Gradient-Boosting Algorithm for Microwave Breast Lesion Classification—SAFE Clinical Investigation
Abstract
:1. Introduction
2. Materials and Methods
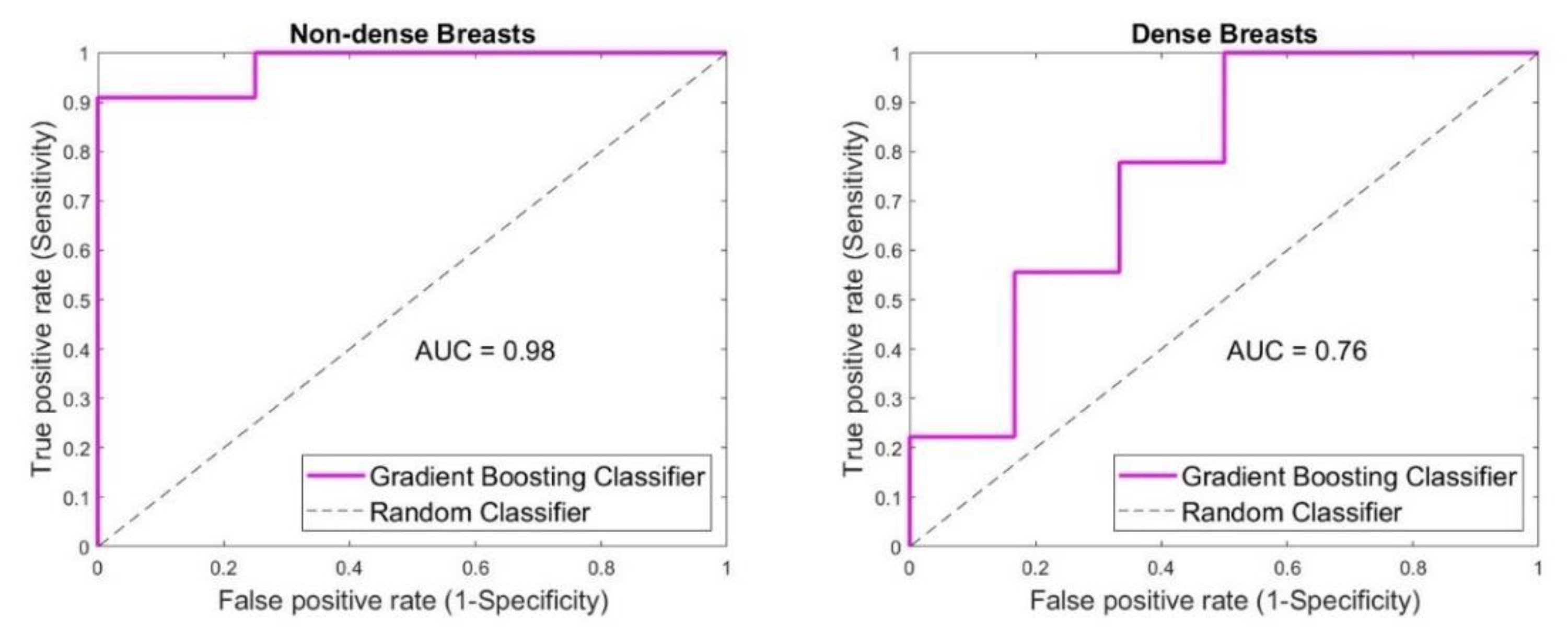
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society. Survival Rates for Breast Cancer. March 2022. Available online: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html (accessed on 10 November 2022).
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keemers-Gels, M.; Groenendijk, R.; Heuvel, J.V.D.; Boetes, C.; Peer, P.; Wobbes, T. Pain experienced by women attending breast cancer screening. Breast Cancer Res. Treat. 2000, 60, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.H.; Helvie, M.A. Overdiagnosis and Risks of Breast Cancer Screening. Radiol. Clin. N. Am. 2021, 59, 19–27. [Google Scholar] [CrossRef]
- Von Euler-Chelpin, M.; Lillholm, M.; Vejborg, I.; Nielsen, M.; Lynge, E. Sensitivity of screening mammography by density and texture: A cohort study from a population-based screening program in Denmark. Breast Cancer Res. 2019, 21, 111. [Google Scholar] [CrossRef] [Green Version]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic Density and the Risk and Detection of Breast Cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Nass, S.J.; Henderson, I.C.; Lashof, J.C. (Eds.) Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Boyd, N.F.; Rommens, J.M.; Vogt, K.; Lee, V.; Hopper, J.L.; Yaffe, M.J.; Paterson, A. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005, 6, 798–808. [Google Scholar] [CrossRef]
- Davey, B. Pain during mammography: Possible risk factors and ways to alleviate pain. Radiography 2007, 13, 229–234. [Google Scholar] [CrossRef]
- Freer, P. Mammographic Breast Density: Impact on Breast Cancer Risk and Implications for Screening. Radiographics 2015, 35, 302–315. [Google Scholar] [CrossRef]
- Mann, R.M.; Athanasiou, A.; Baltzer, P.A.; Camps-Herrero, J.; Clauser, P.; Fallenberg, E.M.; Forrai, G.; Fuchsjäger, M.H.; Helbich, T.H.; Killburn-Toppin, F.; et al. Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI) On behalf of the European Society of Breast Imaging (EUSOBI). Eur. Radiol. 2022, 32, 4036–4045. [Google Scholar] [CrossRef]
- Huynh, P.T.; Jarolimek, A.M.; Daye, S. The false-negative mammogram. RadioGraphics 1998, 18, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Slanetz, P.J.; Lee, C.I. Breast Imaging for Cancer Screening: Mammography and Ultrasonography. 2020. Available online: https://www.uptodate.com/contents/breast-imaging-for-cancer-screening-mammography-and-ultrasonography (accessed on 1 December 2021).
- Freer, P.E.; Slanetz, P.J. Breast Density and Screening for Breast Cancer. 2020. Available online: https://www.uptodate.com/contents/breast-density-and-screening-for-breast-cancer (accessed on 1 December 2021).
- Lee, J.M.; Arao, R.F.; Sprague, B.L.; Kerlikowske, K.; Lehman, C.D.; Smith, R.A.; Henderson, L.M.; Rauscher, G.H.; Miglioretti, D.L. Performance of Screening Ultrasonography as an Adjunct to Screening Mammography in Women Across the Spectrum of Breast Cancer Risk. JAMA Intern. Med. 2019, 179, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Esserman, L.J.; Joe, B.N. Diagnostic Evaluation of Women with Suspected Breast Cancer. 2019. Available online: https://www.uptodate.com/contents/diagnostic-evaluation-of-suspected-breast-cancer (accessed on 1 December 2021).
- Elmore, J.G.; Lee, C.I. Screening for Breast Cancer: Strategies and Recommendations. 2021. Available online: https://www.uptodate.com/contents/screening-for-breast-cancer-strategies-and-recommendations (accessed on 1 December 2021).
- Elmore, J.G.; Lee, C.I. Screening for Breast Cancer: Evidence for Effectiveness and Harms. 2020. Available online: https://www.uptodate.com/contents/screening-for-breast-cancer-evidence-for-effectiveness-and-harms (accessed on 1 December 2021).
- Gabriel, C.; Gabriel, S.; Corthout, Y.E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [Green Version]
- Lazebnik, M.; McCartney, L.; Popovic, D.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Magliocco, A.; Booske, J.H.; Okoniewski, M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys. Med. Biol. 2007, 52, 2637–2656. [Google Scholar] [CrossRef] [Green Version]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093. [Google Scholar] [CrossRef]
- Surowiec, A.; Stuchly, S.; Barr, J.; Swarup, A. Dielectric properties of breast carcinoma and the surrounding tissues. IEEE Trans. Biomed. Eng. 1988, 35, 257–263. [Google Scholar] [CrossRef]
- Campbell, A.M.; Land, D.V. Dielectric properties of female human breast tissue measured in vitro at 3.2 GHz. Phys. Med. Biol. 1992, 37, 193–210. [Google Scholar] [CrossRef]
- Chaudhary, S.S. Dielectric properties of normal and malignant human breast tissues at radiowave and microwave frequencies. Indian J. Biochem. Biophs. 1984, 21, 76–79. [Google Scholar]
- Kwon, S.; Lee, S. Recent Advances in Microwave Imaging for Breast Cancer Detection. Int. J. Biomed. Imaging 2016, 2016, 5054912. [Google Scholar] [CrossRef]
- O'Loughlin, D.; O'Halloran, M.J.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave Breast Imaging: Clinical Advances and Remaining Challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Benny, R.; Anjit, T.A.; Mythili, P. An overview of microwave imaging for breast tumor detection. Prog. Electromagn. Res. B 2020, 87, 61–91. [Google Scholar] [CrossRef]
- Shere, M.; Lyburn, I.; Sidebottom, R.; Massey, H.; Gillett, C.; Jones, L. MARIA® M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system (MARIA®) to detect lesions in the symptomatic breast. Eur. J. Radiol. 2019, 116, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Preece, A.W.; Craddock, I.; Shere, M.; Jones, L.; Winton, H.L. MARIA M4: Clinical evaluation of a prototype ultrawideband radar scanner for breast cancer detection. J. Med. Imaging 2016, 3, 033502. [Google Scholar] [CrossRef]
- Sani, L.; Vispa, A.; Loretoni, R.; Duranti, M.; Ghavami, N.; Sánchez-Bayuela, D.A.; Caschera, S.; Paoli, M.; Bigotti, A.; Badia, M.; et al. Breast lesion detection through MammoWave device: Empirical detection capability assessment of microwave images’ parameters. PLoS ONE 2021, 16, e0250005. [Google Scholar] [CrossRef]
- Rana, S.P.; Dey, M.; Loretoni, R.; Duranti, M.; Sani, L.; Vispa, A.; Ghavami, M.; Dudley, S.; Tiberi, G. Radial Basis Function for Breast Lesion Detection from MammoWave Clinical Data. Diagnostics 2021, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Janjic, A.; Cayoren, M.; Akduman, I.; Yilmaz, T.; Onemli, E.; Bugdayci, O.; Aribal, M.E. SAFE: A Novel Microwave Imaging System Design for Breast Cancer Screening and Early Detection—Clinical Evaluation. Diagnostics 2021, 11, 533. [Google Scholar] [CrossRef]
- Janjic, A.; Akduman, I.; Cayoren, M.; Bugdayci, O.; Erkin, M.A. Microwave Breast Lesion Classification—Results from Clinical Investigation of the SAFE Microwave Breast Cancer System. Acad. Radiol. (Rev.) 2022. [Google Scholar]
- Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Moloney, B.M.; Elwahab, S.M.A.; Kerin, M.J. Automated Breast Lesion Detection and Characterization with the Wavelia Microwave Breast Imaging System: Methodological Proof-of-Concept on First-in-Human Patient Data. Appl. Sci. 2021, 11, 9998. [Google Scholar] [CrossRef]
- Fasoula, A.; Duchesne, L.; Moloney, B.M.; Gil Cano, J.D.; Chenot, C.; Oliveira, B.L.; Bernard, J.-G.; Abd Elwahab, S.M.; Kerin, M.J. Pilot patient study with the Wavelia Microwave Breast Imaging system for breast cancer detection: Clinical feasibility and identified technical challenges. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020. [Google Scholar]
- Moloney, B.M.; McAnena, P.F.; Elwahab, S.M.A.; Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Glynn, C.; O'Connell, A.; Ennis, R.; Lowery, A.J.; et al. Microwave Imaging in Breast Cancer—Results from the First-In-Human Clinical Investigation of the Wavelia System. Acad. Radiol. 2021, 29, S211–S222. [Google Scholar] [CrossRef] [PubMed]
- Moloney, B.M.; McAnena, P.F.; Elwahab, S.M.; Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Glynn, C.; O'Connell, A.; Ennis, R.; Lowery, A.J.; et al. The Wavelia Microwave Breast Imaging system–tumour discriminating features and their clinical usefulness. Br. J. Radiol. 2021, 94, 20210907. [Google Scholar] [CrossRef] [PubMed]
- ACR BI-RADS® ATLAS-MAMMOGRAPHY. Available online: https://downloads.regulations.gov/FDA-2013-N-0134-0184/content.pdf (accessed on 11 November 2022).
- Keysight Technologies. Streamline Series P937XA USB Vector Network Analyzer 2-Port, Up to 26.5 GHz—Data Sheet. Available online: https://www.keysight.com/us/en/assets/7018-06033/data-sheets/5992-2765.pdf (accessed on 11 November 2022).







| Patient Group | Number of Cases |
|---|---|
| All cases | 54 |
| Benign cases | 29 |
| Malignant cases | 24 |
| Small breast cases | 32 |
| Large breast cases | 22 |
| Non-dense cases | 15 |
| Dense cases | 15 |
| Young cases | 21 |
| Older cases | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janjic, A.; Akduman, I.; Cayoren, M.; Bugdayci, O.; Aribal, M.E. Gradient-Boosting Algorithm for Microwave Breast Lesion Classification—SAFE Clinical Investigation. Diagnostics 2022, 12, 3151. https://doi.org/10.3390/diagnostics12123151
Janjic A, Akduman I, Cayoren M, Bugdayci O, Aribal ME. Gradient-Boosting Algorithm for Microwave Breast Lesion Classification—SAFE Clinical Investigation. Diagnostics. 2022; 12(12):3151. https://doi.org/10.3390/diagnostics12123151
Chicago/Turabian StyleJanjic, Aleksandar, Ibrahim Akduman, Mehmet Cayoren, Onur Bugdayci, and Mustafa Erkin Aribal. 2022. "Gradient-Boosting Algorithm for Microwave Breast Lesion Classification—SAFE Clinical Investigation" Diagnostics 12, no. 12: 3151. https://doi.org/10.3390/diagnostics12123151
APA StyleJanjic, A., Akduman, I., Cayoren, M., Bugdayci, O., & Aribal, M. E. (2022). Gradient-Boosting Algorithm for Microwave Breast Lesion Classification—SAFE Clinical Investigation. Diagnostics, 12(12), 3151. https://doi.org/10.3390/diagnostics12123151








