A Modified Run-Off Resistance Score from Cross-Sectional Imaging Discriminates Chronic Critical Limb Ischemia from Intermittent Claudication in Peripheral Arterial Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics
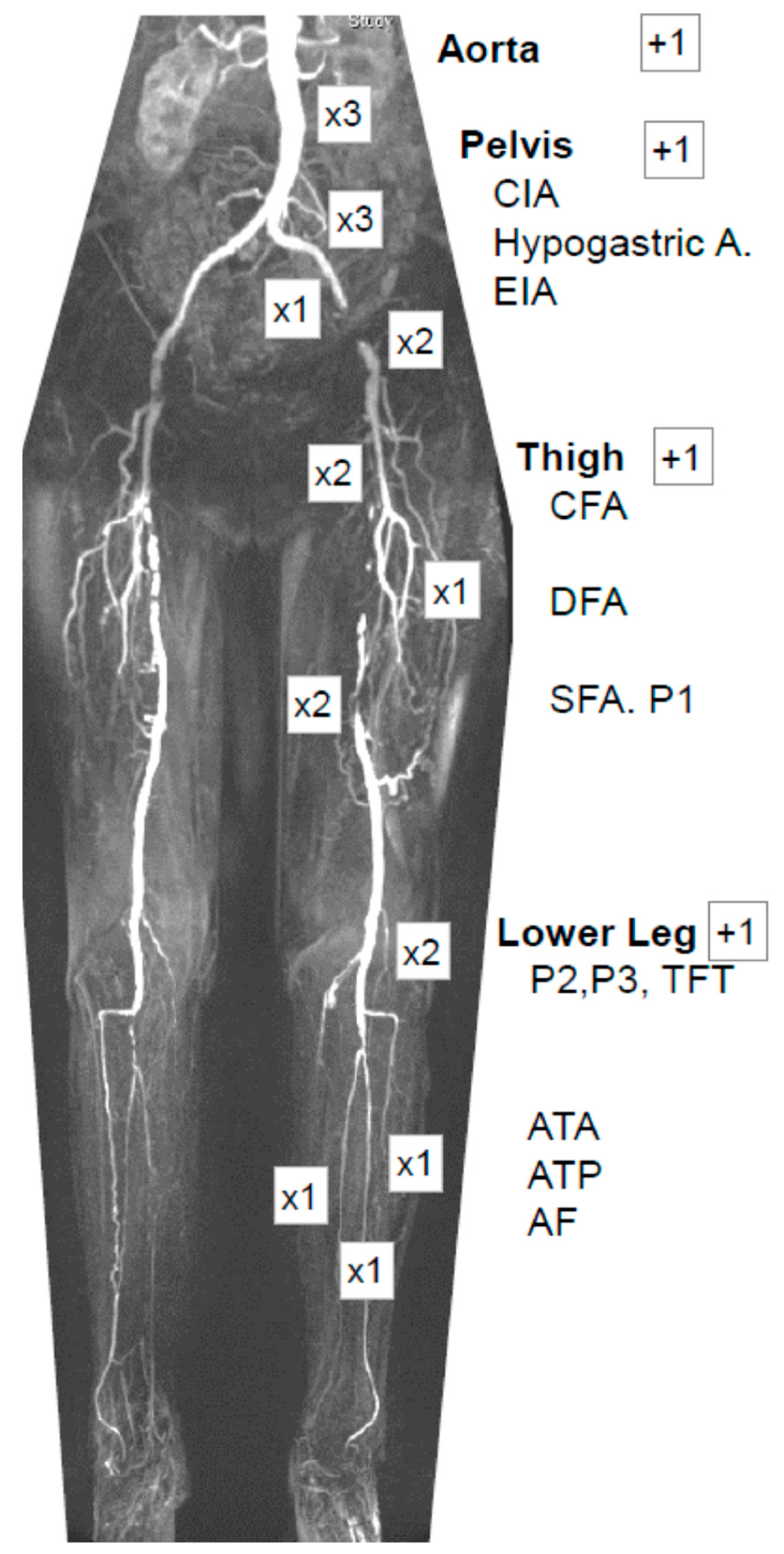
2.3. Modified Run-Off Resistance Score (mROR)
2.4. Statistics
3. Results
3.1. Patient Characteristics
3.2. mROR Sum Scores and Subgroup Analysis
3.3. Univariate Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Kengne, A.P.; Echouffo-Tcheugui, J.B. Differential burden of peripheral artery disease. Lancet Glob. Health 2019, 7, e980–e981. [Google Scholar] [CrossRef] [PubMed]
- Achim, A.; Stanek, A.; Homorodean, C.; Spinu, M.; Onea, H.L.; Lazăr, L.; Marc, M.; Ruzsa, Z.; Olinic, D.M. Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences? Int. J. Environ. Res. Public Health 2022, 19, 9801. [Google Scholar] [CrossRef] [PubMed]
- Diehm, C.; Kareem, S.; Lawall, H. Epidemiology of peripheral arterial disease. Vasa 2004, 33, 183–189. [Google Scholar] [CrossRef]
- Gratl, A.; Wipper, S.; Frese, J.P.; Raude, B.; Greiner, A.; Pesta, D. The Role of Mitochondrial Function in Peripheral Arterial Disease: Insights from Translational Studies. Int. J. Mol. Sci. 2021, 22, 8478. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. Editor’s Choice—2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R.; Jazaeri, O.; Yi, J.; Smith, M.; Gupta, R. Overview of Classification Systems in Peripheral Artery Disease. Semin. Interv. Radiol. 2014, 31, 378–388. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 2007, 33, S1–S75. [Google Scholar] [CrossRef]
- TASC Steering Committee; Jaff, M.R.; White, C.J.; Hiatt, W.R.; Fowkes, G.R.; Dormandy, J.; Razavi, M.; Reekers, J.; Norgren, L. An Update on Methods for Revascularization and Expansion of the TASC Lesion Classification to Include Below-the-Knee Arteries: A Supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Endovasc Ther. 2015, 22, 663–677. [Google Scholar] [CrossRef]
- Bollinger, A.; Breddin, K.; Hess, H.; Heystraten, F.M.; Kollath, J.; Konttila, A.; Pouliadis, G.; Marshall, M.; Mey, T.; Mietaschk, A.; et al. Semiquantitative assessment of lower limb atherosclerosis from routine angiographic images. Atherosclerosis 1981, 38, 339–346. [Google Scholar] [CrossRef]
- Bradbury, A.W.; Adam, D.J.; Bell, J.; Forbes, J.F.; Fowkes, F.G.R.; Gillespie, I.; Ruckley, C.V.; Raab, G.M. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: A description of the severity and extent of disease using the Bollinger angiogram scoring method and the TransAtlantic Inter-Society Consensus II classification. J. Vasc. Surg. 2010, 51, 32S–42S. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S. [Google Scholar] [CrossRef] [PubMed]
- Peterkin, G.A.; Manabe, S.; Lamorte, W.W.; Menzoian, J.O. Evaluation of a proposed standard reporting system for preoperative angiograms in infrainguinal bypass procedures: Angiographic correlates of measured runoff resistance. J. Vasc. Surg. 1988, 7, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Suggested standards for reports dealing with lower extremity ischemia. J. Vasc. Surg. 1986, 4, 80–94. [Google Scholar] [CrossRef]
- Williams, D.M.; Fencil, L.; Chenevert, T.L. Peripheral arterial occlusive disease: P-31 MR spectroscopy of calf muscle. Radiology 1990, 175, 381–385. [Google Scholar] [CrossRef]
- Esterhammer, R.; Schocke, M.; Gorny, O.; Posch, L.; Messner, H.; Jaschke, W.; Fraedrich, G.; Greiner, A. Phosphocreatine Kinetics in the Calf Muscle of Patients with Bilateral Symptomatic Peripheral Arterial Disease during Exhaustive Incremental Exercise. Mol. Imaging Biol. 2008, 10, 30–39. [Google Scholar] [CrossRef]
- Gorny, O.; Santner, W.; Fraedrich, G.; Jaschke, W.; Greiner, A.; Schocke, M.F. The run-off resistance (ROR) assessed on MR angiograms may serve as a valid scoring system in patients with symptomatic peripheral arterial disease (PAD) and correlates with the ankle-brachial pressure index (ABI). Eur. J. Radiol. 2012, 81, 1155–1157. [Google Scholar] [CrossRef]
- Morris, D.R.; Singh, T.P.; Moxon, J.V.; Smith, A.; Stewart, F.; Jones, R.E.; Golledge, J. Assessment and validation of a novel angiographic scoring system for peripheral artery disease. Br. J. Surg. 2017, 104, 544–554. [Google Scholar] [CrossRef]
- Anderson, J.D.; Epstein, F.H.; Meyer, C.H.; Hagspiel, K.D.; Wang, H.; Berr, S.S.; Harthun, N.L.; Weltman, A.; DiMaria, J.M.; West, A.M.; et al. Multifactorial determinants of functional capacity in peripheral arterial disease: Uncoupling of calf muscle perfusion and metabolism. J. Am. Coll. Cardiol. 2009, 54, 628–635. [Google Scholar] [CrossRef]
- Greiner, A.; Esterhammer, R.; Messner, H.; Biebl, M.; Mühlthaler, H.; Fraedrich, G.; Jaschke, W.R.; Schocke, M.F. High-energy phosphate metabolism during incremental calf exercise in patients with unilaterally symptomatic peripheral arterial disease measured by phosphor 31 magnetic resonance spectroscopy. J. Vasc. Surg. 2006, 43, 978–986. [Google Scholar] [CrossRef][Green Version]
- Müller-Bühl, U.; Wiesemann, A.; Oser, B.; Kirchberger, I.; Strecker, E.P. Correlation of hemodynamic and functional variables with the angiographic extent of peripheral arterial occlusive disease. Vasc. Med. 1999, 4, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Lowry, D.; Vitalis, A.; Al Shakarchi, J.; Psarros, V.; Karkhanis, S.; Saeed, M.; Narendran, P.; Tiwari, A. An Extension of the Bollinger Scoring System to Analyse the Distribution of Macrovascular Disease of the Lower Limb in Diabetes. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R. Medical Treatment of Peripheral Arterial Disease and Claudication. N. Engl. J. Med. 2001, 344, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Murabito, J.M.; D’Agostino, R.B.; Silbershatz, H.; Wilson, W.F. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation 1997, 96, 44–49. [Google Scholar] [CrossRef] [PubMed]

| IC (n = 20) | CLTI (n = 20) | p-Value | |||
|---|---|---|---|---|---|
| (%) (IQR) | (%) (IQR) | ||||
| male gender | 15 | (75) | 13 | (65) | 0.490 |
| age [years] | 63.0 | (59.7–72.3) | 70.7 | (66.1–79.5) | 0.010 * |
| PAD Rutherford stage | 2.0 | (2.0–3.0) | 5.0 | (5.0–5.0) | 0.000 * |
| body mass index (BMI) | 27.2 | (20.9–28.9) | 26.1 | (24.1–31.9) | 0.365 |
| overweight (BMI > 25) | 12 | (60) | 12 | (60) | 1.000 |
| ASA 2 | 9 | (45) | 2 | (10) | |
| ASA 3 | 11 | (55) | 12 | (60) | |
| ASA 4 | 0 | (0) | 6 | (30) | 0.005 * |
| Diabetes | 3 | (15) | 10 | (50) | 0.018 * |
| Hypertension | 15 | (75) | 18 | (90) | 0.212 |
| active smoker | 13 | (65) | 10 | (50) | 0.337 |
| coronary heart disease | 5 | (25) | 11 | (55) | 0.053 |
| dyslipoproteinemia | 13 | (65) | 12 | (60) | 0.744 |
| number of cardiovascular risk factors | 2.0 | (1.0–2.5) | 3.0 | (2.0–4.5) | 0.047 * |
| kidney disease | 4 | (20) | 11 | (55) | 0.022 * |
| permanent dialysis | 0 | (0) | 2 | (10) | 0.147 |
| ankle-brachial index (ABI) before revascularization | 0.6 | (0.5–0.8) | 0.8 | (0.5–1.0) | 0.571 |
| technique of revascularization | |||||
| − Open | 8 | (40) | 12 | (60) | |
| − Endovascular | 4 | (20) | 2 | (10) | |
| − Hybrid | 8 | (40) | 6 | (30) | 0.416 |
| n | mROR Pelvis | mROR Thigh | mROR Lower Leg | mROR Sum | |||||
|---|---|---|---|---|---|---|---|---|---|
| study cohort | 40 | 9.62 | ±5.15 | 8.09 | ±3.28 | 7.53 | ±3.61 | 27.59 | ±9.06 |
| − IC | 20 | 8.90 | ±4.88 | 6.88 | ±3.44 | 5.78 | ±3.82 | 23.45 | ±7.17 |
| − CLTI | 20 | 10.35 | ±5.45 | 9.30 | ±2.68 | 9.28 | ±2.38 | 31.73 | ±9.01 |
| − p-value | 0.432 | 0.029 * | 0.001 * | 0.004 * | |||||
| IC | |||||||||
| − diabetics | 3 | 8.00 | ±6.56 | 8.67 | ±0.58 | 7.00 | ±5.57 | 25.67 | ±12.42 |
| − non-diabetics | 17 | 9.06 | ±4.76 | 6.56 | ±3.65 | 5.56 | ±3.62 | 23.06 | ±6.38 |
| − p-value | 0.750 | 0.309 | 0.670 | 0.874 | |||||
| CLTI | |||||||||
| − diabetics | 10 | 6.90 | ±3.41 | 8.80 | ±2.25 | 8.05 | ±1.64 | 25.65 | ±4.96 |
| − non-diabetics | 10 | 13.80 | ±4.95 | 9.80 | ±3.09 | 10.50 | ±2.44 | 37.80 | ±8.05 |
| − p-value | 0.004 * | 0.493 | 0.033 * | 0.001 * | |||||
| diabetics | |||||||||
| − IC | 3 | 8.00 | ±6.56 | 8.67 | ±0.58 | 7.00 | ±5.57 | 25.67 | ±12.42 |
| − CLTI | 10 | 6.90 | ±3.41 | 8.80 | ±2.25 | 8.05 | ±1.64 | 25.65 | ±4.96 |
| − p-value | 0.798 | 0.729 | 0.550 | 0.612 | |||||
| non-diabetics | |||||||||
| − IC | 17 | 9.06 | ±4.76 | 6.56 | ±3.65 | 5.56 | ±3.62 | 23.06 | ±6.38 |
| − CLTI | 10 | 13.80 | ±4.95 | 9.80 | ±3.09 | 10.50 | ±2.44 | 37.80 | ±8.05 |
| − p-value | 0.029 * | 0.038 * | 0.001 * | <0.001 * | |||||
| IC | CLTI | p-Value | |||
|---|---|---|---|---|---|
| n = 17 | n = 10 | ||||
| Aorta | 1.88 | ±1.41 | 3.70 | ±1.70 | 0.009 * |
| Common iliac artery | 3.00 | ±2.81 | 4.05 | ±3.00 | 0.358 |
| Hypogastric artery | 0.82 | ±0.95 | 1.65 | ±0.85 | 0.028 * |
| External iliac artery | 2.35 | ±1.62 | 3.40 | ±1.35 | 0.086 |
| Sum mROR pelvis | 8.06 | ±4.76 | 12.80 | ±4.95 | 0.029 * |
| Common femoral artery | 2.24 | ±1.71 | 3.00 | ±1.70 | 0.324 |
| Deep femoral artery | 0.50 | ±0.79 | 1.10 | ±1.07 | 0.133 |
| Superficial femoral artery and supragenual popliteal artery (P1) | 2.82 | ±2.13 | 4.70 | ±1.64 | 0.028 * |
| Sum mROR thigh | 6.56 | ±3.65 | 9.80 | ±3.09 | 0.038 * |
| Infragenual popliteal artery (P2–P3) and tibiofibular trunk | 0.94 | ±1.09 | 2.00 | ±0.78 | 0.014 * |
| Anterior tibial artery | 1.06 | ±0.97 | 1.90 | ±0.70 | 0.025 * |
| Posterior tibial artery | 0.79 | ±0.95 | 2.05 | ±0.93 | 0.004 * |
| Peroneal artery | 0.82 | ±1.17 | 1.55 | ±1.01 | 0.054 |
| Sum mROR lower leg | 5.56 | ±3.62 | 10.50 | ±2.44 | 0.001 * |
| Sum mROR | 23.06 | ±6.38 | 37.80 | ±8.05 | <0.001 * |
| Study Cohort | B | 95% CI | p-Value | |
|---|---|---|---|---|
| CLTI over IC | 8.28 | (3.06–13.49) | 0.003 * | |
| Rutherford stage | 2.20 | (0.24–4.16) | 0.029 * | |
| CLTI subgroup | ||||
| body mass index (BMI) | −0.77 | (−1.42–−0.12) | 0.023 * | |
| Diabetes | −12.2 | (−18.43–−5.87) | 0.001 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frese, J.P.; Schawe, L.; Carstens, J.; Milbergs, K.; Speichinger, F.; Gratl, A.; Greiner, A.; Raude, B. A Modified Run-Off Resistance Score from Cross-Sectional Imaging Discriminates Chronic Critical Limb Ischemia from Intermittent Claudication in Peripheral Arterial Disease. Diagnostics 2022, 12, 3155. https://doi.org/10.3390/diagnostics12123155
Frese JP, Schawe L, Carstens J, Milbergs K, Speichinger F, Gratl A, Greiner A, Raude B. A Modified Run-Off Resistance Score from Cross-Sectional Imaging Discriminates Chronic Critical Limb Ischemia from Intermittent Claudication in Peripheral Arterial Disease. Diagnostics. 2022; 12(12):3155. https://doi.org/10.3390/diagnostics12123155
Chicago/Turabian StyleFrese, Jan Paul, Larissa Schawe, Jan Carstens, Karlis Milbergs, Fiona Speichinger, Alexandra Gratl, Andreas Greiner, and Ben Raude. 2022. "A Modified Run-Off Resistance Score from Cross-Sectional Imaging Discriminates Chronic Critical Limb Ischemia from Intermittent Claudication in Peripheral Arterial Disease" Diagnostics 12, no. 12: 3155. https://doi.org/10.3390/diagnostics12123155
APA StyleFrese, J. P., Schawe, L., Carstens, J., Milbergs, K., Speichinger, F., Gratl, A., Greiner, A., & Raude, B. (2022). A Modified Run-Off Resistance Score from Cross-Sectional Imaging Discriminates Chronic Critical Limb Ischemia from Intermittent Claudication in Peripheral Arterial Disease. Diagnostics, 12(12), 3155. https://doi.org/10.3390/diagnostics12123155





