Reinducing Radioiodine-Sensitivity in Radioiodine-Refractory Thyroid Cancer Using Lenvatinib (RESET): Study Protocol for a Single-Center, Open Label Phase II Trial
Abstract
:1. Background
2. Methods and Design
2.1. Study Design
2.2. Trial Organization and Coordination
2.3. Ethics, Informed Consent and Monitoring
2.4. Sample Size Calculation
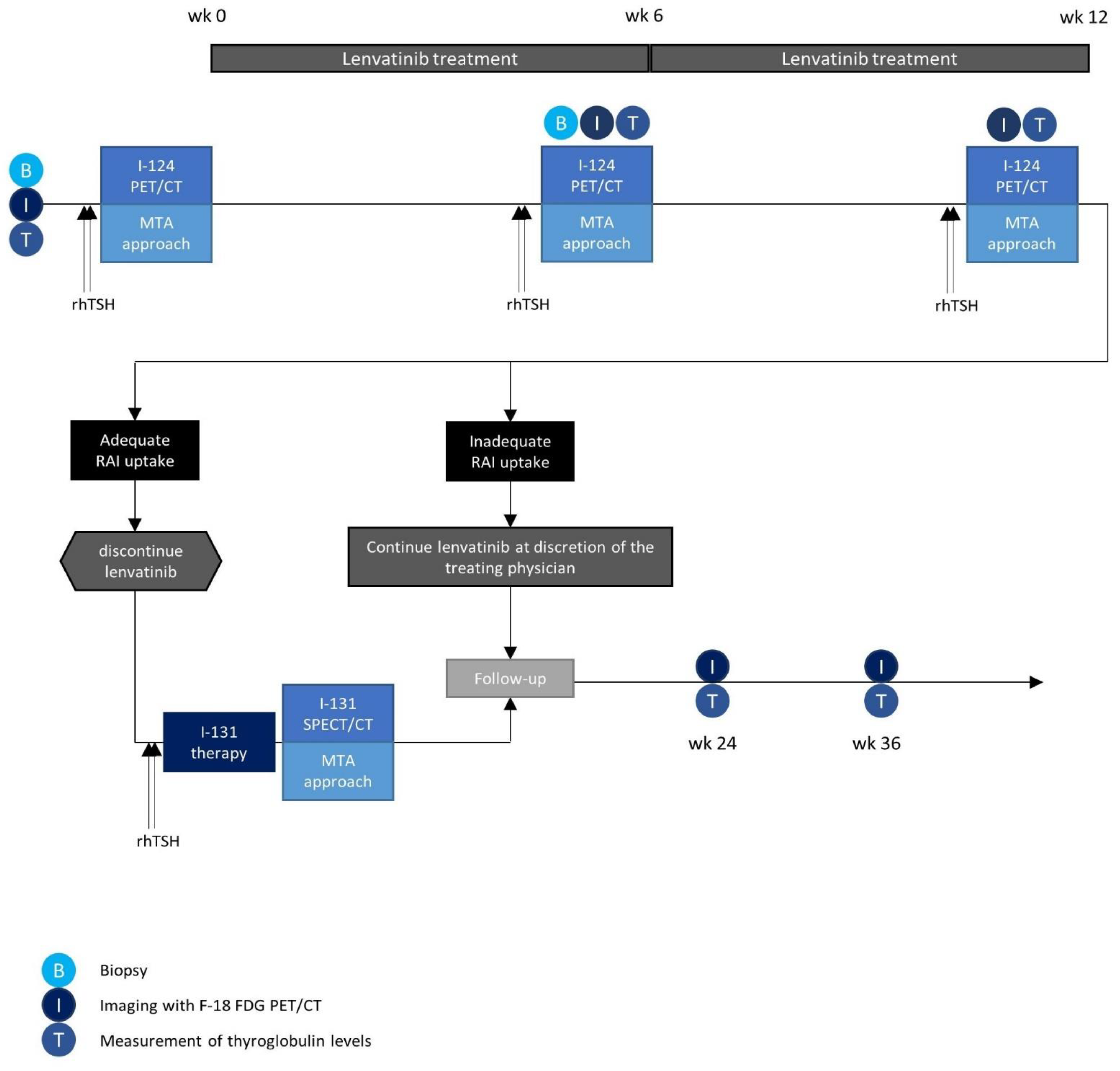
2.5. Study Overview
2.6. Study Endpoints
- Extent of RAI uptake at baseline and after 6- or 12-week lenvatinib;
- Optimal duration of lenvatinib treatment (6 weeks or 12 weeks) for maximum redifferentiation;
- Agreement between expected absorbed dose per lesion predicted by I-124 PET/CT dosimetry and actual absorbed dose per lesion determined by intra-therapeutic I-131 single photon emission computed tomography (SPECT)/CT dosimetry in patients in which I-131 therapy is warranted;
- Metabolic and biochemical treatment response using F-18 FDG PET and unstimulated (thyroid-stimulating hormone (TSH)-suppressed) Tg levels, respectively;
- Progression free survival, best objective response and overall survival;
- Incidence and severity of toxicities according to the Common Terminology Criteria for Adverse Events (CTCAE) 5.0;
- Quality of life (QoL).
2.7. Patient Selection
2.8. Study Procedures
2.8.1. Lenvatinib Treatment
2.8.2. Biopsy
2.8.3. I-124 Dosimetry Procedures
- I-124 PET/CT
- Pre-treatment dosimetry with PET/CT will be performed according to Jentzen et al. [26] This approach requires two PET/CT scans at 24 ± 6 and 96–120 h post-administration.
- Activity in blood and the whole body
- To assess toxicity of the bone marrow and lungs, the activity in blood samples and the whole body is determined according to Jentzen et al. [27] One heparinized blood sample of 2 mL is withdrawn from the patient and whole body counts measured at 2 ± 0.5, 24 ± 6 and 96–120 h post-administration. Whole body counting is performed using an uncollimated gamma camera detector.
2.8.4. I-131 Therapy
- A clinically meaningful lesion dose is expected: ≥20 Gy in at least one lesion;
- Acceptable toxicity is expected: blood dose ≤2 Gy and 48 h post-administration body retention ≤3.0 GBq–4.4 GBq (with and without lung metastases).
2.8.5. Post-Therapeutic Dosimetry
2.8.6. Quality of Life Assessment
- THYCA-QoL: disease-specific health-related QoL questionnaire for thyroid cancer survivors [30];
- RAND-36 V2.0 (SF36-II): a validated translation in Dutch of the Short Form health survey (SF36-II) comprising 36 questions yielding a 9-scale profile of function health, wellbeing and psychometrically based physical and mental health [31];
- EQ-5D-5L: EuroQol 5-dimensional 5-level questionnaire: indexing health status including a single visual analogue scale (VAS) [32];
- DT&PL: an 11-point VAS including a 35-item problem list. We will only use the VAS, since the 35-item problem list mostly overlaps with the content of the other questionnaires [33].
2.8.7. Follow-Up
2.9. Data Analysis and Statistics
3. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebastchi, A.; Callender, G. Thyroid cancer. Curr. Probl. Cancer 2014, 38, 48–74. [Google Scholar] [CrossRef] [PubMed]
- Schmidbauer, B.; Menhart, K.; Hellwig, D.; Grosse, J. Differentiated Thyroid Cancer—Treatment: State of the Art. Int. J. Mol. Sci. 2017, 18, 1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aashiq, M.; Silverman, D.A.; Na’ara, S.; Takahashi, H.; Amit, M. Radioiodine-refractory thryoid cancer: Molecular basis of redifferentation therapies, management and novel therapies. Cancers 2019, 11, 1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, Y.; Lin, Y.; Liang, J. Radioactive iodine-refractory differentiated thryoid cancer and redifferentiation therapy. Endocrinol. Metab. 2019, 34, 215–225. [Google Scholar] [CrossRef]
- Narayanan, S.; Colevas, A.D. Current Standards in Treatment of Radioiodine Refractory Thyroid Cancer. Curr. Treat. Options Oncol. 2016, 17, 30. [Google Scholar] [CrossRef]
- Berdelou, A.; Lamartina, L.; Klain, M.; Leboulleux, S.; Schlumberger, M. Treatment of refractory thyroid cancer. Endocr.-Relat. Cancer 2018, 25, R209–R223. [Google Scholar] [CrossRef] [Green Version]
- Vaisman, F.; Carvalho, D.; Vaisman, M. A new appraisal of iodine refractory thyroid cancer. Endocr.-Relat. Cancer 2015, 22, R301–R310. [Google Scholar] [CrossRef]
- Mu, Z.-Z.; Zhang, X.; Lin, Y.-S. Identification of Radioactive Iodine Refractory Differentiated Thyroid Cancer. Chonnam Med. J. 2019, 55, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Berruti, A. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-Term Outcome of 444 Patients with Distant Metastases from Papillary and Follicular Thyroid Carcinoma: Benefits and Limits of Radioiodine Therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resteghini, C.; Cavalieri, S.; Galbiati, D.; Granata, R.; Alfieri, S.; Bergamini, C.; Bossi, P.; Licitra, L.; Locati, L. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Aydemirli, M.; Kapiteijn, E.; Ferrier, K.R.M.; Ottevanger, P.B.; Links, T.P.; A Van Der Horst-Schrivers, A.N.; Broekman, K.; Groenwold, R.H.H.; Zwaveling, J. Effectiveness and toxicity of lenvatinib in refractory thyroid cancer: Dutch real-life data. Eur. J. Endocrinol. 2020, 182, 131–138. [Google Scholar] [CrossRef]
- Dotinga, M.; Vriens, D.; van Velden, F.; Heijmen, L.; Nagarajah, J.; Hicks, R.; Kapiteijn, E.; de Geus-Oei, L.-F. Managing radioiodine refractory thyroid cancer: The role of dosimetry and redifferentiation on subsequent I-131 therapy. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 250–264. [Google Scholar] [CrossRef]
- Ho, A.L.; Grewal, R.K.; Leboeuf, R.; Sherman, E.J.; Pfister, D.G.; Deandreis, D.; Pentlow, K.S.; Zanzonico, P.B.; Haque, S.; Gavane, S.; et al. Selumetinib-Enhanced Radioiodine Uptake in Advanced Thyroid Cancer. N. Engl. J. Med. 2013, 368, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Dunn, L.; Sherman, E.J.; Baxi, S.S.; Tchekmedyian, V.; Grewal, R.K.; Larson, S.M.; Pentlow, K.S.; Haque, S.; Tuttle, R.M.; Ho, A.L.; et al. Vemurafenib redifferentiation of BRAF mutant, RAI-refractory thyroid cancer. J. Clin. Endocrinol. Metab. 2019, 104, 1417–1428. [Google Scholar] [CrossRef]
- Rothenberg, S.; McFadden, D.G.; Palmer, E.L.; Daniels, G.H.; Wirth, L.J. Redifferentation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin. Cancer Res. 2014, 21, 1028–1035. [Google Scholar] [CrossRef] [Green Version]
- Iravani, A.; Solomon, B.; Pattison, D.A.; Jackson, P.; Ravi Kumar, A.; Kong, G.; Hicks, R.J.; Hofman, M.S.; Akhurst, T. Mitogen-activated protein kinase pathway inhibition for redifferentiation of radioiodine refractory differentiated thryoid cancer: An evolving protocol. Thyroid. Cancer Nodules 2019, 29, 1634–1645. [Google Scholar] [CrossRef]
- Jaber, T.; Waguespack, S.G.; Cabanillas, M.E.; Elbanan, M.; Vu, T.; Dadu, R.; Sherman, S.I.; Amit, M.; Santos, E.B.; Busaidy, N.L.; et al. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive radioiodine. J. Clin. Endocrinol. Metab. 2018, 103, 3698–3705. [Google Scholar] [CrossRef]
- Groussin, L.; Theodon, H.; Bessiene, L.; Bricaire, L.; Bonnet-Serrano, F.; Cochand-Priollet, B.; Leroy, K.; Garinet, S.; Pasmant, E.; Zerbit, J.; et al. Redifferentiating Effect of Larotrectinib in NTRK-Rearranged Advanced Radioactive-Iodine Refractory Thyroid Cancer. Thyroid 2022, 32, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyian, V.; Dunn, L.; Sherman, E.; Baxi, S.S.; Grewal, R.K.; Larson, S.M.; Ho, A.L.; Pentlow, K.S.; Haque, S.; Tuttle, R.M.; et al. Enhancing Radioiodine Incorporation in BRAF-Mutant, Radioiodine-Refractory Thyroid Cancers with Vemurafenib and the Anti-ErbB3 Monoclonal Antibody CDX-3379: Results of a Pilot Clinical Trial. Thyroid 2022, 32, 273–282. [Google Scholar] [CrossRef]
- Weber, M.; Kersting, D.; Riemann, B.; Brandenburg, T.; Führer-Sakel, D.; Grünwald, F.; Kreissl, M.C.; Dralle, H.; Weber, F.; Schmid, K.W.; et al. Enhancing Radioiodine Incorporation into Radioiodine-Refractory Thyroid Cancer with MAPK Inhibition (ERRITI): A Single-Center Prospective Two-Arm Study. Clin. Cancer Res. 2022, 28, 4194–4202. [Google Scholar] [CrossRef] [PubMed]
- Suyama, K.; Iwase, H. Lenvatinib: A Promising Molecular Targeted Agent for Multiple Cancers. Cancer Control 2018, 25, 1073274818789361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anschlag, A.; Greene, B.H.; Könneker, L.; Luster, M.; Nagarajah, J.; Wächter, S.; Wunderlich, A.; Pfestroff, A. Effect of Kinase Inhibitors on the Technetium-99m Uptake into Thyroid Carcinoma Cells In Vitro. In Vivo 2021, 35, 721–729. [Google Scholar] [CrossRef]
- Jentzen, W.; Freudenberg, L.; Eising, E.G.; Sonnenschein, W.; Knust, J.; Bockisch, A. Optimized 124I PET Dosimetry Protocol for Radioiodine Therapy of Differentiated Thyroid Cancer. J. Nucl. Med. 2008, 49, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Jentzen, W.; Bockisch, A.; Ruhlmann, M. Assessment of Simplified Blood Dose Protocols for the Estimation of the Maximum Tolerable Activity in Thyroid Cancer Patients Undergoing Radioiodine Therapy Using 124I. J. Nucl. Med. 2015, 56, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Wadsley, J.; Gregory, R.; Flux, G.; Newbold, K.; Du, Y.; Moss, L.; Hall, A.; Flanagan, L.; Brown, S.R. SELIMETRY—A multicentre I-131 dosimetry trial: A clinical perspective. Br. J. Radiol. 2017, 90, 20160637. [Google Scholar] [CrossRef]
- Lassmann, M.; Hänscheid, H.; Chiesa, C.; Hindorf, C.; Flux, G.; Luster, M. EANM dosimetry committe series on standard operational procedures for pre-therapeutic dosimetry I: Blood and bone marrow dosimetry in differentiated thryoid cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1405–1412. [Google Scholar] [CrossRef]
- Husson, O.; Haak, H.R.; Mols, F.; Nieuwenhuijzen, G.A.; Nieuwlaat, W.A.; Reemst, P.H.; Toorians, A.W.; van de Poll-Franse, L.V. Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors. Acta Oncol. 2013, 52, 447–454. [Google Scholar] [CrossRef]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The rand 36-item health survey 1.0. Health Econ. 1993, 2, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riba, M.B.; Donovan, K.A.; Andersen, B.; Braun, I.; Breitbart, W.S.; Brewer, B.W.; Buchmann, L.O.; Clark, M.M.; Collins, M.; Darlow, S.D.; et al. Distress Management, Version 3. 2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw 2019, 17, 1229–1249. [Google Scholar]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Karapanou, O.; Simeakis, G.; Vlassopoulou, B.; Alevizaki, M.; Saltiki, K. Advanced RAI-refractory thyroid cancer: An update on treatment perspectives. Endocr.-Relat. Cancer 2022, 29, R57–R66. [Google Scholar] [CrossRef]
- Kreissl, M.C.; Janssen, M.J.; Nagarajah, J. Current Treatment Strategies in Metastasized Differentiated Thyroid Cancer. J. Nucl. Med. 2018, 60, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Kuker, R.; Sztejnberg, M.; Gulec, S. I-124 imaging and dosimetry. Mol. Imaging Radionucl. Ther. 2017, 26, 66–73. [Google Scholar] [CrossRef]
- Konijnenberg, M.; Herrmann, K.; Kobe, C.; Verburg, F.; Hindorf, C.; Hustinx, R.; Lassmann, M. EANM position paper on article 56 of the Council Directive 2013/59/Euratom (basic safety standards) for nuclear medicine therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 67–72. [Google Scholar] [CrossRef]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.G.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. 2008, 35, 1941–1949. [Google Scholar] [CrossRef]
- Verburg, F.A.; Biko, J.; Diessl, S.; Demidchik, Y.; Drozd, V.; Rivkees, S.A.; Reiners, C.; Hänscheid, H. I-131 Activities as High as Safely Administrable (AHASA) for the Treatment of Children and Adolescents with Advanced Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2011, 96, E1268–E1271. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Cheng, D.W. What is the role of dosimetry in patients with advanced thyroid cancer? Curr. Opin. Oncol. 2015, 27, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, L.S.; Antoch, G.; Jentzen, W.; Pink, R.; Knust, J.; Bockisch, A.; Debatin, J.F.; Brandau, W. Value of 124I-PET/CT in staging of patients with differentiated thyroid cancer. Eur. Radiol. 2004, 14, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Taprogge, J.; Leek, F.; Schurrat, T.; Tran-Gia, J.; Vallot, D.; Bardiès, M.; Eberlein, U.; Lassmann, M.; Schlögl, S.; Gil, A.V.; et al. Setting up a quantitative SPECT imaging network for a European multi-centre dosimetry study of radioiodine treatment for thyroid cancer as part of the MEDIRAD project. EJNMMI Phys. 2020, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.A.; Murray, I.; Gear, J.; Leek, F.; Chittenden, S.J.; Fenwick, A.; Wevrett, J.; Scuffham, J.; Tipping, J.; Murby, B.; et al. Standardised quantitative radioiodine SPECT/CT Imaging for multicentre dosimetry trials in molecular radiotherapy. Phys. Med. Biol. 2019, 64, 245013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerio, L.; Guidoccio, F.; Giani, C.; Tardelli, E.; Puccini, G.; Puleo, L.; Minaldi, E.; Boni, G.; Elisei, R.; Volterrani, D. [18F]-FDG-PET/CT Correlates With the Response of Radiorefractory Thyroid Cancer to Lenvatinib and Patient Survival. J. Clin. Endocrinol. Metab. 2021, 106, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
| 12-Wks Lenvatinib | 0 | 1–5 | 6 | 7 | 8–11 | 12 | 13 | Follow-Up | |
|---|---|---|---|---|---|---|---|---|---|
| Week | (Wk 24, 36) | ||||||||
| Medical history | X | ||||||||
| Physical examination | X | ||||||||
| Serum pregnancy test (females) | X | X iii | X | X i,ii | |||||
| Hematology and blood chemistry a | X | ||||||||
| Biopsy | X i | X | |||||||
| Lenvatinib treatment | X i | X i | X i | X i | X i | ||||
| rhTSH-stimulated I-124 dosimetry b | X | X iii | X | ||||||
| rhTSH-stimulated I-131 therapy c | X i,ii | ||||||||
| Post-therapeutic I-131 dosimetry d | X ii | ||||||||
| Thyroid Hormone Levels | X | X iii | X | X i,ii | |||||
| Unstimulated Tg levels | X i | X i | X i | X i | |||||
| F-18 FDG PET/CT e | X i | X iii | X i | X i | |||||
| QoL assessment | X | X | X | X ii | X | ||||
| 6-Wks Lenvatinib | Follow-up | ||||||||
| Week | 0 | 1–5 | 6 | 7 | 8–11 | 12 | 13 | (24, 30, 36) | |
| History | X | ||||||||
| Physical examination | X | ||||||||
| Serum pregnancy test (females) | X | X | X i,ii | ||||||
| Hematology and blood chemistry a | X | ||||||||
| Biopsy | X i | X | |||||||
| Lenvatinib treatment | X i | X i | |||||||
| rhTSH-stimulated I-124 dosimetry b | X | X | |||||||
| rhTSH-stimulated I-131 therapy c | X i,ii | ||||||||
| Post-therapeutic I-131 dosimetry d | X ii | ||||||||
| Thyroid Hormone Levels | X | X | X i,ii | ||||||
| Unstimulated Tg levels | X i | X i | X i | X i | |||||
| F-18 FDG PET/CT e | X i | X | X i | X i | |||||
| QoL assessment | X | X | X ii | X | X | ||||
| Inclusion Criteria | |
|---|---|
| General | Age ≥18 years ECOG performance status ≤2 Life expectancy ≥3 months Agreement to use a highly effective method of contraception during the study |
| Tumor characteristics | Histologically or cytologically confirmed DTC Progressive (biochemical or anatomic) disease for which lenvatinib is indicated RAI-refractory disease on structural imaging, defined as:
|
| Blood levels | Creatinine levels indicating renal clearance ≥50 mL/min INR ≤ 1.5 Absolute neutrophil count ≥1.5 × 109/L Hemoglobin ≥9 g/dL (5.6 mmol/L) Platelets ≥100 × 109/L Albumin ≥25 g/L Total bilirubin <1.5× ULN ASAT ≤3× ULN (≤5× ULN in case of liver metastases) ALAT ≤3× ULN (≤5× ULN in case of liver metastases) |
| Exclusion Criteria | |
| General | Known hypersensitivity or idiosyncrasy to lenvatinib and/or to thyrotropin alfa Received iodinated intravenous contrast <6–8 weeks prior to enrollment Inability to follow a low iodine diet Pregnant, lactating or breast-feeding women Inability to give informed consent |
| Concomitant diseases | Other malignancies <3 years prior to enrollment, except completely resected non-melanoma skin cancer or indolent secondary malignancies Symptomatic or untreated leptomeningeal or brain metastases or spinal cord compression Gastrointestinal abnormalities that may alter absorption Evidence of cardiovascular risk including clinically relevant arrhythmias, acute coronary syndromes, severe/unstable angina or symptomatic congestive heart failure Concurrent uncontrolled medical condition |
| Treatments | I-131 therapy <6 months prior to enrollment External beam radiation therapy <4 weeks prior to start lenvatinib Treatment with investigational drugs <4 weeks prior to start lenvatinib Requiring medication with high content in iodide (e.g., amiodarone) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotinga, M.; Vriens, D.; van Velden, F.H.P.; Stam, M.K.; Heemskerk, J.W.T.; Dibbets-Schneider, P.; Pool, M.; Rietbergen, D.D.D.; de Geus-Oei, L.-F.; Kapiteijn, E. Reinducing Radioiodine-Sensitivity in Radioiodine-Refractory Thyroid Cancer Using Lenvatinib (RESET): Study Protocol for a Single-Center, Open Label Phase II Trial. Diagnostics 2022, 12, 3154. https://doi.org/10.3390/diagnostics12123154
Dotinga M, Vriens D, van Velden FHP, Stam MK, Heemskerk JWT, Dibbets-Schneider P, Pool M, Rietbergen DDD, de Geus-Oei L-F, Kapiteijn E. Reinducing Radioiodine-Sensitivity in Radioiodine-Refractory Thyroid Cancer Using Lenvatinib (RESET): Study Protocol for a Single-Center, Open Label Phase II Trial. Diagnostics. 2022; 12(12):3154. https://doi.org/10.3390/diagnostics12123154
Chicago/Turabian StyleDotinga, Maaike, Dennis Vriens, Floris H. P. van Velden, Mette K. Stam, Jan W. T. Heemskerk, Petra Dibbets-Schneider, Martin Pool, Daphne D. D. Rietbergen, Lioe-Fee de Geus-Oei, and Ellen Kapiteijn. 2022. "Reinducing Radioiodine-Sensitivity in Radioiodine-Refractory Thyroid Cancer Using Lenvatinib (RESET): Study Protocol for a Single-Center, Open Label Phase II Trial" Diagnostics 12, no. 12: 3154. https://doi.org/10.3390/diagnostics12123154
APA StyleDotinga, M., Vriens, D., van Velden, F. H. P., Stam, M. K., Heemskerk, J. W. T., Dibbets-Schneider, P., Pool, M., Rietbergen, D. D. D., de Geus-Oei, L.-F., & Kapiteijn, E. (2022). Reinducing Radioiodine-Sensitivity in Radioiodine-Refractory Thyroid Cancer Using Lenvatinib (RESET): Study Protocol for a Single-Center, Open Label Phase II Trial. Diagnostics, 12(12), 3154. https://doi.org/10.3390/diagnostics12123154










