The Effect of Primary Aldosteronism on Carotid Artery Texture in Ultrasound Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Pressure Monitoring
2.3. Laboratory
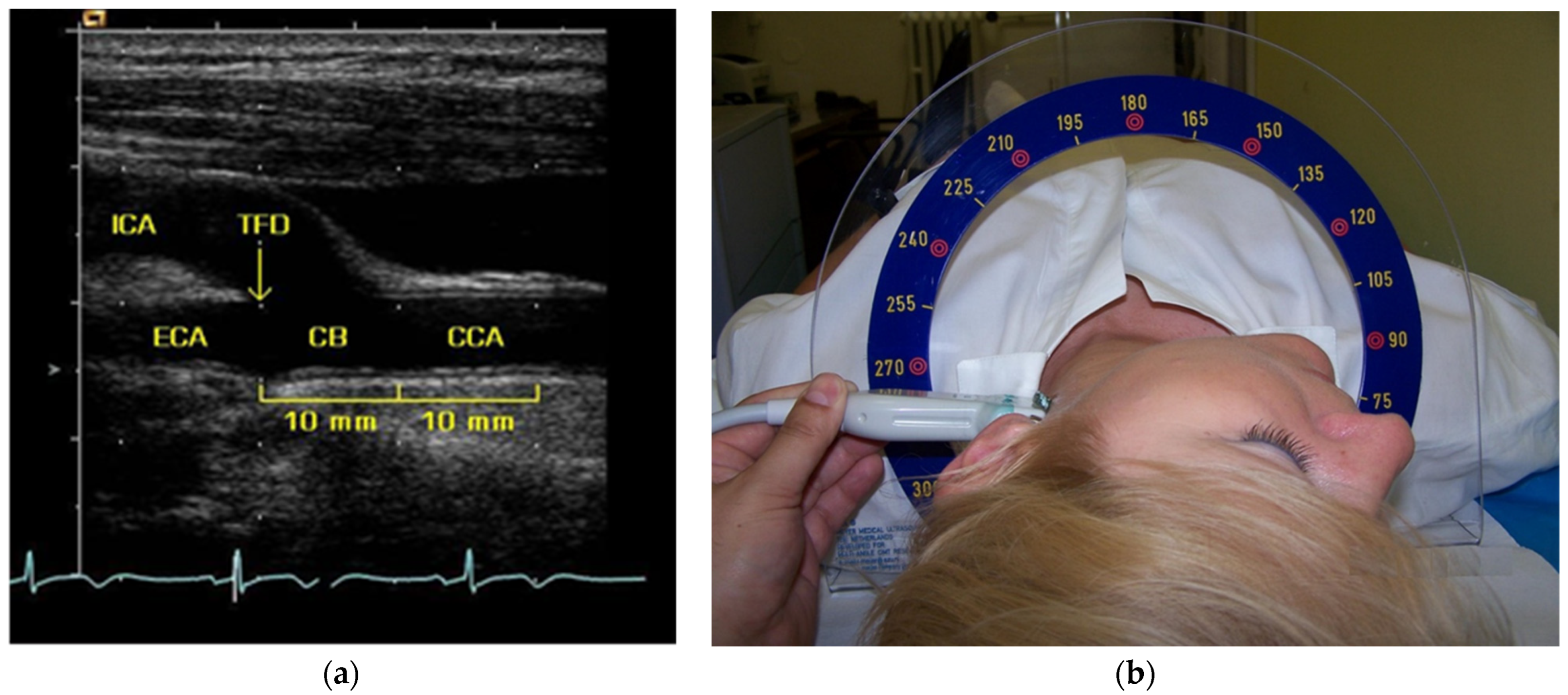
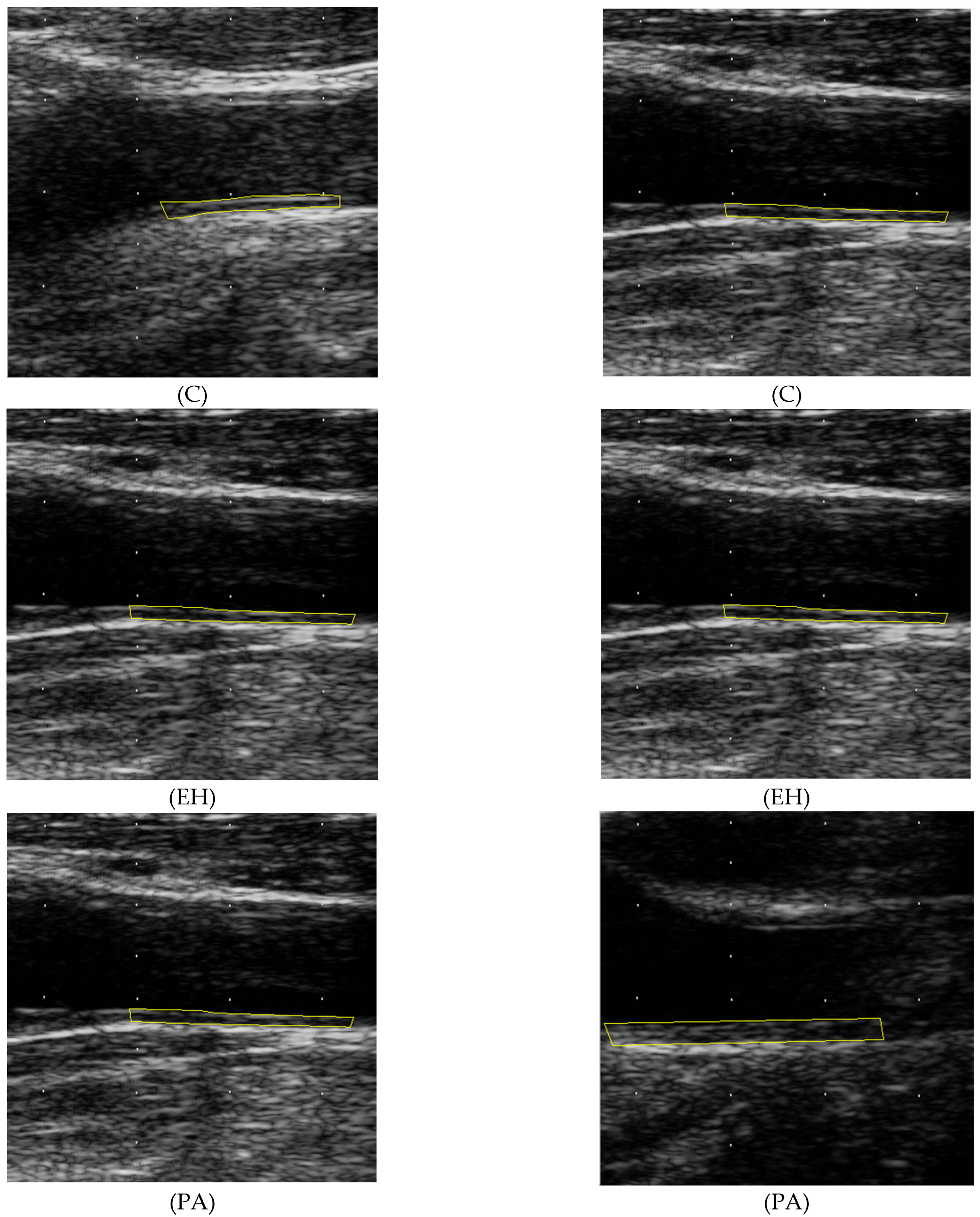
2.4. Carotid Ultrasound and IMT Measurement
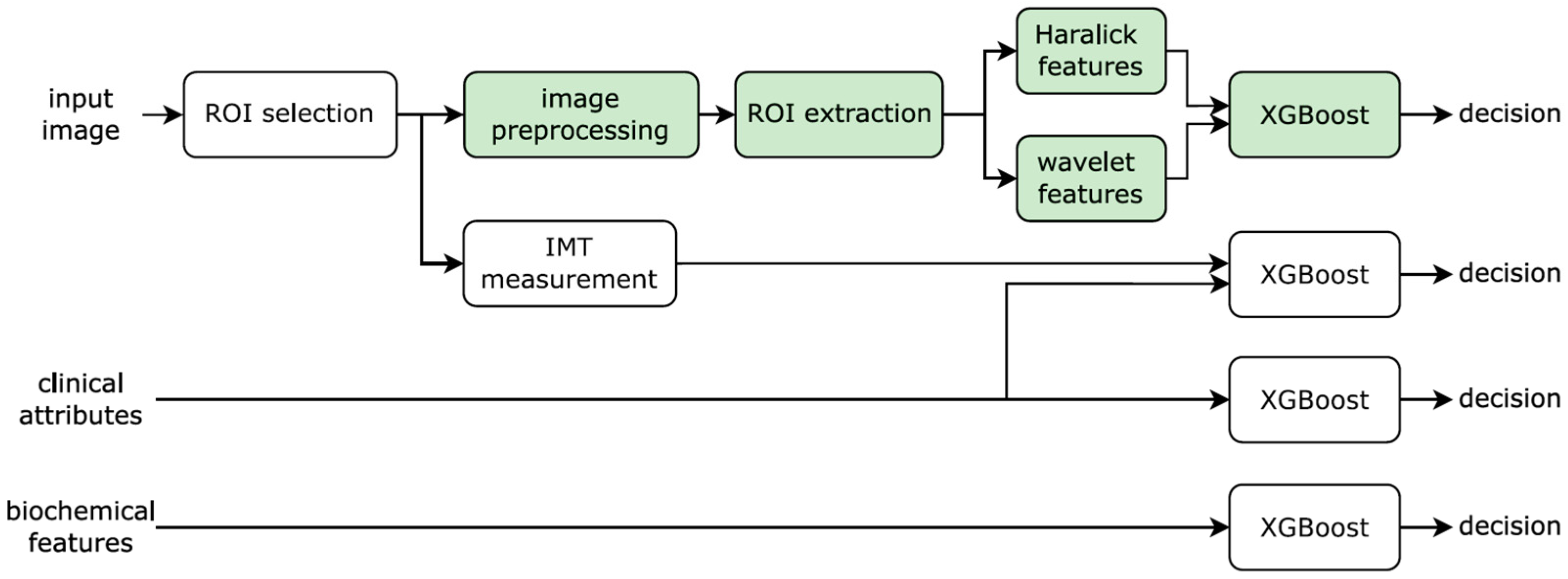
2.5. Texture Analysis
- •
- Inverse difference moment
- •
- Correlation
- •
- Contrast
- •
- Maximum
- •
- Energy
- •
- Dissimilarity
- •
- Entropy
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Study Groups
3.2. Statistical Significance Testing
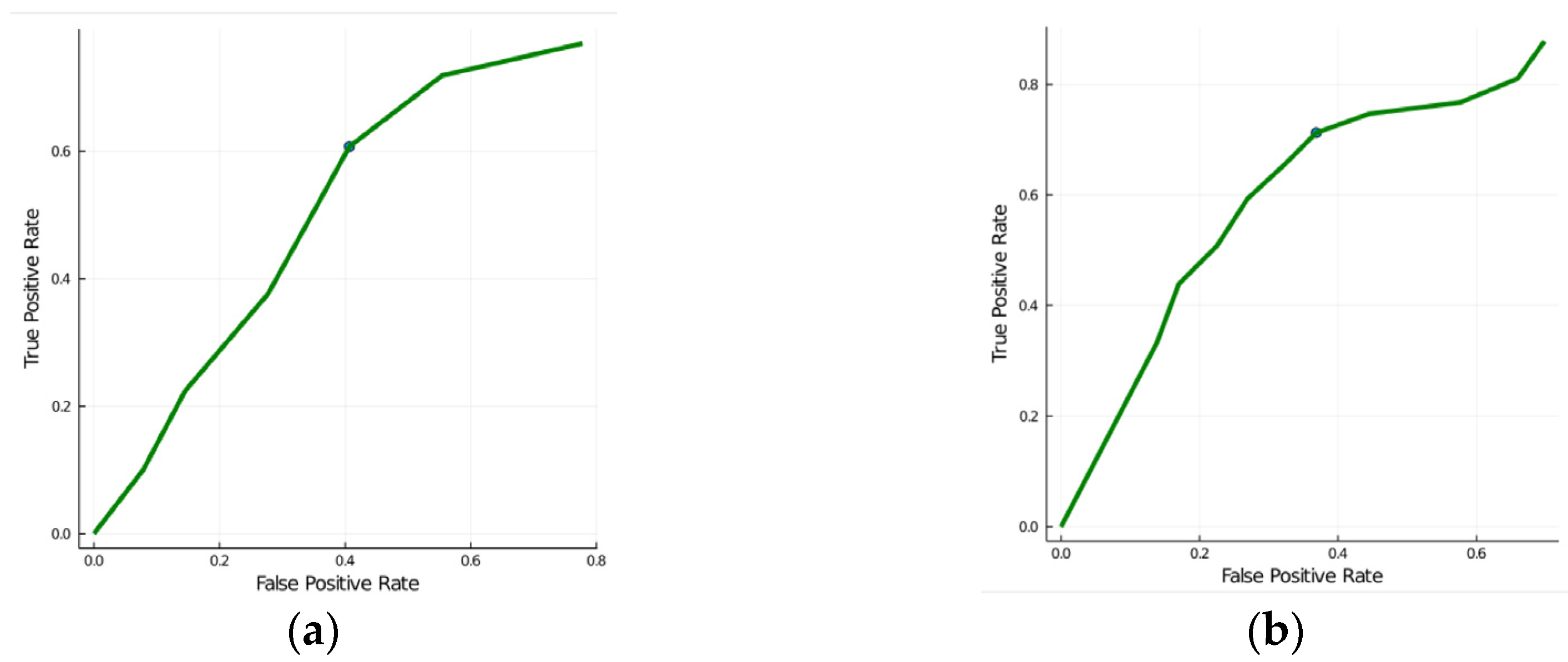
3.3. Classification Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.R.; Goldbourt, U.; Evans, G.; Pinsky, J.; Sharrett, A.R.; Sorlie, P.; Riley, W.; Heiss, G. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke A J. Cereb. Circ. 1996, 27, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Aronova, A.; Fahey, T.J., III; Zarnegar, R. Management of hypertension in primary aldosteronism. World J. Cardiol. 2014, 6, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Fardella, C.E.; Mosso, L.; Gomez-Sanchez, C.; Cortes, P.; Soto, J.; Gomez, L.; Pinto, M.; Huete, A.; Oestreicher, E.; Foradori, A.; et al. Primary hyperaldosteronism in essential hypertensives: Prevalence, biochemical profile, and molecular biology. J. Clin. Endocrinol. Metab. 2000, 85, 1863–1867. [Google Scholar] [CrossRef]
- Rossi, G.P.; Bernini, G.; Caliumi, C.; Desideri, G.; Fabris, B.; Ferri, C.; Ganzaroli, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J. Am. Coll. Cardiol. 2006, 48, 2293–2300. [Google Scholar] [CrossRef]
- Kayser, S.C.; Deinum, J.; de Grauw, W.J.; Schalk, B.W.; Bor, H.J.; Lenders, J.W.; Schermer, T.R.; Biermans, M.C. Prevalence of primary aldosteronism in primary care: A cross-sectional study. Br. J. Gen. Pract. 2018, 68, e114–e122. [Google Scholar] [CrossRef]
- Štrauch, B.; Zelinka, T.; Hampf, M.; Bernhardt, R.; Widimský, J., Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J. Hum. Hypertens. 2003, 17, 349–352. [Google Scholar] [CrossRef]
- Milliez, P.; Girerd, X.; Plouin, P.F.; Blacher, J.; Safar, M.E.; Mourad, J.J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef]
- Oberleithner, H.; Ludwig, T.; Riethmuller, C.; Hillebrand, U.; Albermann, L.; Schafer, C.; Shahin, V.; Schillers, H. Human endothelium: Target for aldosterone. Hypertension 2004, 43, 952–956. [Google Scholar] [CrossRef]
- Rizzoni, D.; Porteri, E.; Castellano, M.; Bettoni, G.; Muiesan, M.L.; Muiesan, P.; Giulini, S.M.; Agabiti-Rosei, E. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension 1996, 28, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Paiardi, S.; Rodella, L.; Porteri, E.; De Ciuceis, C.; Rezzani, R.; Boari, G.E.M.; Zani, F.; Miclini, M.; Tiberio, G.A.M.; et al. Changes in Extracellular Matrix in Subcutaneous Small Resistance Arteries of Patients with Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2006, 91, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Pignoli, P.; Tremoli, E.; Poli, A.; Oreste, P.; Paoletti, R. Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation 1986, 74, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; Sharrett, A.R.; Heiss, G.; Evans, G.W.; Chambless, L.E.; Riley, W.A.; Burke, G.L. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC Investigators. Stroke A J. Cereb. Circ. 1993, 24, 1297–1304. [Google Scholar] [CrossRef]
- Gariepy, J.; Massonneau, M.; Levenson, J.; Heudes, D.; Simon, A. Evidence for in vivo carotid and femoral wall thickening in human hypertension. Groupe de Prevention Cardio-vasculaire en Medecine du Travail. Hypertension 1993, 22, 111–118. [Google Scholar] [CrossRef]
- Psaty, B.M.; Furberg, C.D.; Kuller, L.H.; Borhani, N.O.; Rautaharju, P.M.; O’Leary, D.H.; Bild, D.E.; Robbins, J.; Fried, L.P.; Reid, C. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. Jama 1992, 268, 1287–1291. [Google Scholar] [CrossRef]
- Bots, M.L.; Hofman, A.; de Bruyn, A.M.; de Jong, P.T.; Grobbee, D.E. Isolated systolic hypertension and vessel wall thickness of the carotid artery. The Rotterdam Elderly Study. Arter. Thromb 1993, 13, 64–69. [Google Scholar] [CrossRef]
- Pit’ha, J.; Krajickova, D.; Cifkova, R.; Hubacek, J.; Petrzilkova, Z.; Hejl, Z.; Stavek, P.; Skibova, J.; Poledne, R. Intima-media thickness of carotid arteries in borderline hypertensives. J. Neuroimaging 1999, 9, 19–22. [Google Scholar] [CrossRef]
- Štrauch, B.; Petrák, O.; Wichterle, D.; Zelinka, T.; Holaj, R.; Widimský, J., Jr. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am. J. Hypertens. 2006, 19, 909–914. [Google Scholar] [CrossRef]
- Holaj, R.; Zelinka, T.; Wichterle, D.; Petrák, O.; Štrauch, B.; Widimský, J., Jr. Increased intima-media thickness of the common carotid artery in primary aldosteronism in comparison with essential hypertension. J. Hypertens. 2007, 25, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.C.; Korcarz, C.E.; Tattersall, M.C.; Gepner, A.D.; Young, R.L.; Post, W.S.; Kaufman, J.D.; McClelland, R.L.; Stein, J.H. Carotid artery ultrasound texture, cardiovascular risk factors, and subclinical arterial disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Br. J. Radiol. 2018, 91, 20170637. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Bots, M.L.; Evans, G.W.; Riley, W.A.; Grobbee, D.E. Carotid intima-media thickness measurements in intervention studies: Design options, progression rates, and sample size considerations: A point of view. Stroke A J. Cereb. Circ. 2003, 34, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Haralick, R.M. Statistical and structural approaches to texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar] [CrossRef]
- Unser, M. Texture classification and segmentation using wavelet frames. IEEE Trans. Image Process. 1995, 4, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd Acm Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Bernini, G.; Galetta, F.; Franzoni, F.; Bardini, M.; Taurino, C.; Bernardini, M.; Ghiadoni, L.; Bernini, M.; Santoro, G.; Salvetti, A. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J. Hypertens. 2008, 26, 2399–2405. [Google Scholar] [CrossRef]
- Smutek, D.; Sucharda, P.; Sara, R. Quantitative indicators of sonographic image of thyroid gland and their relation to antithyroid antibodies in Hashimoto’s lymphocytic thyroiditis. Stud. Health Technol. Inform. 2002, 90, 8–12. [Google Scholar] [CrossRef]
- Virmani, J.; Kumar, V.; Kalra, N.; Khandelwal, N. SVM-based characterization of liver ultrasound images using wavelet packet texture descriptors. J. Digit. Imaging 2013, 26, 530–543. [Google Scholar] [CrossRef]
- Berroug, J.; Korcarz, C.E.; Mitchell, C.K.; Weber, J.M.; Tian, L.; McDermott, M.M.; Stein, J.H. Brachial artery intima-media thickness and grayscale texture changes in patients with peripheral artery disease receiving supervised exercise training in the PROPEL randomized clinical trial. Vasc. Med. 2019, 24, 12–22. [Google Scholar] [CrossRef]
- Loizou, C.P.; Pantziaris, M.; Pattichis, M.S.; Kyriacou, E.; Pattichis, C.S. Ultrasound image texture analysis of the intima and media layers of the common carotid artery and its correlation with age and gender. Comput. Med. Imaging Graph. 2009, 33, 317–324. [Google Scholar] [CrossRef]
- Gordon, R.D.; Stowasser, M.; Rutherford, J.C. Primary aldosteronism: Are we diagnosing and operating on too few patients? World J. Surg. 2001, 25, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Murtza, I.; Hira, A.; Ali, S.; Kifayat, K. Robust spatial fuzzy GMM based MRI segmentation and carotid artery plaque detection in ultrasound images. Comput. Methods Programs Biomed. 2019, 175, 179–192. [Google Scholar] [CrossRef] [PubMed]
| Feature | Description |
|---|---|
| μ | mean intensity (n = 1) |
| σ | standard deviation of the intensity (n = 1) |
| Haralick texture descriptors (n = 5 × 4 × 7 = 140) | |
| d displacement in pixels (1, 2, 3, 4, and 5) | |
| Hd,θ,a | θ displacement direction (0°, 45°, 90°, and 135°) |
| a texture descriptor (1: inverse difference moment, 2: correlation, 3: contrast, 4: maximum, 5: energy, 6: dissimilarity, and 7: entropy) | |
| Wavelet texture descriptors (n = (3 × 3) + 1 = 10) | |
| i decomposition level (1, 2, and 3) | |
| Wi,j | j filter orientation (1: GxGx, 2: HxGy, and 3: GxHy) |
| W0 | low-pass subband wavelet energy |
| Primary Aldosteronism (n = 33) | Essential Hypertension (n = 52) | Hypertensive Patients (n = 85) | Normotensive Controls (n = 33) | |
|---|---|---|---|---|
| Age (years) | 57 ± 8 | 55 ± 7 | 56 ± 7 | 54 ± 8 |
| Gender (F/M (%F)) | 13/20 (39) | 20/32 (38) | 33/52 (39) | 16/17 (48) |
| Body mass index (kg·m−2) | 29.0 ± 3.9 # | 28.4 ± 4.6 # | 28.7±4.3 # | 26.6 ± 4.2 |
| Office systolic blood pressure (mm Hg) | 164 ± 23 ## | 170 ± 30 ## | 168±28 ## | 125 ±15 |
| Office diastolic blood pressure (mm Hg) | 97 ± 14 ## | 99 ± 17 ## | 98±16 ## | 78 ± 9 |
| Current cigarette smoking (n (%)) | 11 (33) | 20 (38) | 31 (36) | 8 (24) |
| Lipid lowering medication (n (%)) | 10 (30) | 21 (40) | 31 (36) | 9 (27) |
| Diabetes mellitus (n (%)) | 8 (24) | 11 (21) | 19 (22) | - |
| Plasma cholesterol (mmol/L) | 4.98 ± 1.05 # | 5.28 ± 1.00 # | 5.16 ± 1.02 # | 5.59 ± 0.97 |
| LDL cholesterol (mmol/L) | 2.99 ± 0.87 | 3.06 ± 0.77 | 3.03 ± 0.81 | 3.19 ± 0.84 |
| HDL cholesterol (mmol/L) | 1.30 ± 0.34 # | 1.38 ± 0.33 # | 1.35 ± 0.34 # | 1.61 ± 0.40 |
| Triglycerides (mmol/L) | 1.54 ± 0.54 | 1.85 ± 0.97 | 1.73 ± 1.02 | 1.73 ± 1.02 |
| Fasting plasma glucose (mmol/L) | 4.9 ± 0.5 | 5.2 ± 0.9 | 5.1 ± 0.8 | 5.0 ± 0.6 |
| Plasma potassium (mmol/L) | 3.6 ± 0.5 **,## | 4.0 ± 0.3 ## | 3.8 ± 0.4 ## | 4.4 ± 0.4 |
| Plasma sodium (mmol/L) | 143 ± 3 | 142 ± 3 | 143 ± 3 | 141 ± 2 |
| Plasma aldosterone-upright (ng/dL) | 38.9 (27.4–64.9) ** | 15.7 (9.2–23.1) | NA | |
| Plasma renin activity-upright (ng/mL per h) | 0.36 (0.25–0.56) ** | 0.66 (0.37–1.68) | NA | |
| Aldosterone to plasma renin activity ratio-upright (ng/dL)/(ng/mL per h) | 103 (69–157) ** | 17 (8–41) | NA | |
| Urine potassium/day (mmol/24 h) | 62 (47–100) * | 46 (31–58) | NA | |
| CCA IMT mean-max (mm) | 0.987 ± 0.152 *,## | 0.892 ± 0.155 # | 0.93 ± 0.16 # | 0.812 ± 0.126 |
| CB IMT mean-max (mm) | 1.157 ± 0.243 # | 1.131 ± 0.275 # | 1.14 ± 0.26 # | 0.994 ± 0.203 |
| Combined IMT mean-max (mm) | 1.066 ± 0.162 ## | 0.974 ± 0.196 # | 1.01 ± 0.19 # | 0.884 ± 0.141 |
| Primary Aldosteronism (n = 33) | Essential Hypertension (n = 52) | |
|---|---|---|
| Estimated duration of hypertension (years) | 11.5 ± 7.8 | 14.5 ± 11.2 |
| Chronic antihypertensive therapy | ||
| Diuretics (n (%)) | 20 (61) | 31 (60) |
| β-Blockers (n (%)) | 20 (61) | 39 (75) |
| Calcium channel blockers (n (%)) | 28 (85) | 41 (79) |
| Angiotensin-converting enzyme inhibitors (n (%)) | 19 (58) | 33 (63) |
| Angiotensin receptor blockers (n (%)) | 15 (45) | 18 (35) |
| α-Blockers (n (%)) | 8 (24) | 17 (33) |
| Central agonists (n (%)) | 18 (55) | 23 (44) |
| Aldosterone antagonists | - | - |
| Number of antihypertensive drugs | 4.0 ± 1.5 | 4.0 ± 1.8 |
| Blood pressure after discontinuation of therapy | ||
| Office systolic blood pressure (mm Hg) | 164 ± 23 | 170 ± 30 |
| Office diastolic blood pressure (mm Hg) | 97 ± 14 | 99 ± 17 |
| Mean 24 h systolic blood pressure (mm Hg) | 150 ± 14 | 148 ± 15 |
| Mean 24 h diastolic blood pressure (mm Hg) | 90 ± 8 | 89 ± 10 |
| PA Versus EH | PA + EH Versus Controls | |||||
|---|---|---|---|---|---|---|
| Parameter | t-Test | KS Test | MW Test | t-Test | KS Test | MW Test |
| Plasma aldosterone | <0.001 | <0.001 | <0.001 | NA | NA | NA |
| ARR | <0.001 | <0.001 | <0.001 | NA | NA | NA |
| Plasma renin activity | 0.004 | 0.002 | <0.001 | NA | NA | NA |
| CCA–IMT | 0.007 | 0.012 | 0.013 | <0.001 | <0.001 | <0.001 |
| Combined mean IMT | 0.022 | 0.025 | <0.001 | 0.008 | 0.001 | |
| Plasma cholesterol | 0.041 | |||||
| HDL cholesterol | 0.002 | 0.004 | <0.001 | |||
| Systolic blood pressure | <0.001 | <0.001 | <0.001 | |||
| Body mass index | 0.024 | 0.019 | ||||
| Diastolic blood pressure | <0.001 | <0.001 | <0.001 | |||
| CB–IMT | 0.004 | 0.016 | ||||
| PA Versus EH | PA + EH Versus Controls | |||||
|---|---|---|---|---|---|---|
| Parameter | t-Test | KS Test | MW Test | t-Test | KS Test | MW Test |
| W2,2 | <0.001 | <0.001 | <0.001 | |||
| W3,3 | <0.001 | 0.034 | 0.003 | |||
| W3,2 | <0.001 | <0.001 | <0.001 | |||
| W3,1 | <0.001 | 0.001 | <0.001 | |||
| H1,0,2 | 0.001 | <0.001 | <0.001 | |||
| H1,135,2 | 0.001 | <0.001 | <0.001 | |||
| W2,3 | <0.001 | 0.017 | 0.005 | |||
| H2,135,2 | <0.001 | <0.001 | <0.001 | |||
| H2,0,2 | <0.001 | <0.001 | <0.001 | |||
| W0 | <0.001 | <0.001 | <0.001 | |||
| Accuracy | ||
|---|---|---|
| Parameters | PA Versus EH | PA + EH Versus Controls |
| Clinical characteristics | 0.57 | 0.89 |
| Clinical characteristics + IMT parameters | 0.63 | 0.92 |
| Texture features | 0.73 | 0.66 |
| “Aldo” parameters | 0.92 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushik, S.; Majtan, B.; Holaj, R.; Baručić, D.; Kološová, B.; Widimský, J., Jr.; Kybic, J. The Effect of Primary Aldosteronism on Carotid Artery Texture in Ultrasound Images. Diagnostics 2022, 12, 3206. https://doi.org/10.3390/diagnostics12123206
Kaushik S, Majtan B, Holaj R, Baručić D, Kološová B, Widimský J Jr., Kybic J. The Effect of Primary Aldosteronism on Carotid Artery Texture in Ultrasound Images. Diagnostics. 2022; 12(12):3206. https://doi.org/10.3390/diagnostics12123206
Chicago/Turabian StyleKaushik, Sumit, Bohumil Majtan, Robert Holaj, Denis Baručić, Barbora Kološová, Jiří Widimský, Jr., and Jan Kybic. 2022. "The Effect of Primary Aldosteronism on Carotid Artery Texture in Ultrasound Images" Diagnostics 12, no. 12: 3206. https://doi.org/10.3390/diagnostics12123206
APA StyleKaushik, S., Majtan, B., Holaj, R., Baručić, D., Kološová, B., Widimský, J., Jr., & Kybic, J. (2022). The Effect of Primary Aldosteronism on Carotid Artery Texture in Ultrasound Images. Diagnostics, 12(12), 3206. https://doi.org/10.3390/diagnostics12123206





