Clinical, Sonographic, and Hysteroscopic Features of Endometrial Carcinoma Diagnosed after Hysterectomy in Patients with a Preoperative Diagnosis of Atypical Hyperplasia: A Single-Center Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
- -
- An interview with the patient to collect anamnestic and clinical data.
- -
- A TVUS performed by an expert highly-trained sonographer (L.M.) with an Affiniti 70 ultrasound machine (Philips, Amsterdam, The Netherlands, 2013) equipped either with a C10-3v Endocavitary Probe with a 3.0–10.0 MHz frequency range; all examinations were performed according to the recommendations of the main international guidelines [7,8].
- -
- A hysteroscopy was performed in an outpatient setting by two highly trained expert operators (A.F. and G.P.) with an endometrial biopsy. All the procedures included vaginoscopy, distension of the uterine cavity with normal saline, diagnostic evaluation of the cervical canal and uterine cavity with visualization of tubal ostia, and targeted biopsy on any suspicious area of the endometrium using a BETTOCCHI® Hysteroscope equipped with bipolar electrode systems [9]. Diagnosis of AEH was made on endometrial specimens according to WHO 2014 criteria [1].
- -
- The anamnestic features, including age, body mass index (BMI), parity, menopausal status, the prevalence of diabetes and hypertension, use of hormone replacement therapy or tamoxifen, and symptoms;
- -
- The ultrasound characteristics regarding endometrial thickness and echogenicity, endometrial–myometrial junction, presence of intracavitary fluid, vascularization at color Doppler (CD) study, size and appearance of the lesion, posterior sliding sign, uterine volume calculated by the formula ellipsoid volume [12], and presence of leiomyomas;
- -
- The hysteroscopic reports about the appearance of the lesion (protruding into the uterine cavity vs. superficial anomaly of the endometrium), presence of necrosis or atypical vascular pattern, subjective assessment indicative of carcinoma by the operator, and visualization of tubal ostia;
- -
- The histopathological reports on the endometrial biopsy regarding the presence of endometrial intraepithelial neoplasia, multiple foci of hyperplasia, and endometrial polyp with AEH arising on its surface, and the number of specimens retrieved by the hysteroscopy operator;
- -
- Histopathological reports on the uterus, most notably the presence of endometrial carcinoma and its features according to WHO 2014 classification [13].
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sobczuk, K.; Sobczuk, A. New classification system of endometrial hyperplasia WHO 2014 and its clinical implications. Menopausal Rev. 2017, 16, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, P.A.; Critchley, H.O.; Williams, A.R.; Arends, M.J.; Saunders, P.T. New concepts for an old problem: The diagnosis of endometrial hyperplasia. Hum. Reprod. Updat. 2017, 23, 232–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetter, M.H.; Smith, B.; Benedict, J.; Hade, E.M.; Bixel, K.; Copeland, L.J.; Cohn, D.E.; Fowler, J.M.; O’Malley, D.; Salani, R.; et al. Preoperative predictors of endometrial cancer at time of hysterectomy for endometrial intraepithelial neoplasia or complex atypical hyperplasia. Am. J. Obstet. Gynecol. 2020, 222, 60.e1–60.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimble, C.L.; Kauderer, J.; Zaino, R.; Silverberg, S.; Lim, P.C.; Burke, J.J.; Alberts, D.; Curtin, J. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: A Gynecologic Oncology Group study. Cancer 2006, 106, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Manap, N.A.; Ng, B.K.; Phon, S.E.; Karim, A.K.A.; Lim, P.S.; Fadhil, M. Endometrial Cancer in Pre-Menopausal Women and Younger: Risk Factors and Outcome. Int. J. Environ. Res. Public Health 2022, 19, 9059. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Leone, F.P.G.; Timmerman, D.; Bourne, T.; Valentin, L.; Epstein, E.; Goldstein, S.R.; Marret, H.; Parsons, A.K.; Gull, B.; Istre, O.; et al. Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: A consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet. Gynecol. 2010, 35, 103–112. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Carugno, J.; Grimbizis, G.; Franchini, M.; Alonso, L.; Bradley, L.; Campo, R.; Catena, U.; De Angelis, C.; Sardo, A.D.S.; Farrugia, M.; et al. International Consensus Statement for recommended terminology describing hysteroscopic procedures. Facts Views Vis. Obgyn. 2021, 13, 287–294. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. Management of Endometrial Hyperplasia (Green-top Guideline No. 67); RCOG/BSGE Joint Guideline|February 2016; Royal College of Obstetricians and Gynaecologists: London, UK, 2016. [Google Scholar]
- Parkash, V.; Fadare, O.; Tornos, C.; McCluggage, W.G. Committee Opinion No. 631: Endometrial Intraepithelial Neoplasia. Obstet. Gynecol. 2015, 126, 897. [Google Scholar] [CrossRef]
- Casikar, I.; Mongelli, M.; Reid, S.; Condous, G. Estimation of uterine volume: A comparison between Viewpoint and 3D ultrasound estimation in women undergoing laparoscopic hysterectomy. Australas. J. Ultrasound Med. 2015, 18, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Creutzberg, C.L.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.A.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 478, 153–190. [Google Scholar]
- Koskas, M.; Amant, F.; Mirza, M.R.; Creutzberg, C.L. Cancer of the corpus uteri: 2021 update. Int. J. Gynecol. Obstet. 2021, 155 (Suppl. 1), 45–60. [Google Scholar] [CrossRef]
- Touboul, C.; Piel, B.; Koskas, M.; Gonthier, C.; Ballester, M.; Cortez, A.; Daraï, E. Factors predictive of endometrial carcinoma in patients with atypical endometrial hyperplasia on preoperative histology. Anticancer Res. 2014, 34, 5671–5676. [Google Scholar]
- Abt, D.; Macharia, A.; Hacker, M.R.; Baig, R.; Esselen, K.M.; Ducie, J. Endometrial stripe thickness: A preoperative marker to identify patients with endometrial intraepithelial neoplasia who may benefit from sentinel lymph node mapping and biopsy. Int. J. Gynecol. Cancer 2022, 32, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Weiss, E.; Feldstein, V. How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound Obstet. Gynecol. 2004, 24, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.M.; Li, Y.; Yu, H.X.; Li, Y.K.; Du, J.H.; Chen, H. Diagnostic value of endometrial volume and flow parameters under 3D ultrasound acquisition in combination with serum CA125 in endometrial lesions. Taiwan Obstet. Gynecol. 2021, 60, 492–497. [Google Scholar] [CrossRef]
- Gharibvand, M.M.; Ahmadzade, A.; Azhine, S. Correlation of color Doppler ultrasound and pathological grading in endometrial carcinoma. J. Fam. Med. Prim. Care 2020, 9, 5188–5192. [Google Scholar] [CrossRef]
- Bosch, T.V.D.; Verbakel, J.Y.; Valentin, L.; Wynants, L.; De Cock, B.; Pascual, M.A.; Leone, F.P.G.; Sladkevicius, P.; Alcazar, J.L.; Votino, A.; et al. Typical ultrasound features of various endometrial pathologies described using International Endometrial Tumor Analysis (IETA) terminology in women with abnormal uterine bleeding. Ultrasound Obstet. Gynecol. 2021, 57, 164–172. [Google Scholar] [CrossRef]
- Garuti, G.; Mirra, M.; Luerti, M. Hysteroscopic view in atypical endometrial hyperplasias: A correlation with pathologic findings on hysterectomy specimens. J. Minim. Invasive Gynecol. 2006, 13, 325–330. [Google Scholar] [CrossRef]
- Daniele, A.; Ferrero, A.; Maggiorotto, F.; Perrini, G.; Volpi, E.; Sismondi, P. Suspecting malignancy in endometrial polyps: Value of hysteroscopy. Tumori J. 2013, 99, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.; Hjorth, I.M.; Secher, P.; Jørgensen, A.; Ørtoft, G. Structured Hysteroscopic Evaluation of Endometrium in Women With Postmenopausal Bleeding. J. Minim. Invasive Gynecol. 2015, 22, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Riemma, G.; Carugno, J.; Chiofalo, B.; Vilos, G.A.; Cianci, S.; Budak, M.S.; Lasmar, B.P.; Raffone, A.; Kahramanoglu, I. Hysteroscopy in the management of endometrial hyperplasia and cancer in reproductive aged women: New developments and current perspectives. Transl. Cancer Res. 2020, 9, 7767–7777. [Google Scholar] [CrossRef] [PubMed]
- Spadoto-Dias, D.; Dias, F.N.B.; Dias, R.; Nahás-Neto, J.; Nahas, E.; Leite, N.J.; Domingues, M.A.C.; Angela, S.P.B.; Padovani, C.R. Usefulness of clinical, ultrasonographic, hysteroscopic, and immunohistochemical parameters in differentiating endometrial polyps from endometrial cancer. J. Minim. Invasive Gynecol. 2014, 21, 296–302. [Google Scholar] [CrossRef]
- Shutter, J.; Wright, T.C., Jr. Prevalence of underlying adenocarcinoma in women with atypical endometrial hyperplasia. Int. J. Gynecol. Pathol. 2005, 24, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Bidus, M.A.; Pulcini, J.P.; Maxwell, G.L.; Cosin, J.A.; Rose, G.S. The ability of endometrial biopsies with atypical complex hyperplasia to guide surgical management. Am. J. Obstet. Gynecol. 2008, 199, 69.e1–69.e4. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.W.; Philp, L.; Kanbergs, A.N.; Safdar, N.; Oliva, E.; Bregar, A.; del Carmen, M.G.; Eisenhauer, E.L.; Goodman, A.; Muto, M.; et al. Lymph node assessment at the time of hysterectomy has limited clinical utility for patients with pre-cancerous endometrial lesions. Gynecol. Oncol. 2021, 162, 613–618. [Google Scholar] [CrossRef]
- Touhami, O.; Grégoire, J.; Renaud, M.C.; Sebastianelli, A.; Grondin, K.; Plante, M. The utility of sentinel lymph node mapping in the management of endometrial atypical hyperplasia. Gynecol. Oncol. 2018, 148, 485–490. [Google Scholar] [CrossRef]
- Lim, S.L.; Moss, H.A.; Secord, A.A.; Lee, P.S.; Havrilesky, L.J.; Davidson, B.A. Hysterectomy with sentinel lymph node biopsy in the setting of pre-operative diagnosis of endometrial intraepithelial neoplasia: A cost-effectiveness analysis. Gynecol. Oncol. 2018, 151, 506–512. [Google Scholar] [CrossRef]
- ASTEC study group; Kitchener, H.; Swart, A.M.C.; Qian, Q.; Amos, C.; Parmar, M.K.B. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised studys. Lancet 2009, 373, 125–136, Erratum in: Lancet 2009, 373, 1764. [Google Scholar] [CrossRef]
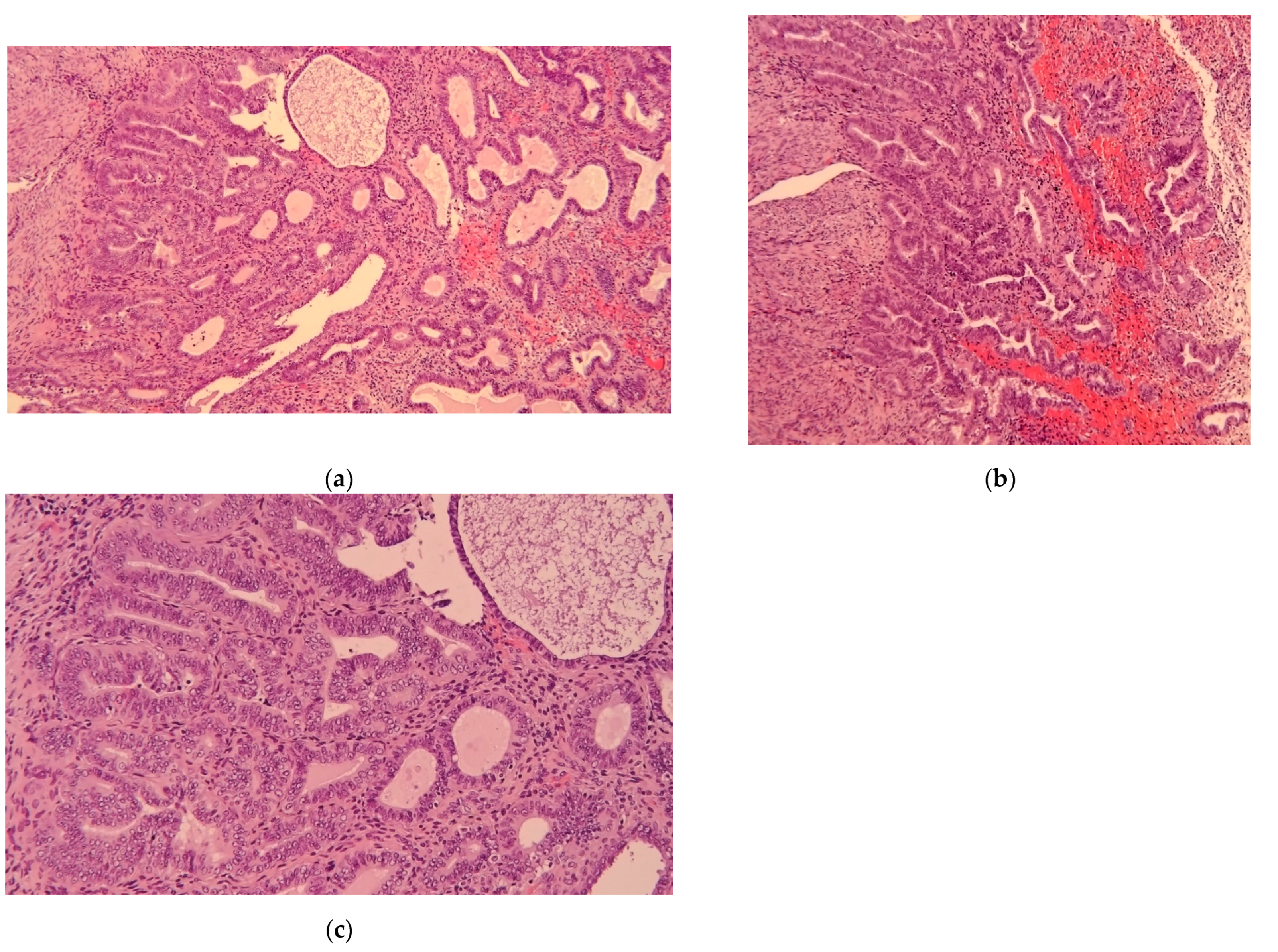
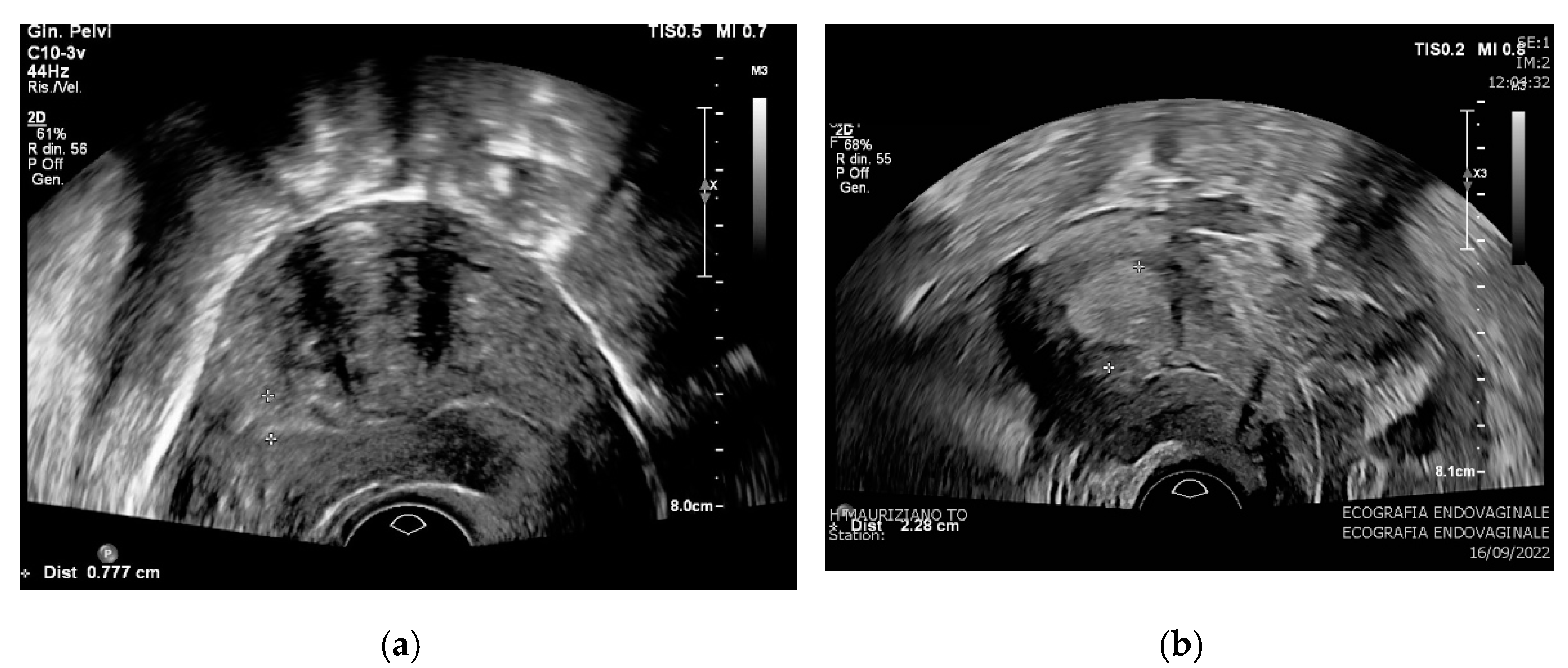

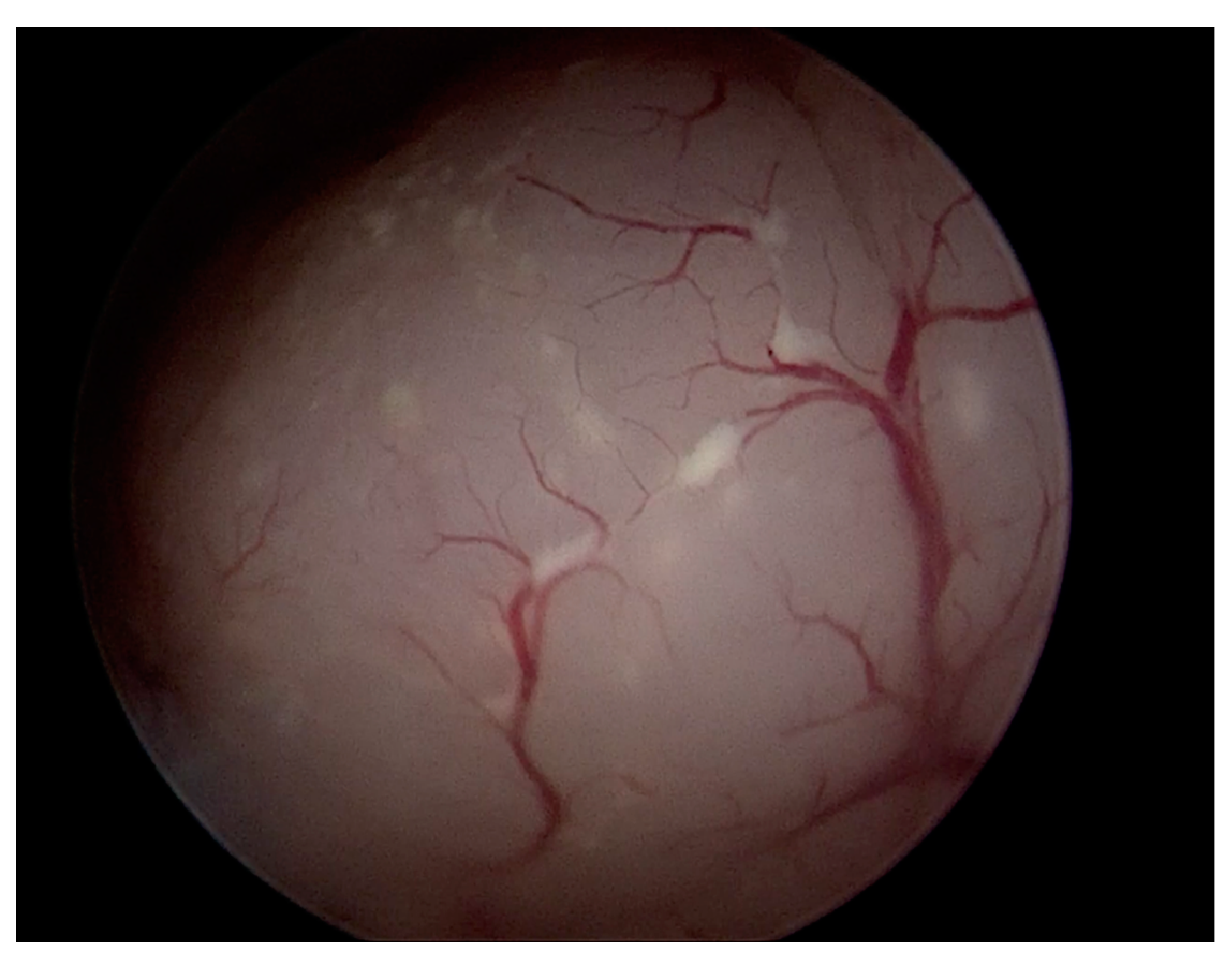
| Category | Characteristics | |
|---|---|---|
| Clinical features | Age at diagnosis (years) * | 64.9 ± 1.1 |
| BMI (kg/m2) * | 30.7 ± 1.0 | |
| Obesity (%) | 32 (45.1) | |
| Diabetes (%) | 16 (21.6) | |
| Hypertension (%) | 40 (54.1) | |
| Number of VD * | 1.2 ± 0.1 | |
| Post-menopausal status (%) | 67 (87.0) | |
| Time between menopause and diagnosis (years) | 14.7 ± 1.1 | |
| Use of HRT (%) | 0 (0) | |
| Use of tamoxifene (%) | 5 (6.9) | |
| Presence of AUB (%) | 52 (67.5) | |
| Ultrasonography features | Endometrial thickness (mm) * | 16.3 ± 1.7 |
| Non-uniform endometrial echogenicity (%) | 10 (12.5) | |
| Irregular endometrial–myometrial junction (%) | 18 (31.6) | |
| Intracavitary fluid (%) | 6 (8.5) | |
| Intracavitary vascularization at CD (%) | 43 (54.4) | |
| Focal endometrial lesion (%) | 24 (37.5) | |
| Maximum diameter of the lesion (ml) * | 22.0 ± 2.5 | |
| Volume of the uterus (cm3) * | 76.4 ± 6.6 | |
| Presence of uterine fibroids (%) | 29 (41.4) | |
| Hysteroscopy features | Protruding intracavitary lesion (%) | 48 (60) |
| Necrosis (%) | 24 (31.6) | |
| Atypical vascularization (%) | 44 (58.7) | |
| Visualization of tubal ostia (%) | 80 (100) | |
| Subjective assessment suggesting cancer (%) | 43 (58.1) | |
| EH on endometrial polyps (%) | 41 (52.6) | |
| EIN (%) | 6 (7.7) | |
| Multiple foci of hyperplasia (%) | 30 (42.9) | |
| Number of endometrial biopsies * | 1.7 ± 0.07 |
| Variables | Endometrial Hyperplasia (N = 27) | Endometrial Carcinoma (N = 53) | p § |
|---|---|---|---|
| Age at diagnosis (years) * | 62.3 ± 1.8 | 66.2 ± 1.4 | 0.09 |
| BMI (kg/m2) * | 29.3 ± 1.5 | 31.4 ± 1.2 | 0.29 |
| Obesity (%) | 11 (47.8) | 21 (43.8) | 0.80 |
| Diabetes (%) | 5 (20.8) | 11 (22.0) | 0.91 |
| Hypertension (%) | 11 (45.8) | 29 (58) | 0.46 |
| Number of VD * | 1.1 ± 0.2 | 1.3 ± 0.2 | 0.42 |
| Post-menopausal status (%) | 23 (88.5) | 44 (86.3) | 0.79 |
| Time between menopause and diagnosis (years) * | 12.6 ± 1.9 | 15.7 ± 1.4 | 0.19 |
| Use of HRT (%) | 0 (0) | 0 (0) | - |
| Use of tamoxifene (%) | 1 (4.0) | 4 (8.5) | 0.65 |
| Presence of AUB (%) | 15 (57.7) | 37 (72.5) | 0.21 |
| Variables | Endometrial Hyperplasia (N = 27) | Endometrial Carcinoma (N = 53) | p § |
|---|---|---|---|
| Endometrial thickness (mm) * | 10.3 ± 1.3 | 20.3 ± 2.4 | 0.001 |
| Non-uniform endometrial echogenicity (%) | 2 (7.4) | 8 (15.1) | 0.48 |
| Irregular endometrial-myometrial junction (%) | 1 (6.7) | 17 (40.5) | 0.022 |
| Intracavitary fluid (%) | 2 (8.7) | 4 (8.3) | 0.96 |
| Intracavitary vascularization at CD (%) | 9 (34.6) | 34 (64.2) | 0.017 |
| Focal endometrial lesion (%) | 8 (44.4) | 16 (34.8) | 0.57 |
| Maximum diameter of the lesion (mm) * | 10.6 ± 2.5 | 25.2 ± 3.0 | 0.001 |
| Volume of the uterus (cm3) * | 78.5 ± 10.4 | 75.6 ± 8.3 | 0.83 |
| Presence of uterine fibroids (%) | 6 (27.3) | 23 (47.9) | 0.12 |
| Variables | Endometrial Hyperplasia (N = 27) | Endometrial Carcinoma (N = 53) | p § |
|---|---|---|---|
| Protruding intracavitary lesion (%) | 21 (77.8) | 27 (50.9) | 0.029 |
| Necrosis (%) | 1 (4.2) | 23 (44.2) | 0.001 |
| Atypical vascularization (%) | 8 (33.3) | 36 (70.6) | 0.003 |
| Visualization of tubal ostia (%) | 27 (100) | 53 (100) | - |
| Subjective assessment suggesting cancer (%) | 3 (12.5) | 40 (80.0) | 0.001 |
| Variables | Endometrial Hyperplasia (N = 27) | Endometrial Carcinoma (N = 53) | p § |
|---|---|---|---|
| EH on endometrial polyp (%) | 19 (73.1) | 22 (42.3) | 0.016 |
| Multiple foci of hyperplasia (%) | 11 (44.0) | 19 (42.2) | 0.86 |
| Number of endometrial biopsies * | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pace, L.; Actis, S.; Mancarella, M.; Novara, L.; Mariani, L.; Perrini, G.; Govone, F.; Testi, A.; Campisi, P.; Ferrero, A.; et al. Clinical, Sonographic, and Hysteroscopic Features of Endometrial Carcinoma Diagnosed after Hysterectomy in Patients with a Preoperative Diagnosis of Atypical Hyperplasia: A Single-Center Retrospective Study. Diagnostics 2022, 12, 3029. https://doi.org/10.3390/diagnostics12123029
Pace L, Actis S, Mancarella M, Novara L, Mariani L, Perrini G, Govone F, Testi A, Campisi P, Ferrero A, et al. Clinical, Sonographic, and Hysteroscopic Features of Endometrial Carcinoma Diagnosed after Hysterectomy in Patients with a Preoperative Diagnosis of Atypical Hyperplasia: A Single-Center Retrospective Study. Diagnostics. 2022; 12(12):3029. https://doi.org/10.3390/diagnostics12123029
Chicago/Turabian StylePace, Luca, Silvia Actis, Matteo Mancarella, Lorenzo Novara, Luca Mariani, Gaetano Perrini, Francesca Govone, Alessandra Testi, Paola Campisi, Annamaria Ferrero, and et al. 2022. "Clinical, Sonographic, and Hysteroscopic Features of Endometrial Carcinoma Diagnosed after Hysterectomy in Patients with a Preoperative Diagnosis of Atypical Hyperplasia: A Single-Center Retrospective Study" Diagnostics 12, no. 12: 3029. https://doi.org/10.3390/diagnostics12123029
APA StylePace, L., Actis, S., Mancarella, M., Novara, L., Mariani, L., Perrini, G., Govone, F., Testi, A., Campisi, P., Ferrero, A., & Biglia, N. (2022). Clinical, Sonographic, and Hysteroscopic Features of Endometrial Carcinoma Diagnosed after Hysterectomy in Patients with a Preoperative Diagnosis of Atypical Hyperplasia: A Single-Center Retrospective Study. Diagnostics, 12(12), 3029. https://doi.org/10.3390/diagnostics12123029







