Individualized Management of Coagulopathy in Patients with End-Stage Liver Disease
Abstract
:1. End-Stage Liver Disease (ESLD)
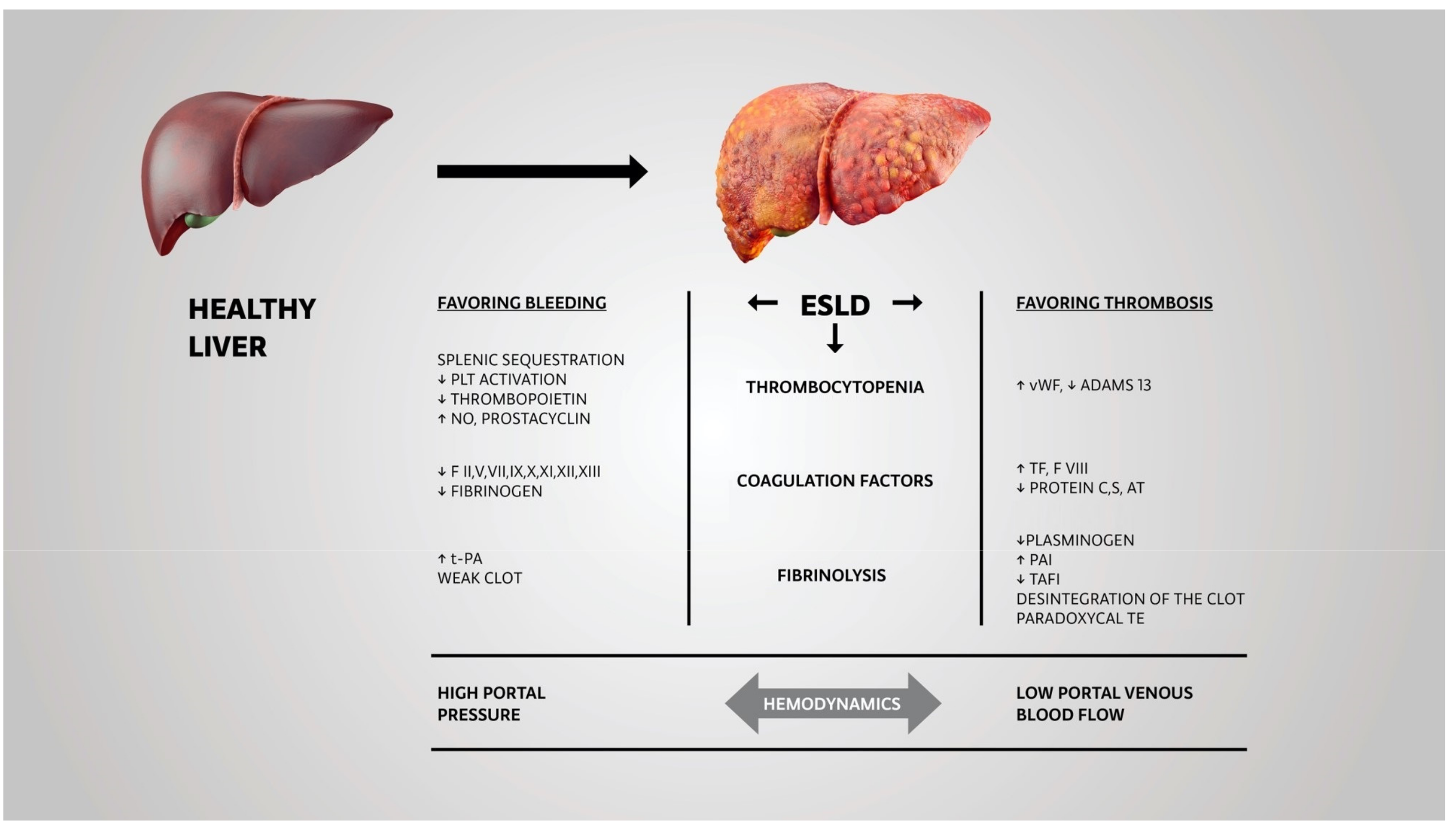
2. Pathophysiology of Deranged Hemostasis in End-Stage Liver Disease
3. Difficulties with Evaluating the Coagulopathies in ESLD Patients
4. Use of Viscoelastic Tests Assays
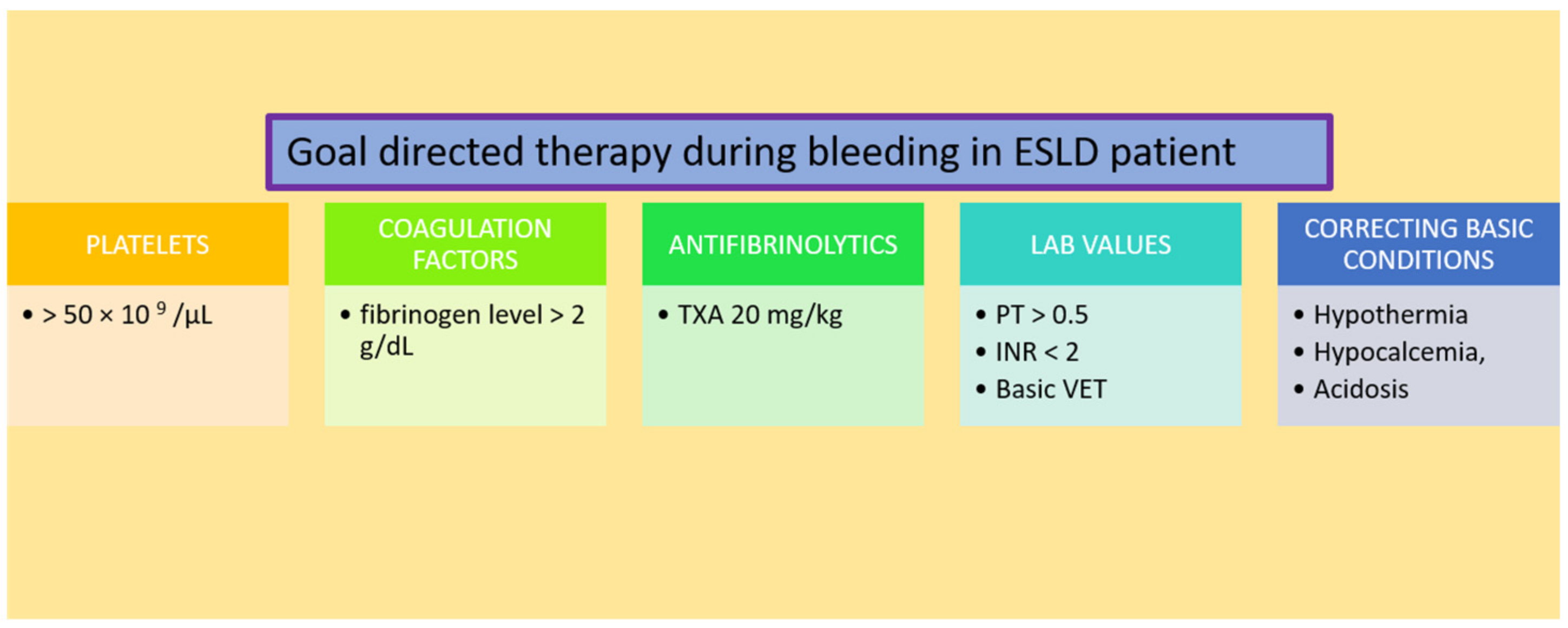
5. Timely Management of Coagulopathy by Using Coagulation Factors
5.1. Platelets
5.2. Coagulation Factors
5.3. Antifibrinolytics
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corrao, G.; Ferrari, P.; Zambon, A.; Torchio, P.; Aricò, S.; Decarli, A. Trends of Liver Cirrhosis Mortality in Europe, 1970–1989: Age-Period-Cohort Analysis and Changing Alcohol Consumption. Int. J. Epidemiol. 1997, 26, 100–109. [Google Scholar] [CrossRef]
- Ye, F.; Zhai, M.; Long, J.; Gong, Y.; Ren, C.; Zhang, D.; Lin, X.; Liu, S. The Burden of Liver Cirrhosis in Mortality: Results from the Global Burden of Disease Study. Front. Public Health 2022, 10, 909455. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, J.; Kühne, M.; Pradat, P.; Mössner, J.; Trepo, C.; Tillmann, H.L. Different Patterns of Decompensation in Patients with Alcoholic vs. Non-Alcoholic Liver Cirrhosis. Aliment. Pharmacol. Ther. 2012, 35, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Choudhury, A. Management of Acute-on-Chronic Liver Failure: An Algorithmic Approach. Hepatol. Int. 2018, 12, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Sakiani, S.; Heller, T.; Koh, C. Current and Investigational Drugs in Early Clinical Development for Portal Hypertension. Front. Med. 2022, 9, 974182. [Google Scholar] [CrossRef]
- Northup, P.G.; Caldwell, S.H. Coagulation in Liver Disease: A Guide for the Clinician. Clin. Gastroenterol. Hepatol. 2013, 11, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Martin Mateos, R.; Garcia de la Filia Molina, I.; Albillos, A. Pre-Surgical Risk Assessment in Patients with Cirrhosis. Acta Gastroenterol. Belg. 2020, 83, 449–453. [Google Scholar]
- Sakai, T. Viscoelastic Testing in Liver Transplantation. Transfusion 2020, 60 (Suppl. S6), S61–S69. [Google Scholar] [CrossRef]
- Maheshwari, A.; Bajpai, M.; Patidar, G.K. Effects of Therapeutic Plasma Exchange on Liver Function Test and Coagulation Parameters in Acute Liver Failure Patients. Hematol. Transfus. Cell Ther. 2020, 42, 125–128. [Google Scholar] [CrossRef]
- Schaden, E.; Saner, F.H.; Goerlinger, K. Coagulation Pattern in Critical Liver Dysfunction . Curr. Opin. Crit. Care 2013, 19, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Tripodi, A.; Mannucci, P.M. The Coagulopathy of Chronic Liver Disease. N. Engl. J. Med. 2011, 365, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.; Monroe, D.M. A Cell-Based Model of Hemostasis. Thromb. Haemost. 2001, 85, 958–965. [Google Scholar] [PubMed]
- Saner, F.H.; Gieseler, R.K.; Akız, H.; Canbay, A.; Görlinger, K. Delicate Balance of Bleeding and Thrombosis in End-Stage Liver Disease and Liver Transplantation. Digestion 2013, 88, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Pereboom, I.T.A.; Adelmeijer, J.; van Leeuwen, Y.; Hendriks, H.G.D.; Porte, R.J.; Lisman, T. No Evidence for Systemic Platelet Activation during or after Orthotopic Liver Transplantation: Platelet Activation During Liver Transplantation. Liver Transpl. 2009, 15, 956–962. [Google Scholar] [CrossRef]
- Lisman, T.; Bongers, T.N.; Adelmeijer, J.; Janssen, H.L.A.; de Maat, M.P.M.; de Groot, P.G.; Leebeek, F.W.G. Elevated Levels of von Willebrand Factor in Cirrhosis Support Platelet Adhesion despite Reduced Functional Capacity. Hepatology 2006, 44, 53–61. [Google Scholar] [CrossRef]
- Napolitano, G.; Iacobellis, A.; Merla, A.; Niro, G.; Valvano, M.R.; Terracciano, F.; Siena, D.; Caruso, M.; Ippolito, A.; Mannuccio, P.M.; et al. Bleeding after Invasive Procedures Is Rare and Unpredicted by Platelet Counts in Cirrhotic Patients with Thrombocytopenia. Eur. J. Intern. Med. 2017, 38, 79–82. [Google Scholar] [CrossRef]
- Forkin, K.T.; Colquhoun, D.A.; Nemergut, E.C.; Huffmyer, J.L. The Coagulation Profile of End-Stage Liver Disease and Considerations for Intraoperative Management. Anesth. Analg. 2018, 126, 46–61. [Google Scholar] [CrossRef]
- Tripodi, A.; Salerno, F.; Chantarangkul, V.; Clerici, M.; Cazzaniga, M.; Primignani, M.; Mannuccio Mannucci, P. Evidence of Normal Thrombin Generation in Cirrhosis despite Abnormal Conventional Coagulation Tests. Hepatology 2005, 41, 553–558. [Google Scholar] [CrossRef]
- Valla, D.-C.; Rautou, P.-E. The Coagulation System in Patients with End-Stage Liver Disease. Liver Int. 2015, 35 (Suppl. S1), 139–144. [Google Scholar] [CrossRef]
- Saner, F.H.; Kirchner, C. Monitoring and Treatment of Coagulation Disorders in End-Stage Liver Disease. Visc. Med. 2016, 32, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Søgaard, K.K.; Horváth-Puhó, E.; Grønbæk, H.; Jepsen, P.; Vilstrup, H.; Sørensen, H.T. Risk of Venous Thromboembolism in Patients with Liver Disease: A Nationwide Population-Based Case–Control Study. Am. J. Gastroenterol. 2009, 104, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Z.; Panagou, M.; Patch, D.; Bates, S.; Osman, E.; Pasi, J.; Burroughs, A. Hypercoagulability in Patients with Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis Evaluated by Thrombelastography. J. Hepatol. 1997, 26, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Zermatten, M.G.; Fraga, M.; Calderara, D.B.; Aliotta, A.; Moradpour, D.; Alberio, L. Biomarkers of Liver Dysfunction Correlate with a Prothrombotic and Not with a Prohaemorrhagic Profile in Patients with Cirrhosis. JHEP Rep. 2020, 2, 100120. [Google Scholar] [CrossRef]
- Zanetto, A.; Campello, E.; Bulato, C.; Gavasso, S.; Saggiorato, G.; Shalaby, S.; Spiezia, L.; Cillo, U.; Farinati, F.; Russo, F.P.; et al. More Pronounced Hypercoagulable State and Hypofibrinolysis in Patients with Cirrhosis with versus without HCC. Hepatol. Commun. 2021, 5, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Kundu, S.; Jha, S.B.; Rivera, A.P.; Flores Monar, G.V.; Islam, H.; Puttagunta, S.M.; Sange, I. Cirrhosis and Coagulopathy: Mechanisms of Hemostasis Changes in Liver Failure and Their Management. Cureus 2022, 14, e23785. [Google Scholar] [CrossRef] [PubMed]
- Schepis, F.; Turco, L.; Bianchini, M.; Villa, E. Prevention and Management of Bleeding Risk Related to Invasive Procedures in Cirrhosis. Semin. Liver Dis. 2018, 38, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Montalvá, E.; Rodríguez-Perálvarez, M.; Blasi, A.; Bonanad, S.; Gavín, O.; Hierro, L.; Lladó, L.; Llop, E.; Pozo-Laderas, J.C.; Colmenero, J.; et al. Consensus Statement on Hemostatic Management, Anticoagulation, and Antiplatelet Therapy in Liver Transplantation. Transplantation 2022, 106, 1123–1131. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, H.; Ikram, S.; Sarfraz, S.; Yousaf, A. Spontaneous Bilateral Subdural Hematomas in a Patient with Cryptogenic Liver Cirrhosis. Cureus 2021, 13, e16100. [Google Scholar] [CrossRef]
- Crager, S. Critically Ill Patients with End-Stage Liver Disease. Emerg. Med. Clin. N. Am. 2019, 37, 511–527. [Google Scholar] [CrossRef]
- Kovacs, T.O.G.; Jensen, D.M. Varices. Clin. Liver Dis. 2019, 23, 625–642. [Google Scholar] [CrossRef]
- Radadiya, D.; Devani, K.; Rockey, D.C. The Impact of Red Blood Cell Transfusion Practices on Inpatient Mortality in Variceal and Non-variceal Gastrointestinal Bleeding Patients: A 20-year US Nationwide Retrospective Analysis. Aliment. Pharmacol. Ther. 2022, 56, 41–55. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.G.; Greenberg, C.S.; Patton, H.M.; Caldwell, S.H. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology 2019, 157, 34–43.e1. [Google Scholar] [CrossRef] [PubMed]
- Markin, N.W.; Ringenberg, K.J.; Kassel, C.A.; Walcutt, C.R.; Chacon, M.M. 2018 Clinical Update in Liver Transplantation. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.B.; Dzik, W.H. Transfusion Medicine/Hemostasis Clinical Trials Network1 Paucity of Studies to Support That Abnormal Coagulation Test Results Predict Bleeding in the Setting of Invasive Procedures: An Evidence-Based Review. Transfusion 2005, 45, 1413–1425. [Google Scholar] [CrossRef]
- Saner, F.H.; Bezinover, D. Assessment and Management of Coagulopathy in Critically-Ill Patients with Liver Failure. Curr. Opin. Crit. Care 2019, 25, 179–186. [Google Scholar] [CrossRef]
- Buliarca, A.; Horhat, A.; Mocan, T.; Craciun, R.; Procopet, B.; Sparchez, Z. Viscoelastic Tests in Liver Disease: Where Do We Stand Now? World J. Gastroenterol. 2021, 27, 3290–3302. [Google Scholar] [CrossRef]
- Nguyen, G.; Lejeune, M.; Crichi, B.; Frere, C. Hemostasis Testing in Patients with Liver Dysfunction: Advantages and Caveats. World J. Gastroenterol. 2021, 27, 7285–7298. [Google Scholar] [CrossRef]
- Northup, P.; Reutemann, B. Management of Coagulation and Anticoagulation in Liver Transplantation Candidates. Liver Transpl. 2018, 24, 1119–1132. [Google Scholar] [CrossRef] [Green Version]
- Lisman, T.; Porte, R.J. Pathogenesis, Prevention, and Management of Bleeding and Thrombosis in Patients with Liver Diseases. Res. Pract. Thromb. Haemost. 2017, 1, 150–161. [Google Scholar] [CrossRef]
- Bienholz, A.; Canbay, A.; Saner, F.H. Coagulation management in patients with liver disease. Med. Klin. Intensivmed. Notfmed. 2016, 111, 224–234. [Google Scholar] [CrossRef]
- McMurry, H.S.; Jou, J.; Shatzel, J. The Hemostatic and Thrombotic Complications of Liver Disease. Eur. J. Haematol. 2021, 107, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.H.; Möhlmann, M.; Herrmann, E.; Schneider, S.; Zacharowski, K.; Zeuzem, S.; Weber, C.F.; Weiler, N. Assessment of Hemostatic Profile in Patients with Mild to Advanced Liver Cirrhosis. World J. Gastroenterol. 2020, 26, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Saner, F.H.; Abeysundara, L.; Hartmann, M.; Mallett, S.V. Rational Approach to Transfusion in Liver Transplantation. Minerva Anestesiol. 2018, 84, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, B.; Li, H.; Deng, H.; Méndez-Sánchez, N.; Guo, X.; Qi, X. Association of Coagulopathy with the Risk of Bleeding after Invasive Procedures in Liver Cirrhosis. Saudi J. Gastroenterol. 2018, 24, 220–227. [Google Scholar] [CrossRef]
- Shah, A.; Amarapurkar, D.; Dharod, M.; Chandnani, M.; Baijal, R.; Kumar, P.; Jain, M.; Patel, N.; Kamani, P.; Gautam, S.; et al. Coagulopathy in Cirrhosis: A Prospective Study to Correlate Conventional Tests of Coagulation and Bleeding Following Invasive Procedures in Cirrhotics. Indian J. Gastroenterol. 2015, 34, 359–364. [Google Scholar] [CrossRef]
- Janko, N.; Majeed, A.; Commins, I.; Kemp, W.; Roberts, S.K. Procedural Bleeding Risk, Rather than Conventional Coagulation Tests, Predicts Procedure Related Bleeding in Cirrhosis. Eur. J. Gastroenterol. Hepatol. 2022, 34, 192–199. [Google Scholar] [CrossRef]
- Harrison, M.F. The Misunderstood Coagulopathy of Liver Disease: A Review for the Acute Setting. West. J. Emerg. Med. 2018, 19, 863–871. [Google Scholar] [CrossRef]
- Haas, T.; Fries, D.; Tanaka, K.A.; Asmis, L.; Curry, N.S.; Schöchl, H. Usefulness of Standard Plasma Coagulation Tests in the Management of Perioperative Coagulopathic Bleeding: Is There Any Evidence? Br. J. Anaesth. 2015, 114, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Child, L.J. Clinical Utility of Viscoelastic Testing in Chronic Liver Disease: A Systematic Review. World J. Hepatol. 2020, 12, 1115–1127. [Google Scholar] [CrossRef]
- Curry, N.S.; Davenport, R.; Pavord, S.; Mallett, S.V.; Kitchen, D.; Klein, A.A.; Maybury, H.; Collins, P.W.; Laffan, M. The Use of Viscoelastic Haemostatic Assays in the Management of Major Bleeding: A British Society for Haematology Guideline. Br. J. Haematol. 2018, 182, 789–806. [Google Scholar] [CrossRef] [Green Version]
- Lawson, P.J.; Moore, H.B.; Moore, E.E.; Stettler, G.R.; Pshak, T.J.; Kam, I.; Silliman, C.C.; Nydam, T.L. Preoperative Thrombelastography Maximum Amplitude Predicts Massive Transfusion in Liver Transplantation. J. Surg. Res. 2017, 220, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, M.; Anand, A.C. Overview of Complications in Cirrhosis. J. Clin. Exp. Hepatol. 2022, 12, 1150–1174. [Google Scholar] [CrossRef] [PubMed]
- Hartert, H. Blutgerinnungsstudien mit der Thrombelastographie, einem neuen Untersuchungsverfahren. Klin. Wochenschr. 1948, 26, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Spring, A.; Saran, J.S.; McCarthy, S.; McCluskey, S.A. Anesthesia for the Patient with Severe Liver Failure. Anesthesiol. Clin. 2020, 38, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Shamseddeen, H.; Patidar, K.R.; Ghabril, M.; Desai, A.P.; Nephew, L.; Kuehl, S.; Chalasani, N.; Orman, E.S. Features of Blood Clotting on Thromboelastography in Hospitalized Patients with Cirrhosis. Am. J. Med. 2020, 133, 1479–1487.e2. [Google Scholar] [CrossRef]
- Kohli, R.; Shingina, A.; New, S.; Chaturvedi, S.; Benson, A.; Biggins, S.W.; Bambha, K. Thromboelastography Parameters Are Associated with Cirrhosis Severity. Dig. Dis. Sci. 2019, 64, 2661–2670. [Google Scholar] [CrossRef]
- Bedreli, S.; Sowa, J.-P.; Gerken, G.; Saner, F.H.; Canbay, A. Management of Acute-on-Chronic Liver Failure: Rotational Thromboelastometry May Reduce Substitution of Coagulation Factors in Liver Cirrhosis. Gut 2016, 65, 357–358. [Google Scholar] [CrossRef]
- De Pietri, L.; Bianchini, M.; Montalti, R.; De Maria, N.; Di Maira, T.; Begliomini, B.; Gerunda, G.E.; di Benedetto, F.; Garcia-Tsao, G.; Villa, E. Thrombelastography-Guided Blood Product Use before Invasive Procedures in Cirrhosis with Severe Coagulopathy: A Randomized, Controlled Trial: Thrombelastography-Guided Blood Product. Hepatology 2016, 63, 566–573. [Google Scholar] [CrossRef] [Green Version]
- Blaine, K.P.; Sakai, T. Viscoelastic Monitoring to Guide Hemostatic Resuscitation in Liver Transplantation Surgery. Semin. Cardiothorac. Vasc. Anesth. 2018, 22, 150–163. [Google Scholar] [CrossRef]
- Kang, Y.G.; Martin, D.J.; Marquez, J.; Lewis, J.H.; Bontempo, F.A.; Shaw, B.W.; Starzl, T.E.; Winter, P.M. Intraoperative Changes in Blood Coagulation and Thrombelastographic Monitoring in Liver Transplantation. Anesth. Analg. 1985, 64, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-C.; Shieh, J.-F.; Chang, K.-Y.; Chu, Y.-C.; Liu, C.-S.; Loong, C.-C.; Chan, K.-H.; Mandell, S.; Tsou, M.-Y. Thromboelastography-Guided Transfusion Decreases Intraoperative Blood Transfusion During Orthotopic Liver Transplantation: Randomized Clinical Trial. Transplant. Proc. 2010, 42, 2590–2593. [Google Scholar] [CrossRef]
- Leon-Justel, A.; Noval-Padillo, J.A.; Alvarez-Rios, A.I.; Mellado, P.; Gomez-Bravo, M.A.; Álamo, J.M.; Porras, M.; Barrero, L.; Hinojosa, R.; Carmona, M.; et al. Point-of-Care Haemostasis Monitoring during Liver Transplantation Reduces Transfusion Requirements and Improves Patient Outcome. Clin. Chim. Acta 2015, 446, 277–283. [Google Scholar] [CrossRef]
- Al Moosawi, M.; Trudeau, J.; Smith, T.; Lefebvre, A.; Shih, A.W. ROTEM in the Setting of Liver Transplant Surgery Reduces Frozen Plasma Transfusion. Transfus. Apher. Sci. 2021, 60, 103125. [Google Scholar] [CrossRef]
- Gaspari, R.; Teofili, L.; Aceto, P.; Valentini, C.G.; Punzo, G.; Sollazzi, L.; Agnes, S.; Avolio, A.W. Thromboelastography Does Not Reduce Transfusion Requirements in Liver Transplantation: A Propensity Score-Matched Study. J. Clin. Anesth. 2021, 69, 110154. [Google Scholar] [CrossRef]
- Hashir, A.; Singh, S.A.; Krishnan, G.; Subramanian, R.; Gupta, S. Correlation of Early ROTEM Parameters with Conventional Coagulation Tests in Patients with Chronic Liver Disease Undergoing Liver Transplant. Indian J. Anaesth. 2019, 63, 21–25. [Google Scholar] [CrossRef]
- Görlinger, K.; Dirkmann, D.; Solomon, C.; Hanke, A.A. Fast Interpretation of Thromboelastometry in Non-Cardiac Surgery: Reliability in Patients with Hypo-, Normo-, and Hypercoagulability. Br. J. Anaesth. 2013, 110, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-G.; Jeong, S.-M.; Jun, I.-G.; Lee, H.-M.; Hwang, G.-S. Five-Minute Parameter of Thromboelastometry Is Sufficient to Detect Thrombocytopenia and Hypofibrinogenaemia in Patients Undergoing Liver Transplantation. Br. J. Anaesth. 2014, 112, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Stegewerth, K.; Weber, C.F.; Moehlmann, M.; Adam, E.H.; Zacharowski, K.; Zeuzem, S.; Weiler, N. Fast Interpretation of Thromboelastometry and Aggregometry in Patients Suffering from Chronic Liver Disease. Clin. Lab. 2019, 65, 190505. [Google Scholar] [CrossRef]
- Yuen, S.W.; Barrios, E.; Moon, T.; Pak, T.; Smith, K.M.; Toomay, S.; Cripps, M.W. Utilization of Rotational Thromboelastometry (ROTEM) in Patients Undergoing Transjugular Intrahepatic Portosystemic Shunt (TIPS). J. Clin. Anesth. 2019, 58, 31–32. [Google Scholar] [CrossRef]
- Yoon, U.; Bartoszko, J.; Bezinover, D.; Biancofiore, G.; Forkin, K.T.; Rahman, S.; Spiro, M.; Raptis, D.A.; Kang, Y. ERAS4OLT.org Working Group Intraoperative Transfusion Management, Antifibrinolytic Therapy, Coagulation Monitoring and the Impact on Short-Term Outcomes after Liver Transplantation—A Systematic Review of the Literature and Expert Panel Recommendations. Clin. Transplant. 2022, 36, e14637. [Google Scholar] [CrossRef]
- Leon-Justel, A.; Alvarez-Rios, A.I.; Noval-Padillo, J.A.; Gomez-Bravo, M.A.; Porras, M.; Gomez-Sosa, L.; Lopez-Romero, J.L.; Guerrero, J.M. Point-of-Care Haemostasis Monitoring during Liver Transplantation Is Cost Effective. Clin. Chem. Lab. Med. 2019, 57, 883–890. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Torrance, H.D.; Pearse, R.M.; Ackland, G.L.; Prowle, J.R.; Owen, H.C.; Hinds, C.J.; O’Dwyer, M.J. Perioperative Blood Transfusion Is Associated with a Gene Transcription Profile Characteristic of Immunosuppression: A Prospective Cohort Study. Crit. Care 2014, 18, 541. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of Severe Perioperative Bleeding: Guidelines from the European Society of Anaesthesiology: First Update 2016. Eur. J. Anaesthesiol. 2017, 34, 332–395. [Google Scholar] [CrossRef] [Green Version]
- Neeff, H.; Mariaskin, D.; Spangenberg, H.-C.; Hopt, U.T.; Makowiec, F. Perioperative Mortality after Non-Hepatic General Surgery in Patients with Liver Cirrhosis: An Analysis of 138 Operations in the 2000s Using Child and MELD Scores. J. Gastrointest. Surg. 2011, 15, 1–11. [Google Scholar] [CrossRef]
- del Olmo, J.A.; Flor-Lorente, B.; Flor-Civera, B.; Rodriguez, F.; Serra, M.A.; Escudero, A.; Lledó, S.; Rodrigo, J.M. Risk Factors for Nonhepatic Surgery in Patients with Cirrhosis. World J. Surg. 2003, 27, 647–652. [Google Scholar] [CrossRef]
- Ziser, A.; Plevak, D.J.; Wiesner, R.H.; Rakela, J.; Offord, K.P.; Brown, D.L. Morbidity and Mortality in Cirrhotic Patients Undergoing Anesthesia and Surgery. Anesthesiology 1999, 90, 42–53. [Google Scholar] [CrossRef]
- Raeven, P.; Baron-Stefaniak, J.; Simbrunner, B.; Stadlmann, A.; Schwabl, P.; Scheiner, B.; Schaden, E.; Eigenbauer, E.; Quehenberger, P.; Mandorfer, M.; et al. Thromboelastometry in Patients with Advanced Chronic Liver Disease Stratified by Severity of Portal Hypertension. Hepatol. Int. 2020, 14, 1083–1092. [Google Scholar] [CrossRef]
- Zanetto, A.; Campello, E.; Bulato, C.; Gavasso, S.; Saggiorato, G.; Shalaby, S.; Burra, P.; Angeli, P.; Senzolo, M.; Simioni, P. Global Hemostatic Profiling in Patients with Decompensated Cirrhosis and Bacterial Infections. JHEP Rep. 2022, 4, 100493. [Google Scholar] [CrossRef]
- Lange, N.W.; Salerno, D.M.; Berger, K.; Cushing, M.M.; Brown, R.S. Management of Hepatic Coagulopathy in Bleeding and Nonbleeding Patients: An Evidence-Based Review. J. Intensive Care Med. 2021, 36, 524–541. [Google Scholar] [CrossRef]
- Neeff, H.P.; Streule, G.C.; Drognitz, O.; Tittelbach-Helmrich, D.; Spangenberg, H.-C.; Hopt, U.T.; Makowiec, F. Early Mortality and Long-Term Survival after Abdominal Surgery in Patients with Liver Cirrhosis. Surgery 2014, 155, 623–632. [Google Scholar] [CrossRef]
- Perkins, L.; Jeffries, M.; Patel, T. Utility of Preoperative Scores for Predicting Morbidity after Cholecystectomy in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2004, 2, 1123–1128. [Google Scholar] [CrossRef]
- Cucchetti, A.; Ercolani, G.; Vivarelli, M.; Cescon, M.; Ravaioli, M.; Ramacciato, G.; Grazi, G.L.; Pinna, A.D. Is Portal Hypertension a Contraindication to Hepatic Resection? Ann. Surg. 2009, 250, 922–928. [Google Scholar] [CrossRef]
- Capussotti, L.; Ferrero, A.; Viganò, L.; Muratore, A.; Polastri, R.; Bouzari, H. Portal Hypertension: Contraindication to Liver Surgery? World J. Surg. 2006, 30, 992–999. [Google Scholar] [CrossRef]
- Dieterich, D.T.; Bernstein, D.; Flamm, S.; Pockros, P.J.; Reau, N. Review Article: A Treatment Algorithm for Patients with Chronic Liver Disease and Severe Thrombocytopenia Undergoing Elective Medical Procedures in the United States. Aliment. Pharmacol. Ther. 2020, 52, 1311–1322. [Google Scholar] [CrossRef]
- Terrault, N.; Chen, Y.-C.; Izumi, N.; Kayali, Z.; Mitrut, P.; Tak, W.Y.; Allen, L.F.; Hassanein, T. Avatrombopag Before Procedures Reduces Need for Platelet Transfusion in Patients with Chronic Liver Disease and Thrombocytopenia. Gastroenterology 2018, 155, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Hu, H.; Huang, F.; Huang, C.; Huang, Q.; Wang, L.; Wu, A.; Yang, J.; Qin, D.; Zou, W.; et al. Comparative Efficacy and Safety of Thrombopoietin Receptor Agonists in Adults with Thrombocytopenia: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trial. Front. Pharmacol. 2021, 12, 704093. [Google Scholar] [CrossRef]
- Wong, M.; Busuttil, R.W. Surgery in Patients with Portal Hypertension. Clin. Liver Dis. 2019, 23, 755–780. [Google Scholar] [CrossRef]
- Sugiyama, A.; Fujii, T.; Okikawa, Y.; Sasaki, F.; Okajima, M.; Hidaka, H.; Iwato, K.; Sato, K.; Kokubunji, A.; Takata, N.; et al. Outcomes of Patients Who Undergo Transfusion of Fresh Frozen Plasma: A Prospective, Observational, Multicentre Cohort Study in Hiroshima, Japan. J. Blood. Med. 2021, 12, 965–973. [Google Scholar] [CrossRef]
- Blasi, A.; Calvo, A.; Prado, V.; Reverter, E.; Reverter, J.C.; Hernández-Tejero, M.; Aziz, F.; Amoros, A.; Cardenas, A.; Fernández, J. Coagulation Failure in Patients with Acute-on-Chronic Liver Failure and Decompensated Cirrhosis: Beyond the International Normalized Ratio. Hepatology 2018, 68, 2325–2337. [Google Scholar] [CrossRef] [Green Version]
- Bezinover, D.; Dirkmann, D.; Findlay, J.; Guta, C.; Hartmann, M.; Nicolau-Raducu, R.; Mukhtar, A.M.; Moguilevitch, M.; Pivalizza, E.; Rosenfeld, D.; et al. Perioperative Coagulation Management in Liver Transplant Recipients. Transplantation 2018, 102, 578–592. [Google Scholar] [CrossRef]
- Latona, A.; Hill, K.; Connelly, A.; Stuart, K.; Wood, P. Prothrombinex®-VF in Chronic Liver Disease: Friend or Foe? Emerg. Med. Australas. 2022. [Google Scholar] [CrossRef]
- Hanke, A.A.; Joch, C.; Görlinger, K. Long-Term Safety and Efficacy of a Pasteurized Nanofiltrated Prothrombin Complex Concentrate (Beriplex P/N): A Pharmacovigilance Study. Br. J. Anaesth. 2013, 110, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Martinez, S.; Garcia, I.; Ruiz, A.; Tàssies, D.; Reverter, J.C.; Colmenero, J.; Beltran, J.; Fondevila, C.; Blasi, A. Is Antivitamin K Reversal Required in Patients with Cirrhosis Undergoing Liver Transplantation? Transfusion 2021, 61, 3008–3016. [Google Scholar] [CrossRef]
- Poon, K.-S.; Chen, C.-C.; Thorat, A.; Chiang, Y.-Y.; Jeng, L.-B.; Yang, H.-R.; Chen, T.-H.; Yeh, C.-C.; Chen, K.-B. Fibrinolysis after Reperfusion of Liver Graft. Acta Anaesthesiol. Taiwanica 2015, 53, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K. Coagulation management during liver transplantation. Hamostaseologie 2006, 26, S64–S76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, J.H.; Lee, K.; Abuelkasem, E.; Udekwu, O.R.; Tanaka, K.A. Coagulation Management During Liver Transplantation: Use of Fibrinogen Concentrate, Recombinant Activated Factor VII, Prothrombin Complex Concentrate, and Antifibrinolytics. Semin. Cardiothorac. Vasc. Anesth. 2018, 22, 164–173. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.R.; Yokoyama, A.P.; Magnus, M.M.; Boin, I.; de Ataide, E.C.; Munhoz, D.C.; Pereira, F.B.; Luzo, A.; Orsi, F.A. Association of Acidosis with Coagulopathy and Transfusion Requirements in Liver Transplantation. J. Thromb. Thrombolysis. 2022, 53, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Arstikyte, K.; Vitkute, G.; Traskaite-Juskeviciene, V.; Macas, A. Disseminated Intravascular Coagulation Following Air Embolism during Orthotropic Liver Transplantation: Is This Just a Coincidence? BMC Anesth. 2021, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Yang, L. Fluids Administration and Coagulation Characteristics in Patients with Different Model for End-Stage Liver Disease Scores Undergoing Orthotopic Liver Transplantation. Chin. Med. J. 2007, 120, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomić Mahečić, T.; Baronica, R.; Mrzljak, A.; Boban, A.; Hanžek, I.; Karmelić, D.; Babić, A.; Mihaljević, S.; Meier, J. Individualized Management of Coagulopathy in Patients with End-Stage Liver Disease. Diagnostics 2022, 12, 3172. https://doi.org/10.3390/diagnostics12123172
Tomić Mahečić T, Baronica R, Mrzljak A, Boban A, Hanžek I, Karmelić D, Babić A, Mihaljević S, Meier J. Individualized Management of Coagulopathy in Patients with End-Stage Liver Disease. Diagnostics. 2022; 12(12):3172. https://doi.org/10.3390/diagnostics12123172
Chicago/Turabian StyleTomić Mahečić, Tina, Robert Baronica, Anna Mrzljak, Ana Boban, Ivona Hanžek, Dora Karmelić, Anđela Babić, Slobodan Mihaljević, and Jens Meier. 2022. "Individualized Management of Coagulopathy in Patients with End-Stage Liver Disease" Diagnostics 12, no. 12: 3172. https://doi.org/10.3390/diagnostics12123172
APA StyleTomić Mahečić, T., Baronica, R., Mrzljak, A., Boban, A., Hanžek, I., Karmelić, D., Babić, A., Mihaljević, S., & Meier, J. (2022). Individualized Management of Coagulopathy in Patients with End-Stage Liver Disease. Diagnostics, 12(12), 3172. https://doi.org/10.3390/diagnostics12123172






