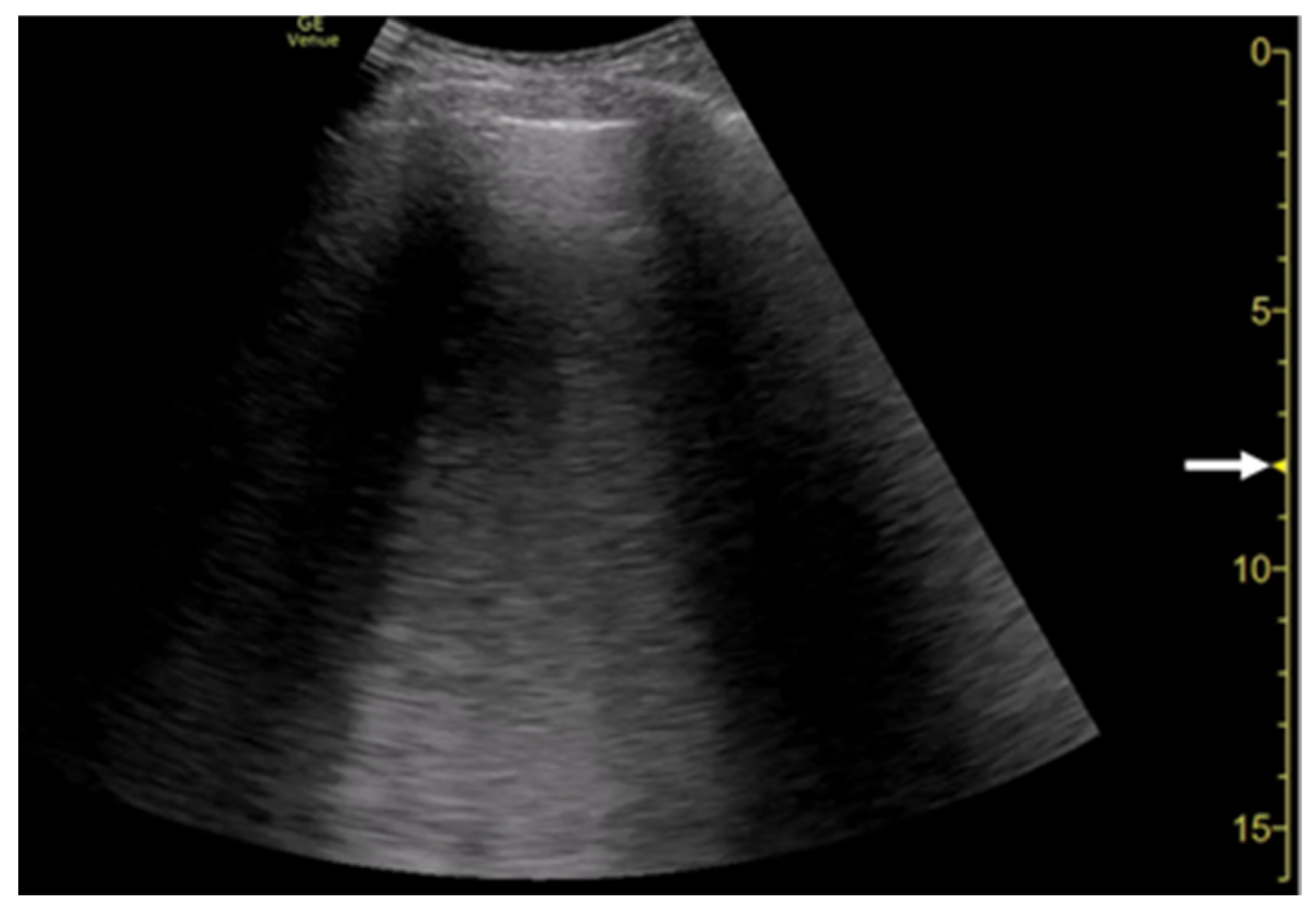
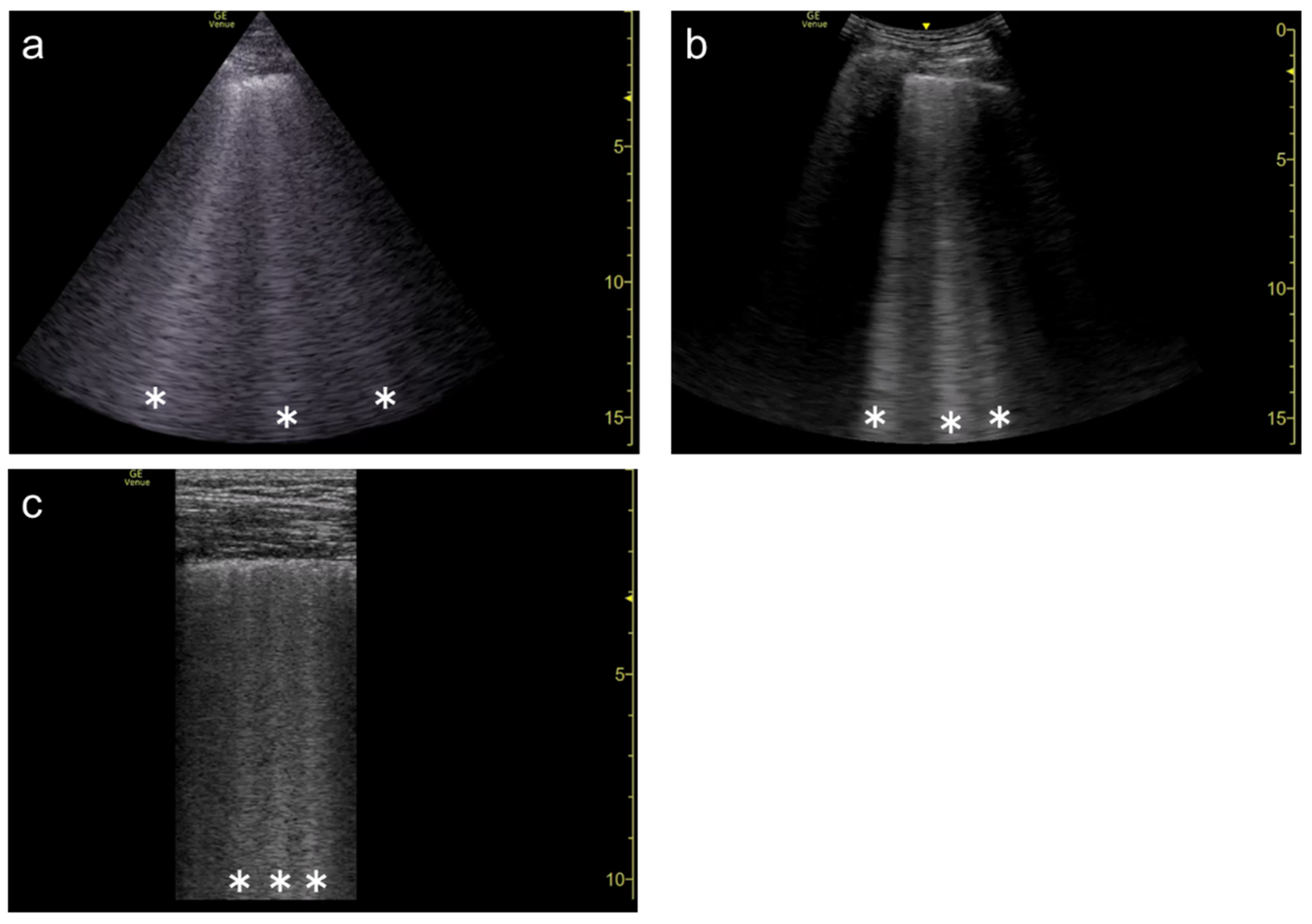
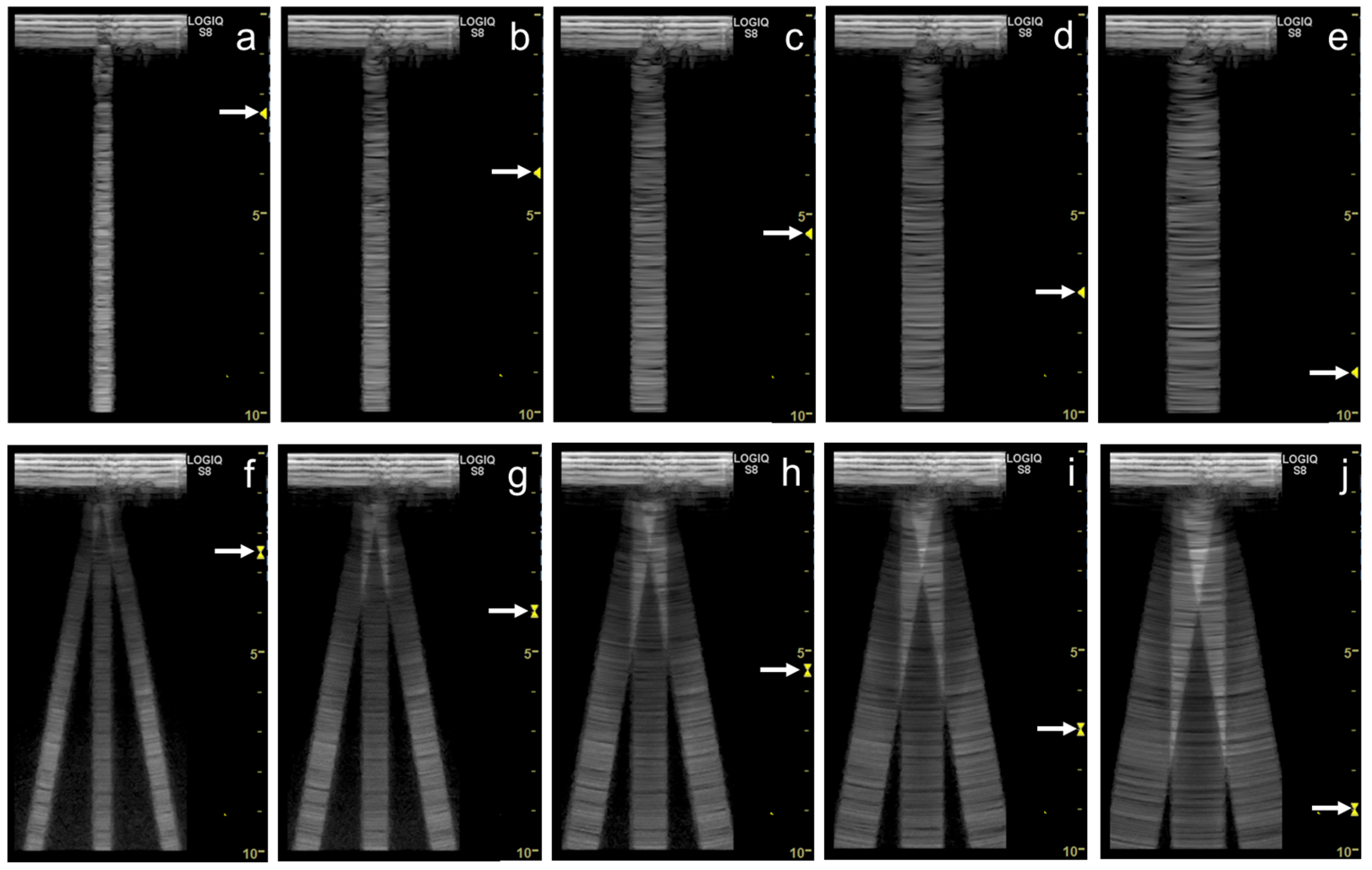
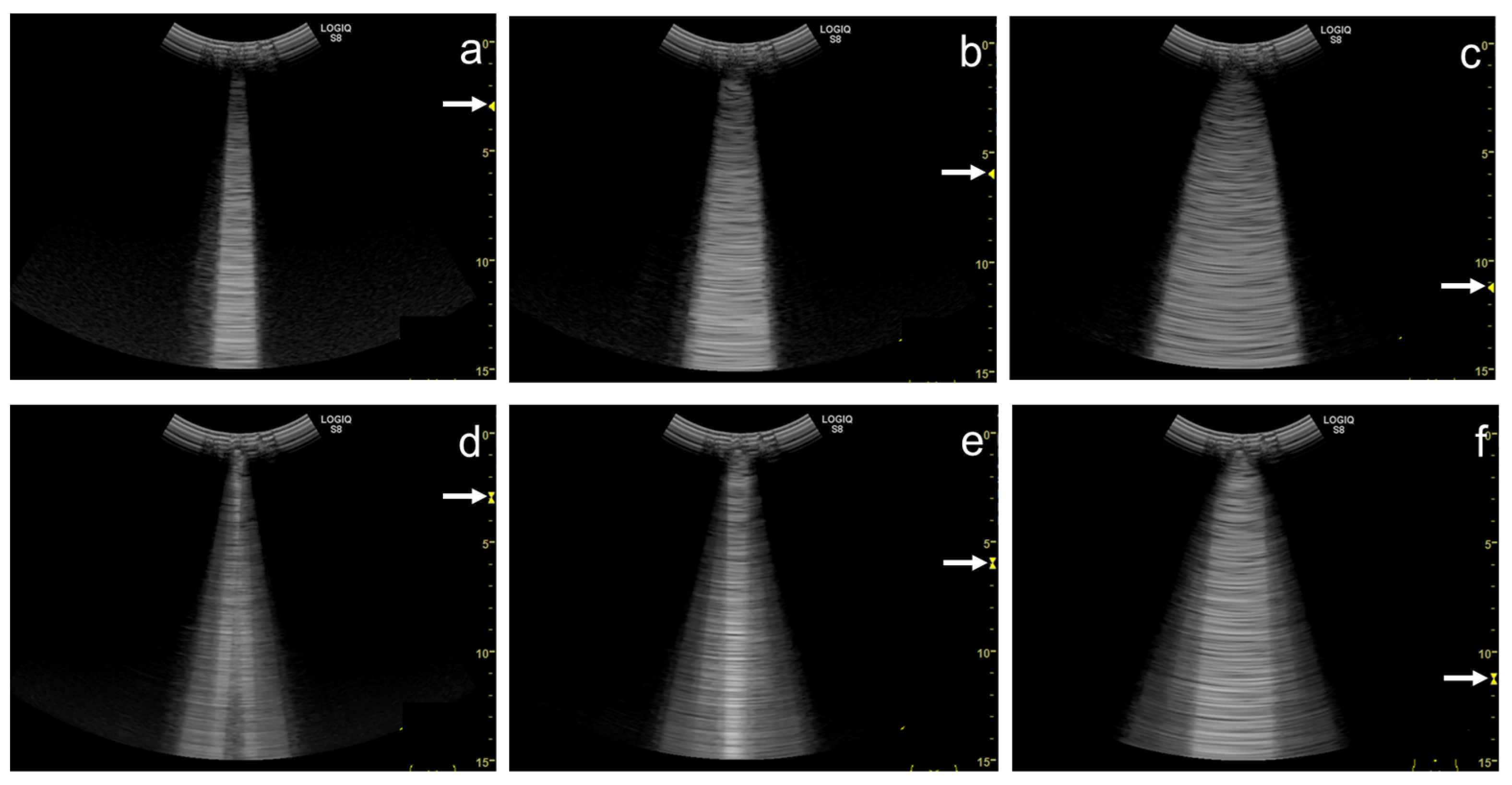
3. Influence of Machine Settings and the Selection of Transducers on Vertical Artifacts
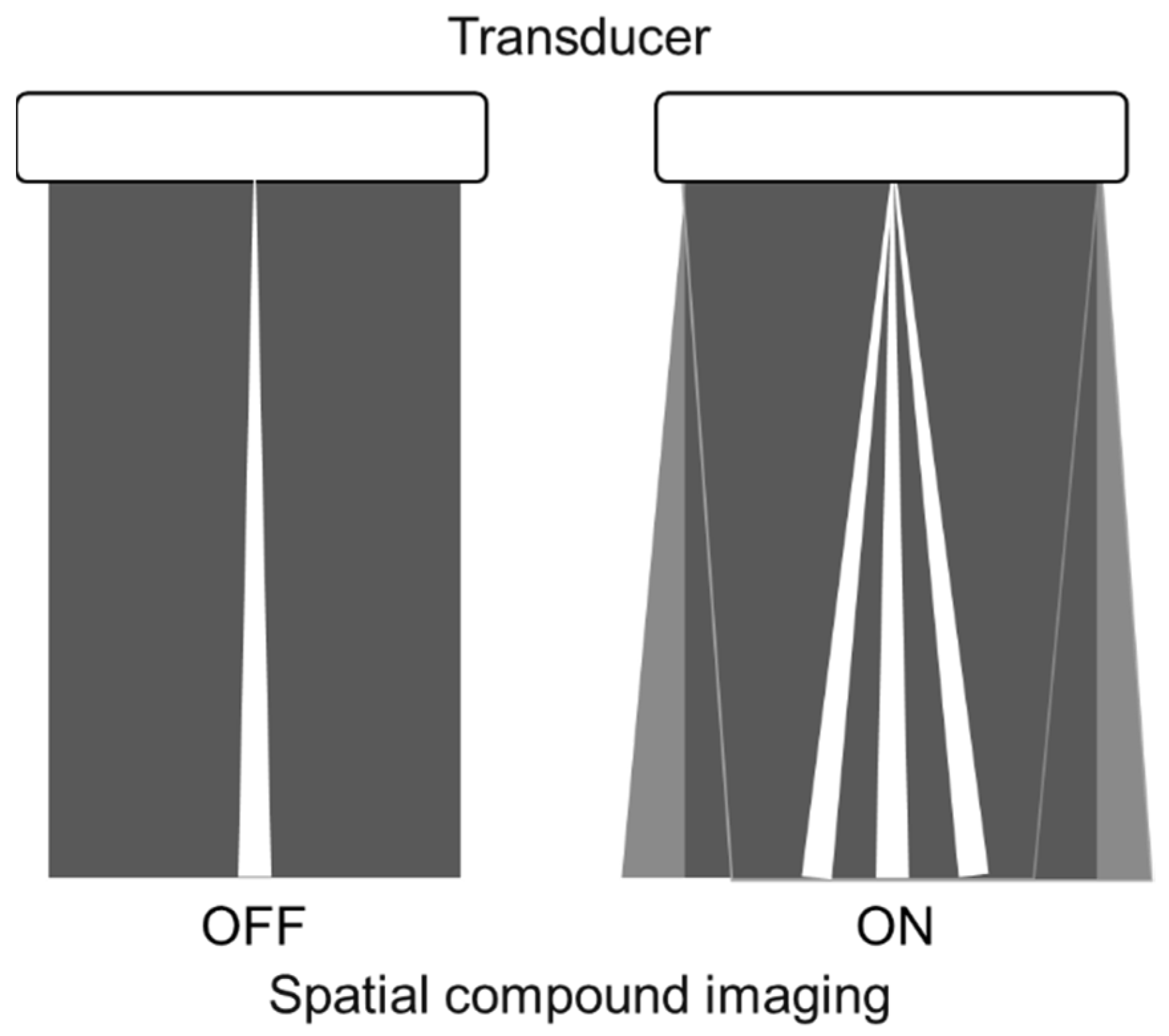
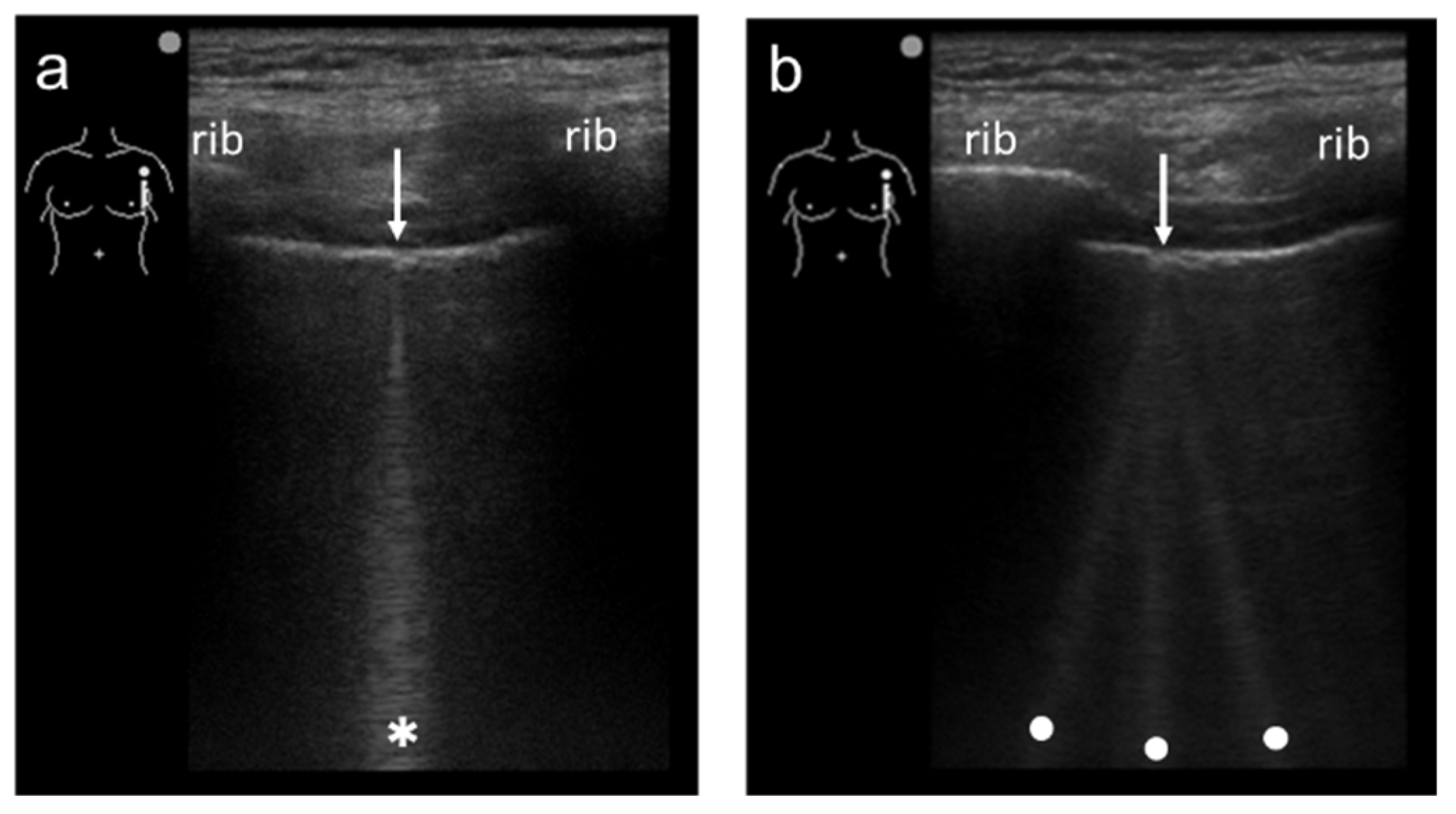
3.1. Spatial Compound Imaging
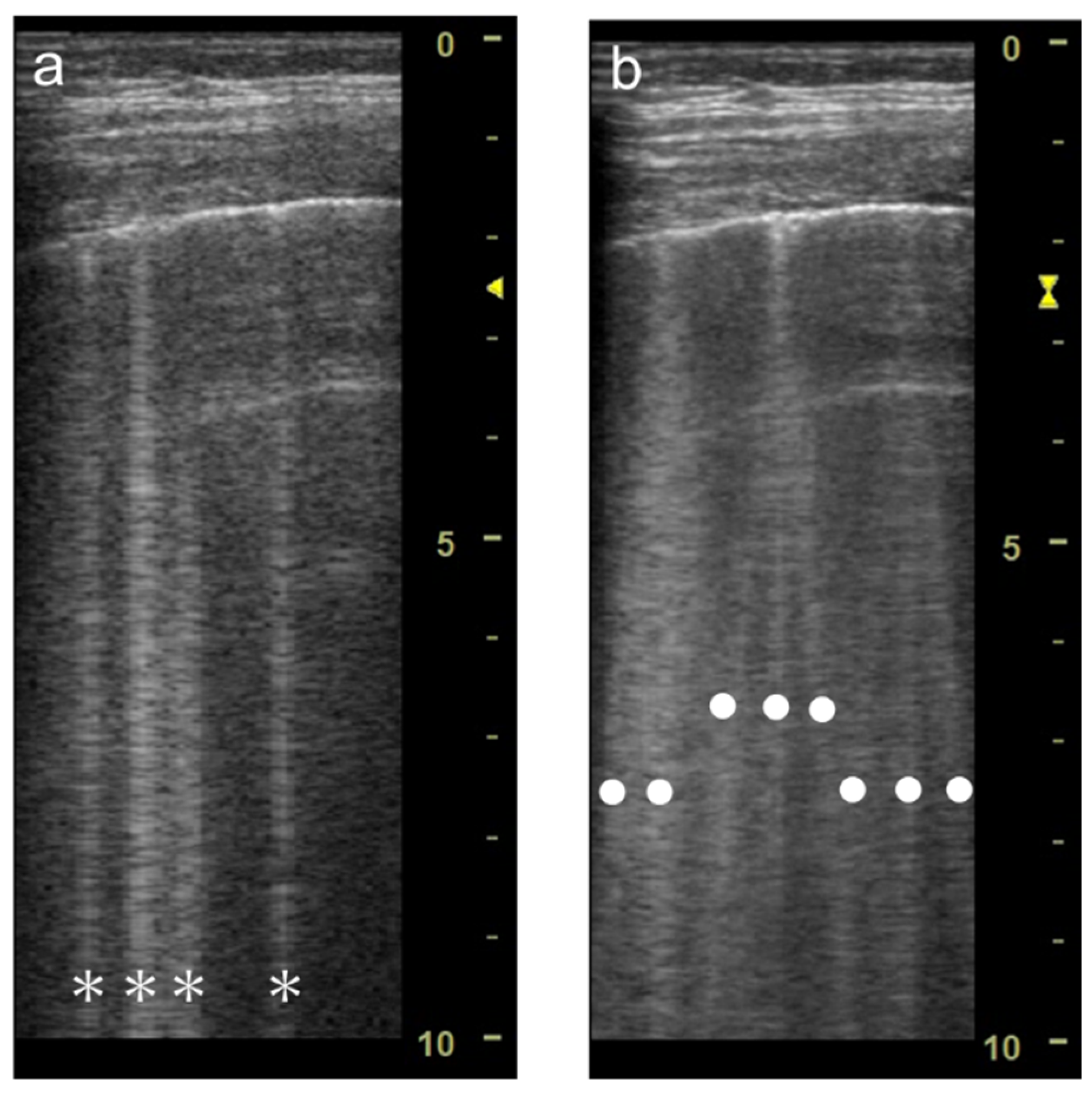
3.2. Focal Point
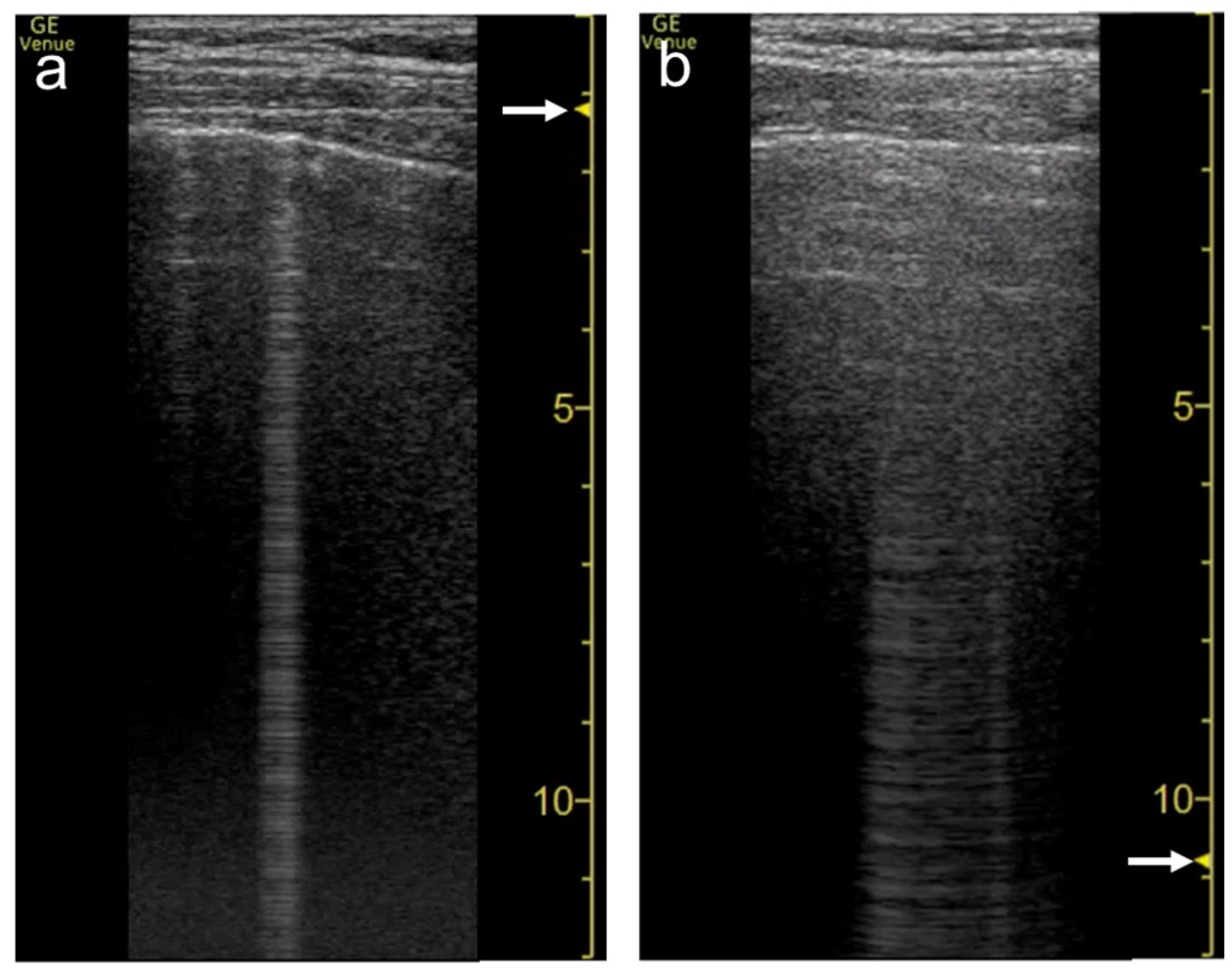
3.3. Frequency
3.4. Selection of Transducers
3.5. Simple Educational Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of Lung Ultrasound in the Diagnosis of Acute Respiratory Failure: The BLUE Protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Pivetta, E.; Goffi, A.; Lupia, E.; Tizzani, M.; Porrino, G.; Ferreri, E.; Volpicelli, G.; Balzaretti, P.; Banderali, A.; Iacobucci, A.; et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: A SIMEU multicenter study. Chest 2015, 148, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Maw, A.M.; Hassanin, A.; Ho, P.M.; McInnes, M.D.F.; Moss, A.; Juarez-Colunga, E.; Soni, N.J.; Miglioranza, M.H.; Platz, E.; DeSanto, K.; et al. Diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: A systematic review and meta-analysis. JAMA Netw. Open 2019, 2, e190703. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Nazerian, P.; Castagno, D.; Tozzetti, C.; Tizzani, P.; Tizzani, M.; Porrino, G.; Ferreri, E.; Busso, V.; et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: A randomized controlled trial. Eur. J. Heart Fail. 2019, 21, 754–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picano, E.; Pellikka, P.A. Ultrasound of extravascular lung water: A new standard for pulmonary congestion. Eur. Heart J. 2016, 37, 2097–2104. [Google Scholar] [CrossRef] [Green Version]
- Kameda, T.; Kamiyama, N.; Taniguchi, N. Simple experimental models for elucidating the mechanism underlying vertical artifacts in lung ultrasound: Tools for revisiting B-Lines. Ultrasound Med. Biol. 2021, 47, 3543–3555. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Kamiyama, N.; Kobayashi, H.; Kanayama, Y.; Taniguchi, N. Ultrasonic B-Line–Like Artifacts Generated with Simple Experimental Models Provide Clues to Solve Key Issues in B-Lines. Ultrasound Med. Biol. 2019, 45, 1617–1626. [Google Scholar] [CrossRef]
- Matthias, I.; Panebianco, N.L.; Maltenfort, M.G.; Dean, A.J.; Baston, C. Effect of Machine Settings on Ultrasound Assessment of B-lines. J. Ultrasound Med. 2020, 40, 2039–2046. [Google Scholar] [CrossRef]
- Nugent, K.; Dobbe, L.; Rahman, R.; Elmassry, M.; Paz, P. Lung morphology and surfactant function in cardiogenic pulmonary edema: A narrative review. J. Thorac. Dis. 2019, 11, 4031–4038. [Google Scholar] [CrossRef]
- Storto, M.L.; Kee, S.T.; Golden, J.A.; Webb, W.R. Hydrostatic pulmonary edema: High-resolution CT findings. Am. J. Roentgenol. 1995, 165, 817–820. [Google Scholar] [CrossRef]
- Parekh, M.; Donuru, A.; Balasubramanya, R.; Kapur, S. Review of the Chest CT Differential Diagnosis of Ground-Glass Opacities in the COVID Era. Radiology 2020, 297, E289–E302. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mézière, G.; Biderman, P.; Gepner, A.; Barré, O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Gargani, L. Interstitial Syndrome. In Chest Sonography, 4th ed.; Mathis, G., Ed.; Springer: Cham, Switzerland, 2017; pp. 45–50. [Google Scholar]
- Soldati, G.; Demi, M.; Inchingolo, R.; Smargiassi, A.; Demi, L. On the Physical Basis of Pulmonary Sonographic Interstitial Syndrome. J. Ultrasound Med. 2016, 35, 2075–2086. [Google Scholar] [CrossRef]
- Demi, M. The impact of multiple concurrent factors on the length of the ultrasound pulmonary vertical artifacts as illustrated through the experimental and numerical analysis of simple models. J. Acoust. Soc. Am. 2021, 150, 2106. [Google Scholar] [CrossRef] [PubMed]
- Buda, N.; Skoczylas, A.; Demi, M.; Wojteczek, A.; Cylwik, J.; Soldati, G. Clinical Impact of Vertical Artifacts Changing with Frequency in Lung Ultrasound. Diagnostics 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Demi, M.; Prediletto, R.; Soldati, G.; Demi, L. Physical Mechanisms Providing Clinical Information from Ultrasound Lung Images: Hypotheses and Early Confirmations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 612–623. [Google Scholar] [CrossRef]
- Mathis, G.; Horn, R.; Morf, S.; Prosch, H.; Rovida, S.; Soldati, G.; Hoffmann, B.; Blaivas, M.; Dietrich, C.F. WFUMB position paper on reverberation artefacts in lung ultrasound: B-lines or comet-tails? Med. Ultrason. 2021, 23, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Mento, F.; Soldati, G.; Prediletto, R.; Demi, M.; Demi, L. Quantitative lung ultrasound spectroscopy applied to the diagnosis of pulmonary fibrosis: The first clinical study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 2265–2273. [Google Scholar] [CrossRef]
- Liu, J.; Guo, G.; Kurepa, D.; Volpicelli, G.; Sorantin, E.; Lovrenski, J.; Alonso-Ojembarrena, A.; Hsieh, K.-S.; Lodha, A.; Yeh, T.F.; et al. Specification and guideline for technical aspects and scanning parameter settings of neonatal lung ultrasound examination. J. Matern. Fetal Neonatal Med. 2021, 1–14. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.Y.; Yoon, J.H.; Youk, J.H.; Moon, H.J.; Son, E.J.; Kwak, J.Y.; Kim, E.-K. How to Find an Isoechoic Lesion with Breast US. RadioGraphics 2011, 31, 663–676. [Google Scholar] [CrossRef]
- Blüthgen, C.; Sanabria, S.; Frauenfelder, T.; Klingmüller, V.; Rominger, M. Economical Sponge Phantom for Teaching, Understanding, and Researching A- and B-Line Reverberation Artifacts in Lung Ultrasound. J. Ultrasound Med. 2017, 36, 2133–2142. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.L.; Fields, J.M.; Panebianco, N.L.; Jenq, K.Y.; Marin, J.; Dean, A.J. Inter-Rater Reliability of Quantifying Pleural B-Lines Using Multiple Counting Methods. J. Ultrasound Med. 2013, 32, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.A.; Minami, T.; Ma, I.W.Y.; Yasukawa, K. Lung Ultrasound for Pleural Line Abnormalities, Confluent B-Lines, and Consolidation: Expert reproducibility and a method of standardization. J. Ultrasound Med. 2021. [Google Scholar] [CrossRef]
- Schmickl, C.N.; Menon, A.A.; Dhokarh, R.; Seth, B.; Schembri, F. Optimizing B-lines on lung ultrasound: An in-vitro to in-vivo pilot study with clinical implications. J. Clin. Monit. Comput. 2020, 34, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.; Van Hoeve, W.; Van Sloun, R.J.G.; Soldati, G.; Demi, M. Determination of a potential quantitative measure of the state of the lung using lung ultrasound spectroscopy. Sci. Rep. 2017, 7, 12746. [Google Scholar] [CrossRef] [Green Version]
- Mento, F.; Demi, L. On the influence of imaging parameters on lung ultrasound B-line artifacts, in vitro study. J. Acoust. Soc. Am. 2020, 148, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Demi, L. Lung ultrasound: The future ahead and the lessons learned from COVID-19. J. Acoust. Soc. Am. 2020, 148, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Smargiassi, A.; Demi, L.; Inchingolo, R. Artifactual Lung Ultrasonography: It Is a Matter of Traps, Order, and Disorder. Appl. Sci. 2020, 10, 1570. [Google Scholar] [CrossRef] [Green Version]
- Tasci, O.; Hatipoglu, O.N.; Cagli, B.; Ermis, V. Sonography of the chest using linear-array versus sector transducers: Correlation with auscultation, chest radiography, and computed tomography. J. Clin. Ultrasound 2016, 44, 383–389. [Google Scholar] [CrossRef]
- Bobbia, X.; Chabannon, M.; Chevallier, T.; de La Coussaye, J.E.; Lefrant, J.Y.; Pujol, S.; Claret, P.-G.; Zieleskiewicz, L.; Roger, C.; Muller, L. Assessment of five different probes for lung ultrasound in critically ill patients: A pilot study. Am. J. Emerg. Med. 2018, 36, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.R.; De Vos, J.; Pisani, L.; Hagens, L.A.; Almondo, C.; Heijnen, N.F.L.; Schnabel, R.M.; Van Der Horst, I.C.C.; Bergmans, D.C.J.J.; Schultz, M.J.; et al. Comparison of Linear and Sector Array Probe for Handheld Lung Ultrasound in Invasively Ventilated ICU Patients. Ultrasound Med. Biol. 2020, 46, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T. Management of shock using point-of-care ultrasound. Jpn. J. Med. Ultrason. 2020, 47, 69–80. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kameda, T.; Kamiyama, N.; Taniguchi, N. The Mechanisms Underlying Vertical Artifacts in Lung Ultrasound and Their Proper Utilization for the Evaluation of Cardiogenic Pulmonary Edema. Diagnostics 2022, 12, 252. https://doi.org/10.3390/diagnostics12020252
Kameda T, Kamiyama N, Taniguchi N. The Mechanisms Underlying Vertical Artifacts in Lung Ultrasound and Their Proper Utilization for the Evaluation of Cardiogenic Pulmonary Edema. Diagnostics. 2022; 12(2):252. https://doi.org/10.3390/diagnostics12020252
Chicago/Turabian StyleKameda, Toru, Naohisa Kamiyama, and Nobuyuki Taniguchi. 2022. "The Mechanisms Underlying Vertical Artifacts in Lung Ultrasound and Their Proper Utilization for the Evaluation of Cardiogenic Pulmonary Edema" Diagnostics 12, no. 2: 252. https://doi.org/10.3390/diagnostics12020252
APA StyleKameda, T., Kamiyama, N., & Taniguchi, N. (2022). The Mechanisms Underlying Vertical Artifacts in Lung Ultrasound and Their Proper Utilization for the Evaluation of Cardiogenic Pulmonary Edema. Diagnostics, 12(2), 252. https://doi.org/10.3390/diagnostics12020252





