Application of Procalcitonin for the Rapid Diagnosis of Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease
Abstract
:1. Background
2. Methods
2.1. Patient Eligibility and Classification
2.2. Detection of Clostridioides Difficile
2.3. Laboratory Measurement of Inflammatory Markers
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Evaluation of Diagnostic Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| PCT | procalcitonin |
| CRP | C-reactive protein |
| WBC | white blood cell |
| CDI | Clostridioides difficile infection |
| IBD | inflammatory bowel disease |
| ROC | receiver operating characteristic |
| CD | Crohn’s disease |
| UC | ulcerative colitis |
| NAAT | nucleic acid amplification test |
| PCR | polymerase chain reaction |
| EIA | enzyme immunoassay |
| GDH | glutamate dehydrogenase |
| EBV | Epstein–Barr |
| CMV | cytomegalovirus |
| HSV-1 | herpes simplex virus type 1 |
| HSV-2 | herpes simplex virus type 2 |
| LR | positive likelihood ratio |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| IFN-γ | interferon-gamma |
References
- Danese, S.; Fiocchi, C. Ulcerative colitis. N. Engl. J. Med. 2011, 365, 1713–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.-P. Long-Term Evolution of Disease Behavior of Crohn’s Disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Lawley, T.D. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 2013, 69, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Purchiaroni, F.; Tortora, A.; Gabrielli, M.; Bertucci, F.; Gigante, G.; Ianiro, G.; Ojetti, V.; Scarpellini, E.; Gasbarrini, A. The role of intestinal microbiota and the immune system. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 323–333. [Google Scholar]
- Enguix, A.; Rey, C.; Concha, A.; Medina, A.; Coto, D.; Diéguez, M.A. Comparison of procalcitonin with C-reactive protein and serum amyloid for the early diagnosis of bacterial sepsis in critically ill neonates and children. Intensiv. Care Med. 2000, 27, 211–215. [Google Scholar] [CrossRef]
- Singh, H.; Nugent, Z.; Yu, B.N.; Lix, L.M.; Targownik, L.E.; Bernstein, C.N. Higher Incidence of Clostridium difficile Infection among individuals with inflammatory bowel disease. Gastroenterology 2017, 153, 430–438. [Google Scholar] [CrossRef]
- Hanada, Y.; Khanna, S.; Loftus, E.V.; Raffals, L.E.; Pardi, D.S. Non–Clostridium difficile Bacterial Infections Are Rare in Patients With Flares of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 528–533. [Google Scholar] [CrossRef]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef]
- Berg, A.M.; Kelly, C.P.; Farraye, F. Clostridium difficile Infection in the Inflammatory Bowel Disease Patient. Inflamm. Bowel Dis. 2013, 19, 194–204. [Google Scholar] [CrossRef]
- Khanna, S.; Shin, A.; Kelly, C.P. Management of Clostridium difficile Infection in Inflammatory Bowel Disease: Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clin. Gastroenterol. Hepatol. 2017, 15, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.H.; Gerding, D.N.; Johnson, S.; Kelly, C.P.; Loo, V.G.; McDonald, L.C.; Pépin, J.L.; Wilcox, M.H. Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control. Hosp. Epidemiol. 2010, 31, 431–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debast, S.; Bauer, M.; Kuijper, E. European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2014, 20 (Suppl. 2), 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planche, T.D.; A Davies, K.; Coen, P.G.; Finney, J.M.; Monahan, I.M.; A Morris, K.; O’Connor, L.; Oakley, S.J.; Pope, C.F.; Wren, M.W.; et al. Differences in outcome according to Clostridium difficile testing method: A prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect. Dis. 2013, 13, 936–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polage, C.R.; Gyorke, C.E.; Kennedy, M.A.; Leslie, J.L.; Chin, D.L.; Wang, S.; Nguyen, H.H.; Huang, B.; Tang, Y.-W.; Lee, L.W.; et al. Overdiagnosis of Clostridium difficile Infection in the Molecular Test Era. JAMA Intern. Med. 2015, 175, 1792–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyne, L.; Warny, M.; Qamar, A.; Kelly, C.P. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 2000, 342, 390–397. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Marquess, J.; Yakob, L.; Riley, T.V.; Paterson, D.L.; Foster, N.F.; Huber, C.A.; Clements, A.C.A. Asymptomatic Clostridium difficile colonization: Epidemiology and clinical implications. BMC Infect. Dis. 2015, 15, 516. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Khanna, S. Repeat Clostridium difficile Testing. JAMA 2016, 316, 2422–2423. [Google Scholar] [CrossRef]
- Tang, Y.M.; Stone, C.D. Clostridium difficile infection in inflammatory bowel disease: Challenges in diagnosis and treatment. Clin. J. Gastroenterol. 2017, 10, 112–123. [Google Scholar] [CrossRef]
- Gupta, A.; Cifu, A.S.; Khanna, S. Diagnosis and Treatment of Clostridium difficile Infection. JAMA 2018, 320, 1031–1032. [Google Scholar] [CrossRef]
- Yi, F.; Wu, J. Biomarkers of inflammatory bowel disease. Dis. Markers 2014, 2014, 710915. [Google Scholar]
- van der Galiën, H.T.; Loeffen, E.A.H.; Miedema, K.G.E.; Tissing, W.J.E. Predictive value of PCT and IL-6 for bacterial infection in children with cancer and febrile neutropenia. Support. Care Cancer 2018, 26, 3819–3826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inflammatory Bowel Disease Group; Chinese Society of Gastroenterology; Chinese Medical Association. Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). J. Dig. Dis. 2021, 22, 298–317. [Google Scholar] [CrossRef] [PubMed]
- A Miller, M.; Louie, T.; Mullane, K.; Weiss, K.; Lentnek, A.; Golan, Y.; Kean, Y.; Sears, P. Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect. Dis. 2013, 13, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jen, M.H.; Saxena, S.; Bottle, A.; Aylin, P.; Pollok, R.C.G. Increased health burden associated with Clostridium difficile diarrhea in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2011, 33, 1322–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitikainen, K.; Haapamäki, J.; Färkkilä, M.; Anttila, V.-J.; Arkkila, P. Clostridium difficile infection in patients with inflammatory bowel disease: A case control study. Scand. J. Gastroenterol. 2018, 53, 947–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crobach, M.; Planche, T.; Eckert, C.; Barbut, F.; Terveer, E.; Dekkers, O.; Wilcox, M.; Kuijper, E. European Society of Clinical Microbiology and Infectious Diseases: Update of the diagnostic guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 2016, 22, S63–S81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, Y.; Yashiro, M.; Yamada, M.; Kikkawa, T.; Nosaka, N.; Saito, Y.; Tsukahara, K.; Ikeda, M.; Morishima, T.; Tsukahara, H. Serum Procalcitonin Levels in Acute Encephalopathy with Biphasic Seizures and Late Reduced Diffusion. J. Dis. Markers 2018, 2018, 2380179. [Google Scholar] [CrossRef] [Green Version]
- Sager, R.; Kutz, A.; Mueller, B.; Schuetz, P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Müller, B.; Becker, K.L.; Schächinger, H.; Rickenbacher, P.R.; Huber, P.R.; Zimmerli, W.; Ritz, R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit. Care Med. 2000, 28, 977–983. [Google Scholar] [CrossRef]
- Muller, F.; Christ-Crain, M.; Bregenzer, T.; Krause, M.; Zimmerli, W.; Mueller, B.; Schuetz, P. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: A prospective cohort trial. Chest 2010, 138, 121–129. [Google Scholar] [CrossRef]

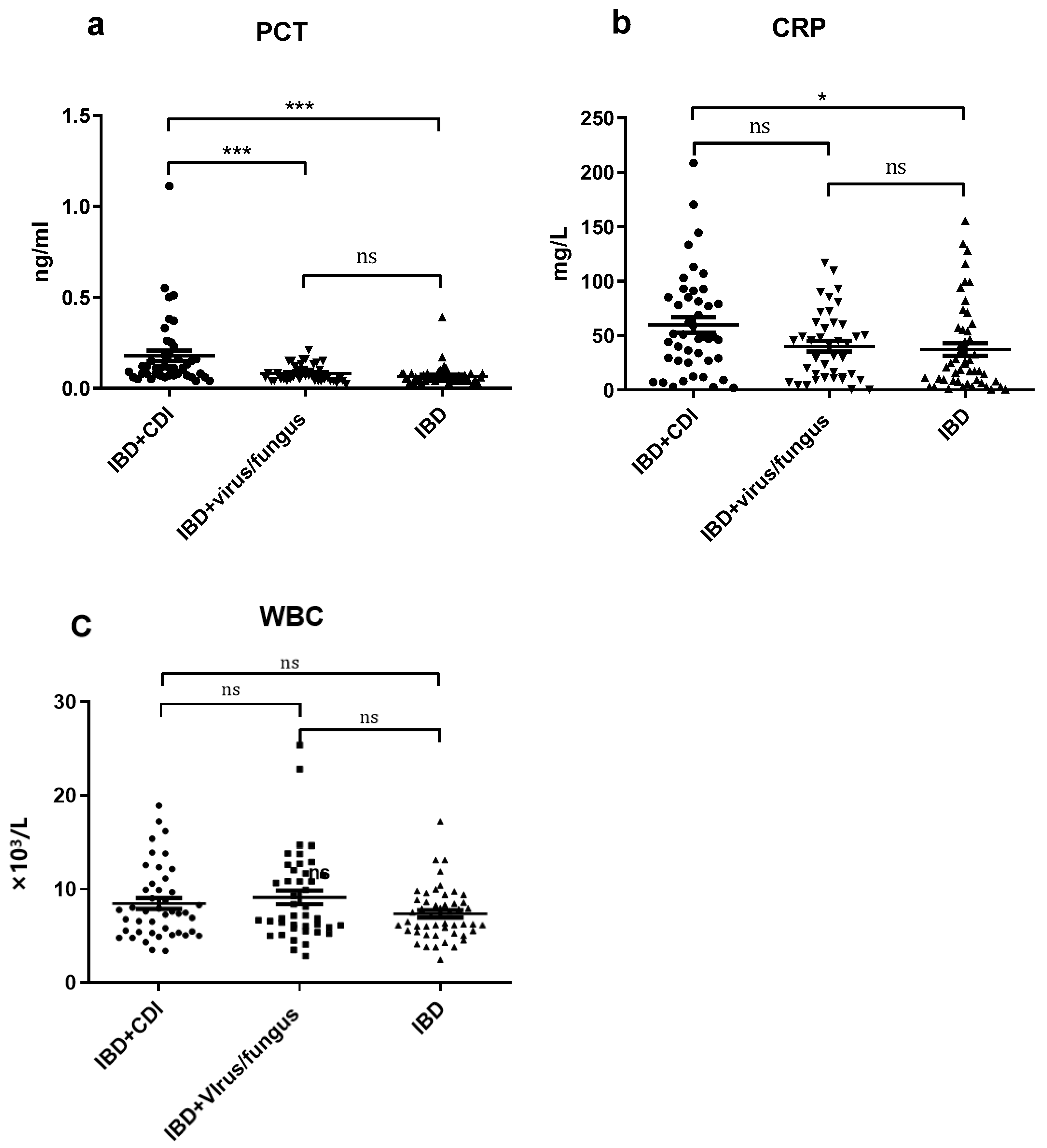

| Characteristic | Crohn’s Disease (n = 83) | Ulcerative Colitis (n = 52) | p-Value |
|---|---|---|---|
| Age, y, median (IQR) | 27.0 (15–61) | 49.5 (18–70) | <0.0001 *** |
| Male, n (%) | 50 (60.2) | 28 (53.8) | 0.391 |
| CDI incidence, n (%) | 32 (38.6) | 12 (23.1) | 0.014 * |
| Colectomy, n (%) | 18 (21.7) | 6 (11.5) | 0.056 |
| Chi-Square Test | Male, n (%) p-Value | Colectomy, n (%) p-Value | Active Period, n (%) p-Value |
|---|---|---|---|
| IBD+CDI vs. IBD+ virus/fungus | 0.031 * | 0.675 | 0.675 |
| IBD+CDI vs. IBD | 0.127 | 0.181 | 0.520 |
| IBD+ virus/fungus vs. IBD | 0.476 | 0.369 | 0.290 |
| Characteristic | IBD with CDI (n = 44) | IBD with Viral/Fungus Infections (n = 42) | IBD Control Group (n = 49) | One-Way ANOVA p-Value |
|---|---|---|---|---|
| PCT (ng/mL) Mean ± SEM | 0.178 ± 0.029 | 0.081 ± 0.007 | 0.066 ± 0.007 | <0.0001 *** |
| CRP (mg/L) Mean ± SEM | 59.70 ± 7.01 | 40.14 ± 4.91 | 37.35 ± 5.71 | 0.0177 * |
| WBC count (×109/L) Mean ± SEM | 8.48 ± 0.57 | 8.93 ± 0.71 | 7.38 ± 0.39 | 0.130 |
| Tukey’s Multiple Comparison Test | PCT p-Value | CRP p-Value | WBC p-Value |
|---|---|---|---|
| IBD+CDI vs. IBD+ virus/fungus | 0.821 | 0.066 | 0.840 |
| IBD+CDI vs. IBD | <0.001 * | 0.022 * | 0.345 |
| IBD+ virus/fungus vs. IBD | <0.001 * | 0.942 | 0.126 |
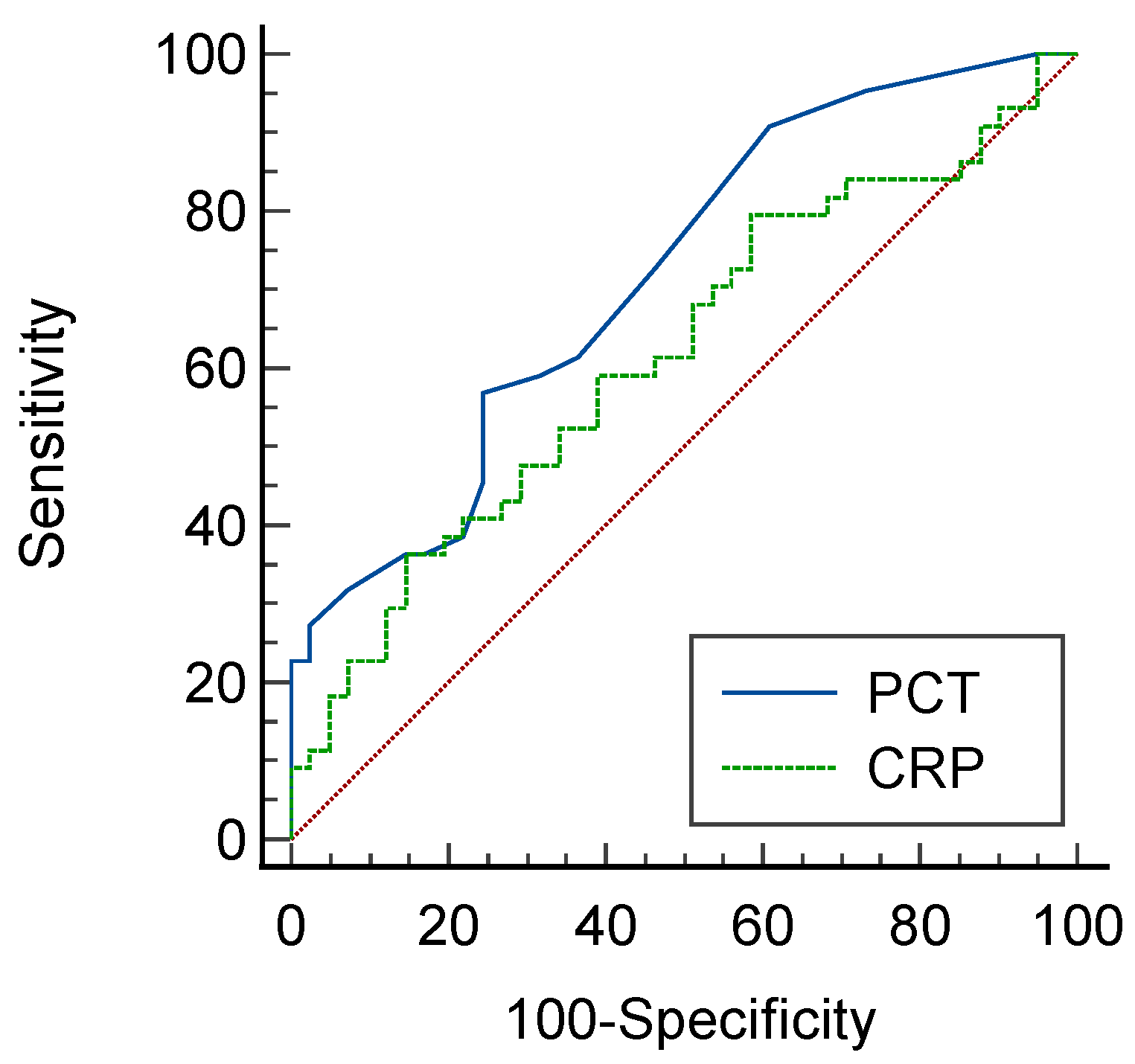
| Sensitivity% | 95%CI | Specificity% | 95%CI | LR+ |
|---|---|---|---|---|
| 59.09% | 43.2–73.7% | 89.80% | 77.8–96.6% | 5.79 |
| ATLAS Score | Number of Patients | PCT | Rate of Colectomy |
|---|---|---|---|
| Mean ± SEM (ng/mL) | n (%) | ||
| 0 | 11 | 0.1255 + 0.0285 | 2 (16.7%) |
| 1 | 17 | 0.1888 + 0.0630 | 3 (17.6%) |
| 2 | 6 | 0.1783 + 0.0715 | 1 (16.7%) |
| 3 | 6 | 0.2317 + 0.0766 | 2 (33.3%) |
| 4 | 2 | 0.1000 + 0.0600 | 1 (50%) |
| 5 | 1 | 0.3800 | 0 |
| 6 | 1 | 0.1800 | 1 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Chen, P.; Wang, D.; Jiang, X.; Wu, Z.; Liao, K.; Liu, M.; Zhang, S.; Chen, Y. Application of Procalcitonin for the Rapid Diagnosis of Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease. Diagnostics 2022, 12, 3108. https://doi.org/10.3390/diagnostics12123108
Xie S, Chen P, Wang D, Jiang X, Wu Z, Liao K, Liu M, Zhang S, Chen Y. Application of Procalcitonin for the Rapid Diagnosis of Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease. Diagnostics. 2022; 12(12):3108. https://doi.org/10.3390/diagnostics12123108
Chicago/Turabian StyleXie, Shuhua, Peisong Chen, Dong Wang, Xiaobing Jiang, Zhongwen Wu, Kang Liao, Min Liu, Shihong Zhang, and Yili Chen. 2022. "Application of Procalcitonin for the Rapid Diagnosis of Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease" Diagnostics 12, no. 12: 3108. https://doi.org/10.3390/diagnostics12123108
APA StyleXie, S., Chen, P., Wang, D., Jiang, X., Wu, Z., Liao, K., Liu, M., Zhang, S., & Chen, Y. (2022). Application of Procalcitonin for the Rapid Diagnosis of Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease. Diagnostics, 12(12), 3108. https://doi.org/10.3390/diagnostics12123108







