Diagnosis and Treatment of Acute Pleural Effusion following Radioiodine Remnant Ablation Post Lobectomy for Thyroid Cancer
Abstract
:1. Introduction
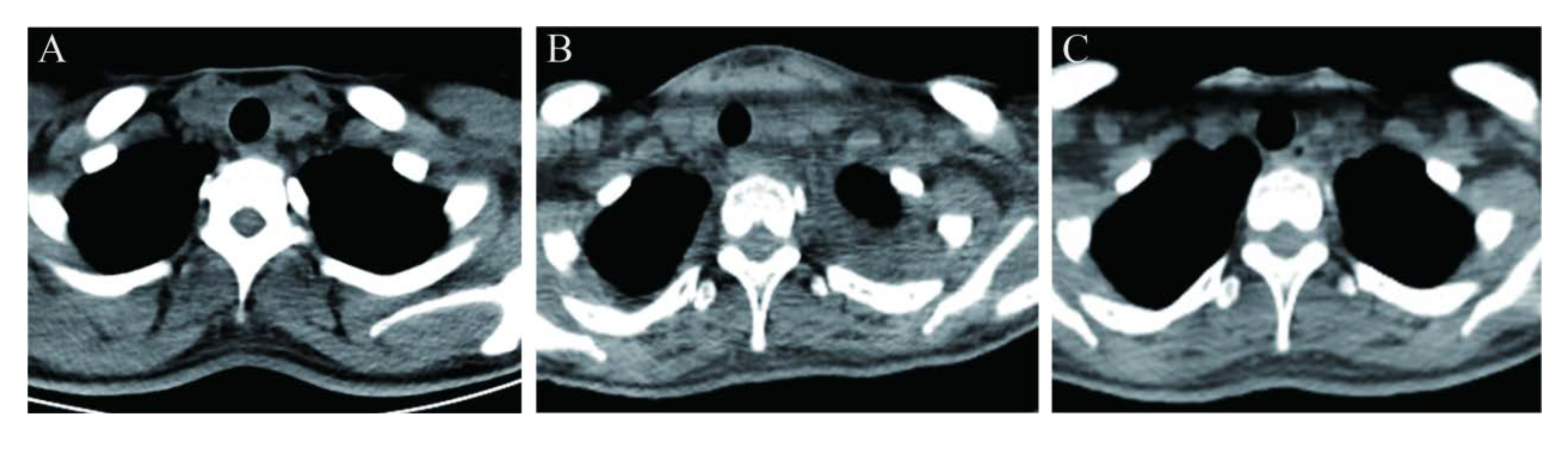
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Nixon, I.J.; Whitcher, M.M.; Palmer, F.L.; Tuttle, R.M.; Shaha, A.R.; Shah, J.P.; Patel, S.G.; Ganly, I. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid 2012, 22, 884–889. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Shiozawa, T.; Satoh, H.; Kurishima, K.; Kagohashi, K.; Takayashiki, N.; Hizawa, N. Pleural fluid due to papillary thyroid cancer. Oncol. Lett. 2019, 18, 962–966. [Google Scholar] [CrossRef] [Green Version]
- Olson, M.T.; Nuransoy, A.; Ali, S.Z. Malignant pleural effusion resulting from metastasis of thyroid primaries: A cytomorphological analysis. Acta Cytol. 2013, 57, 177–183. [Google Scholar] [CrossRef]
- Hsu, K.F.; Hsieh, C.B.; Duh, Q.Y.; Chien, C.F.; Li, H.S.; Shih, M.L. Hürthle cell carcinoma of the thyroid with contralateral malignant pleural effusion. Onkologie 2009, 32, 47–49. [Google Scholar] [CrossRef]
- Vassilopoulou-Sellin, R.; Sneige, N. Pleural effusion in patients with differentiated papillary thyroid cancer. South. Med. J. 1994, 87, 1111–1116. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Sha, S.; Tseng, C.H.; Yang, S.E.; Orr, L.E.; Levin, M.; Wong, C.W.; Livhits, M.J.; Rao, J.; Yeh, M.W. Trends in the Surgical Management of Known or Suspected Differentiated Thyroid Cancer at a Single Institution, 2010–2018. Thyroid 2020, 30, 1639–1645. [Google Scholar] [CrossRef]
- Gulec, S.A.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Draganescu, C.; Elisei, R.; Giovanella, L.; Grant, F.; Greenspan, B.; et al. A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the European Thyroid Association, the Society of Nuclear Medicine and Molecular Imaging on Current Diagnostic and Theranostic Approaches in the Management of Thyroid Cancer. Thyroid 2021, 31, 1009–1019. [Google Scholar] [CrossRef]
- Piccardo, A.; Trimboli, P.; Bottoni, G.; Giovanella, L. Radioiodine Ablation of Remaining Thyroid Lobe in Patients with Differentiated Thyroid Cancer Treated by Lobectomy: A Systematic Review and Metaanalysis. J. Nucl. Med. 2020, 61, 1730–1735. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Pryma, D.A.; Mandel, S.J. Radioiodine therapy for thyroid cancer in the era of risk stratification and alternative targeted therapies. J. Nucl. Med. 2014, 55, 1485–1491. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Ruan, M.; Cheng, L.; Fu, H.; Liu, M.; Sheng, S.; Chen, L. Radioiodine Uptake and Thyroglobulin-Guided Radioiodine Remnant Ablation in Patients with Differentiated Thyroid Cancer: A Prospective, Randomized, Open-Label, Controlled Trial. Thyroid 2019, 29, 101–110. [Google Scholar] [CrossRef]
- Ahn, B.C.; Lee, S.W.; Lee, J.; Kim, C. Pulmonary aspergilloma mimicking metastasis from papillary thyroid cancer. Thyroid 2011, 21, 555–558. [Google Scholar] [CrossRef]
- Tan, M.P.; Agarwal, G.; Reeve, T.S.; Barraclough, B.H.; Delbridge, L.W. Impact of timing on completion thyroidectomy for thyroid cancer. Br. J. Surg. 2002, 89, 802–804. [Google Scholar] [CrossRef]
- Bal, C.S.; Kumar, A.; Chandra, P.; Dwivedi, S.N.; Pant, G.S. A prospective clinical trial to assess the efficacy of radioiodine ablation as an alternative to completion thyroidectomy in patients with differentiated thyroid cancer undergoing sub-total thyroidectomy. Acta Oncol. 2006, 45, 1067–1072. [Google Scholar] [CrossRef]
- Schlumberger, M.; Catargi, B.; Borget, I.; Deandreis, D.; Zerdoud, S.; Bridji, B.; Bardet, S.; Leenhardt, L.; Bastie, D.; Schvartz, C.; et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N. Engl. J. Med. 2012, 366, 1663–1673. [Google Scholar] [CrossRef] [Green Version]
- Untch, B.R.; Palmer, F.L.; Ganly, I.; Patel, S.G.; Michael Tuttle, R.; Shah, J.P.; Shaha, A.A. Oncologic outcomes after completion thyroidectomy for patients with well-differentiated thyroid carcinoma. Ann. Surg. Oncol. 2014, 21, 1374–1378. [Google Scholar] [CrossRef]
- Giovanella, L.; Piccardo, A.; Paone, G.; Foppiani, L.; Treglia, G.; Ceriani, L. Thyroid lobe ablation with iodine- ¹³¹I in patients with differentiated thyroid carcinoma: A randomized comparison between 1.1 and 3.7 GBq activities. Nucl. Med. Commun. 2013, 34, 767–770. [Google Scholar] [CrossRef]
- Hoyes, K.P.; Owens, S.E.; Millns, M.M.; Allan, E. Differentiated thyroid cancer: Radioiodine following lobectomy—A clinical feasibility study. Nucl. Med. Commun. 2004, 25, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.W.; Daniels, G.H. Radioactive iodine lobe ablation as an alternative to completion thyroidectomy for follicular carcinoma of the thyroid. Thyroid 2002, 12, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Ruan, M.; Jin, Y.; Fu, H.; Cheng, L.; Luo, Q.; Liu, Z.; Lv, Z.; Chen, L. Poorly differentiated thyroid carcinoma: A clinician’s perspective. Eur. Thyroid J. 2022, 11, e220021. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, Q.; Shen, Y.; Yu, Y.; Yuan, Z.; Lu, H.; Zhu, R. Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J. Nucl. Med. 2008, 49, 1952–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Fu, W.; Deng, Y.; He, L.; Zhang, W. False-Positive 131I Uptake After Transareola Endoscopic Thyroidectomy in a Patient With Papillary Thyroid Carcinoma. Clin. Nucl. Med. 2022, 47, 324–325. [Google Scholar] [CrossRef]
- Kharroubi, D.; Richa, C.; Saie, C.; Chami, L.; Lussey-Lepoutre, C. Solitary Breast Metastasis From Thyroid Papillary Carcinoma Revealed on Whole-Body Radioactive 131I Scan. Clin. Nucl. Med. 2020, 45, 687–688. [Google Scholar] [CrossRef]
- Bakheet, S.M.; Powe, J.; Hammami, M.M. Radioiodine uptake in the chest. J. Nucl. Med. 1997, 38, 984–986. [Google Scholar]
- Spinapolice, E.G.; Chytiris, S.; Fuccio, C.; Leporati, P.; Volpato, G.; Villani, L.; Trifirò, G.; Chiovato, L. Pulmonary sequestration: A (131)I whole body scintigraphy false-positive result. Ann. Nucl. Med. 2014, 28, 683–687. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, J.M.; Kwak, J.J. A solitary large radioiodine accumulative lung lesion in high-dose 131i therapeutic scan: Bronchial atresia with mucocele. Clin. Nucl. Med. 2015, 40, 149–152. [Google Scholar] [CrossRef]
- Höschl, R.; Choy, D.H.; Gandevia, B. Iodine-131 uptake in inflammatory lung disease: A potential pitfall in treatment of thyroid carcinoma. J. Nucl. Med. 1988, 29, 701–706. [Google Scholar]
- Bakheet, S.; Hammami, M.M. False-positive thyroid cancer metastasis on whole-body radioiodine scanning due to retained radioactivity in the oesophagus. Eur. J. Nucl. Med. 1993, 20, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Duong, R.B.; Fernandez-Ulloa, M.; Planitz, M.K.; Maxon, H.R. I-123 breast uptake in a young primipara with postpartum transient thyrotoxicosis. Clin. Nucl. Med. 1983, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S. Thyroid imaging--mediastinal uptake in thyroid imaging. Semin. Nucl. Med. 1983, 13, 395–396. [Google Scholar] [CrossRef] [PubMed]
- White, J.E.; Flickinger, F.W.; Morgan, M.E. I-131 accumulation in gastric pull-up simulating pulmonary metastases on total-body scan for thyroid cancer. Clin. Nucl. Med. 1990, 15, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Bakheet, S.M.; Hammami, M.M. Patterns of radioiodine uptake by the lactating breast. Eur. J. Nucl. Med. 1994, 21, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Hammami, M.M.; Bakheet, S. Radioiodine breast uptake in nonbreastfeeding women: Clinical and scintigraphic characteristics. J. Nucl. Med. 1996, 37, 26–31. [Google Scholar]
- Schneider, J.A.; Divgi, C.R.; Scott, A.M.; Macapinlac, H.A.; Sonenberg, M.; Goldsmith, S.J.; Larson, S.M. Hiatal hernia on whole-body radioiodine survey mimicking metastatic thyroid cancer. Clin. Nucl. Med. 1993, 18, 751–753. [Google Scholar] [CrossRef]
- Bakheet, S.; Hammami, M.M. Spurious lung metastases on radioiodine thyroid and whole body imaging. Clin. Nucl. Med. 1993, 18, 307–312. [Google Scholar] [CrossRef]
- Bakheet, S.M.; Hammami, M.M. Spurious thyroid cancer bone metastases on radioiodine scan due to external contamination. Eur. J. Radiol. 1993, 16, 239–242. [Google Scholar] [CrossRef]
- Gritters, L.S.; Wissing, J.; Gross, M.D.; Shapiro, B. Extensive salivary contamination due to concurrent use of chewing tobacco during I-131 radioablative therapy. Clin. Nucl. Med. 1993, 18, 115–117. [Google Scholar] [CrossRef]
- Peng, D.; Shao, F. Even Small Pleural Effusion Could Be Potential Pitfall on Posttherapeutic 131I Scintigraphy. Clin. Nucl. Med. 2020, 45, 925–926. [Google Scholar] [CrossRef]
- Gottehrer, A.; Roa, J.; Stanford, G.G.; Chernow, B.; Sahn, S.A. Hypothyroidism and pleural effusions. Chest 1990, 98, 1130–1132. [Google Scholar] [CrossRef]
- Jany, B.; Welte, T. Pleural Effusion in Adults-Etiology, Diagnosis, and Treatment. Dtsch. Ärzteblatt Int. 2019, 116, 377–386. [Google Scholar] [CrossRef]
- Light, R.W. Pleural effusions. Med. Clin. N. Am. 2011, 95, 1055–1070. [Google Scholar] [CrossRef]
- Johnson, O.W.; Chick, J.F.; Chauhan, N.R.; Fairchild, A.H.; Fan, C.M.; Stecker, M.S.; Killoran, T.P.; Suzuki-Han, A. The thoracic duct: Clinical importance, anatomic variation, imaging, and embolization. Eur. Radiol. 2016, 26, 2482–2493. [Google Scholar] [CrossRef]
- Laurberg, P.; Wallin, G.; Tallstedt, L.; Abraham-Nordling, M.; Lundell, G.; Tørring, O. TSH-receptor autoimmunity in Graves’ disease after therapy with anti-thyroid drugs, surgery, or radioiodine: A 5-year prospective randomized study. Eur. J. Endocrinol. 2008, 158, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Rosario, P.W.; Cortes, M.C.S.; Franco Mourao, G. Follow-up of patients with thyroid cancer and antithyroglobulin antibodies: A review for clinicians. Endocr. Relat. Cancer 2021, 28, R111–R119. [Google Scholar] [CrossRef]
- Spencer, C.; Fatemi, S. Thyroglobulin antibody (TgAb) methods-Strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Yin, M.; Li, G. Antithyroglobulin Antibody Variation During Follow-Up Has a Good Prognostic Value for Preoperative Antithyroglobulin Antibody-Positive Differentiated Thyroid Cancer Patients: A Retrospective Study in Southwest China. Front. Endocrinol. 2021, 12, 774275. [Google Scholar] [CrossRef]
- Kim, W.G.; Yoon, J.H.; Kim, W.B.; Kim, T.Y.; Kim, E.Y.; Kim, J.M.; Ryu, J.S.; Gong, G.; Hong, S.J.; Shong, Y.K. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2008, 93, 4683–4689. [Google Scholar] [CrossRef] [Green Version]
- Rosario, P.W.; Carvalho, M.; Mourão, G.F.; Calsolari, M.R. Comparison of Antithyroglobulin Antibody Concentrations Before and After Ablation with 131I as a Predictor of Structural Disease in Differentiated Thyroid Carcinoma Patients with Undetectable Basal Thyroglobulin and Negative Neck Ultrasonography. Thyroid 2016, 26, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Shan, F.; Li, W.; Lu, H. Short-Term Side Effects after Radioiodine Treatment in Patients with Differentiated Thyroid Cancer. BioMed Res. Int. 2016, 2016, 4376720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, B.; Li, L.; Yao, L.; Chen, S.; Yi, H.; Ye, X.; Xu, D.; Wu, P. Combined use of radioiodine therapy and radiofrequency ablation in treating postsurgical thyroid remnant of differentiated thyroid carcinoma. J. Cancer Res. Ther. 2015, 11, C244–C247. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.M.; Kontoyiannis, D.P. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: A retrospective, single-institution study. Cancer 2009, 115, 3919–3923. [Google Scholar] [CrossRef]




| Before 131I Administration | Day 9 after 131I Administration | Day 16 after 131I Administration | |
|---|---|---|---|
| Leukocyte (×109/L) | 4.2 | 4.9 | 5.3 |
| Neutrophil (×109/L) | 2.4 | 4.1 | 4.0 |
| Albumin (g/L) | 55 | 46.5 | 50.0 |
| ALT (U/L) | 18 | 14 | 12 |
| AST (U/L) | 20 | 15 | 16 |
| TBIL (umol/L) | 12.7 | 8.2 | 7.4 |
| DBIL (umol/L) | 2.5 | 2.0 | 1.8 |
| Creatinine (umol/L) | 54 | 49.5 | 55.4 |
| eGFR-EPI (mL/min/1.73 m) | 122.89 | 126.46 | 121.86 |
| proBNP (ng/mL) | NA | 34.21 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Wang, P.; Sa, R.; Cheng, L.; Jin, Y.; Song, H.; Chen, L. Diagnosis and Treatment of Acute Pleural Effusion following Radioiodine Remnant Ablation Post Lobectomy for Thyroid Cancer. Diagnostics 2022, 12, 2982. https://doi.org/10.3390/diagnostics12122982
Qiu X, Wang P, Sa R, Cheng L, Jin Y, Song H, Chen L. Diagnosis and Treatment of Acute Pleural Effusion following Radioiodine Remnant Ablation Post Lobectomy for Thyroid Cancer. Diagnostics. 2022; 12(12):2982. https://doi.org/10.3390/diagnostics12122982
Chicago/Turabian StyleQiu, Xian, Pengwen Wang, Ri Sa, Lin Cheng, Yuchen Jin, Hongjun Song, and Libo Chen. 2022. "Diagnosis and Treatment of Acute Pleural Effusion following Radioiodine Remnant Ablation Post Lobectomy for Thyroid Cancer" Diagnostics 12, no. 12: 2982. https://doi.org/10.3390/diagnostics12122982
APA StyleQiu, X., Wang, P., Sa, R., Cheng, L., Jin, Y., Song, H., & Chen, L. (2022). Diagnosis and Treatment of Acute Pleural Effusion following Radioiodine Remnant Ablation Post Lobectomy for Thyroid Cancer. Diagnostics, 12(12), 2982. https://doi.org/10.3390/diagnostics12122982





