Flat Inferior Vena Cava on Computed Tomography for Predicting Shock and Mortality in Trauma: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Published Study Search and Selection Criteria
2.2. Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
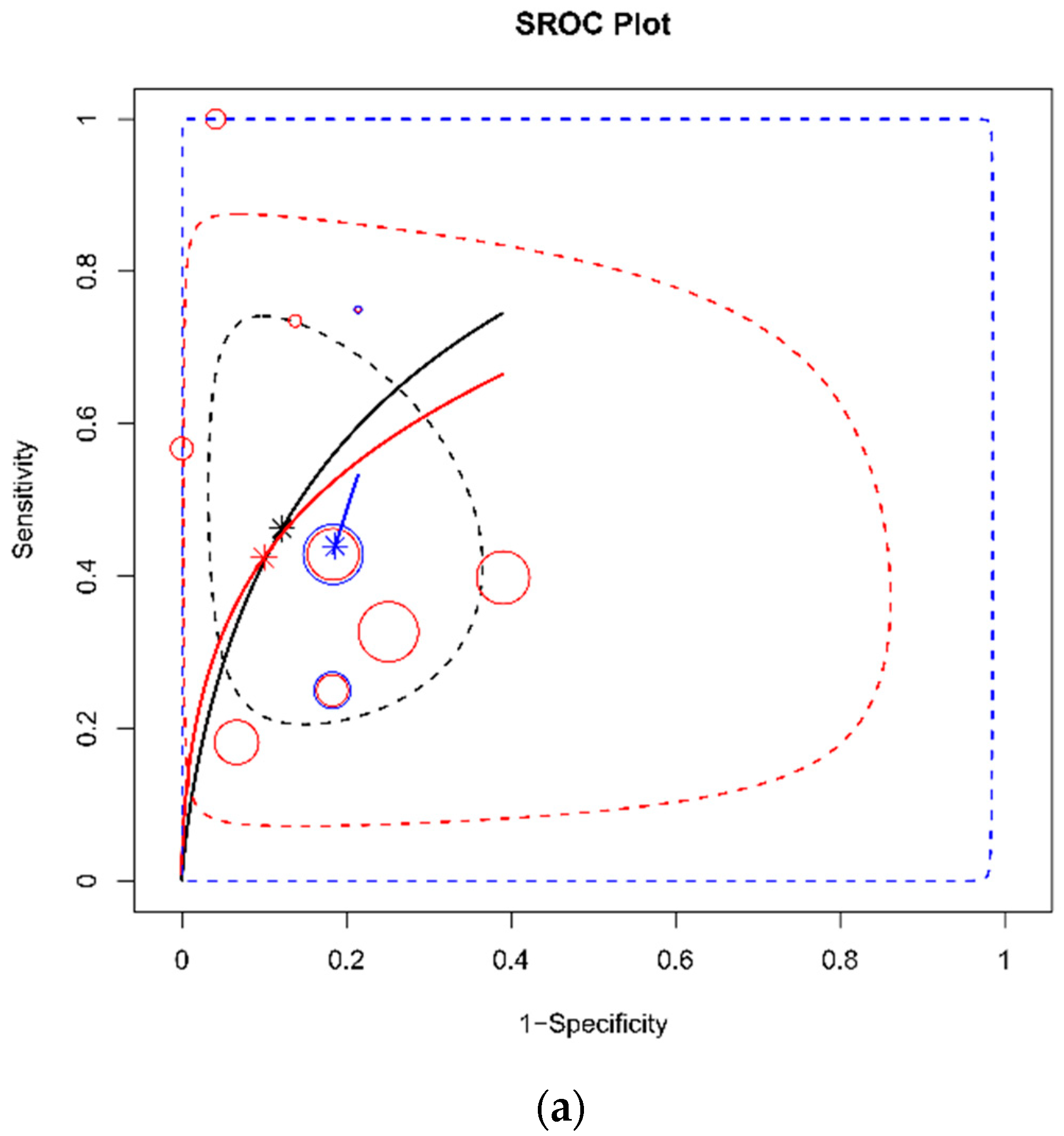
3. Results
3.1. Selection and Characteristics of Included Studies
3.2. Quality Assessment
3.3. DTA Review
3.4. Subgroup Analysis, Sensitivity Analysis, and Evaluation of Heterogeneity
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossaint, R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernández-Mondéjar, E.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Nardi, G.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fourth edition. Crit. Care 2016, 20, 1–55. [Google Scholar] [CrossRef]
- Park, Y.; Lee, G.J.; Lee, A.M.; Choi, K.K.; Gwak, J.; Hyun, S.Y.; Bin Jeon, Y.; Yoon, Y.-C.; Lee, J.; Yu, B. Major Causes of Preventable Death in Trauma Patients. J. Trauma Inj. 2021, 34, 225–232. [Google Scholar] [CrossRef]
- Galvagno, S.M.; Nahmias, J.T.; Young, D.A. Advanced Trauma Life Support® Update 2019: Management and Applications for Adults and Special Populations. Anesthesiol. Clin. 2019, 37, 13–32. [Google Scholar] [CrossRef]
- Shih, A.W.; Al Khan, S.; Wang, A.Y.-H.; Dawe, P.; Young, P.Y.; Greene, A.; Hudoba, M.; Vu, E. Systematic reviews of scores and predictors to trigger activation of massive transfusion protocols. J. Trauma Inj. Infect. Crit. Care 2019, 87, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Chung, S.; Kang, W.-S.; Kim, J. Diagnostic Accuracy of Ultrasonographic Respiratory Variation in the Inferior Vena Cava, Subclavian Vein, Internal Jugular Vein, and Femoral Vein Diameter to Predict Fluid Responsiveness: A Systematic Review and Meta-Analysis. Diagnostics 2021, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Pyo, J.-S.; Kang, W. Accuracy of Contrast Extravasation on Computed Tomography for Diagnosing Severe Pelvic Hemorrhage in Pelvic Trauma Patients: A Meta-Analysis. Medicina 2021, 57, 63. [Google Scholar] [CrossRef] [PubMed]
- Elst, J.; Ghijselings, I.E.; Zuidema, W.P.; Berger, F.H. Signs of post-traumatic hypovolemia on abdominal CT and their clinical importance: A systematic review. Eur. J. Radiol. 2020, 124, 108800. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies. The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Guo, J.; Riebler, A.; Rue, H. Bayesian bivariate meta-analysis of diagnostic test studies with interpretable priors. Stat. Med. 2017, 36, 3039–3058. [Google Scholar] [CrossRef]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 2001, 20, 2865–2884. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, R.; Federle, M. The collapsed inferior vena cava: CT evidence of hypovolemia. Am. J. Roentgenol. 1988, 150, 431–432. [Google Scholar] [CrossRef]
- Wong, Y.-C.; Wang, L.-J.; See, L.-C.; Fang, J.-F.; Ng, C.-J.; Chen, C.-J. Contrast Material Extravasation on Contrast-Enhanced Helical Computed Tomographic Scan of Blunt Abdominal Trauma: Its Significance on the Choice, Time, and Outcome of Treatment. J. Trauma Inj. Infect. Crit. Care 2003, 54, 164–170. [Google Scholar] [CrossRef]
- Ames, J.T.; Federle, M.P. CT Hypotension Complex (Shock Bowel) Is Not Always Due to Traumatic Hypovolemic Shock. Am. J. Roentgenol. 2009, 192, W230–W235. [Google Scholar] [CrossRef]
- Matsumoto, S.; Sekine, K.; Yamazaki, M.; Sasao, K.; Funabiki, T.; Shimizu, M.; Yoshii, H.; Kishikawa, M.; Kitano, M. Predictive Value of a Flat Inferior Vena Cava on Initial Computed Tomography for Hemodynamic Deterioration in Patients with Blunt Torso Trauma. J. Trauma Inj. Infect. Crit. Care 2010, 69, 1398–1402. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Lin, H.-J.; Lu, Y.-H.; Foo, N.-P.; Guo, H.-R.; Chen, K.-T. Does CT Evidence of a Flat Inferior Vena Cava Indicate Hypovolemia in Blunt Trauma Patients with Solid Organ Injuries? J. Trauma Inj. Infect. Crit. Care 2011, 70, 1358–1361. [Google Scholar] [CrossRef]
- Johnson, J.J.; Garwe, T.; Albrecht, R.M.; Adeseye, A.; Bishop, D.; Fails, R.B.; Shepherd, D.W.; Lees, J.S. Initial inferior vena cava diameter on computed tomographic scan independently predicts mortality in severely injured trauma patients. J. Trauma Acute Care Surg. 2013, 74, 741–746. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.-Y.; Wang, Y.; Zhang, W.-G. The Flatness Index of Inferior Vena Cava is Useful in Predicting Hypovolemic Shock in Severe Multiple-Injury Patients. J. Emerg. Med. 2013, 45, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Milia, D.J.; Dua, A.; Paul, J.S.; Tolat, P.; Brasel, K.J. Clinical utility of flat inferior vena cava by axial tomography in severely injured elderly patients. J. Trauma Acute Care Surg. 2013, 75, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Plurad, D.S.; Bricker, S.; Neville, A.; Bongard, F.; Putnam, B.; Kim, D.Y. Flat or fat? Inferior vena cava ratio is a marker for occult shock in trauma patients. J. Surg. Res. 2014, 192, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.; Agnihothri, R.; Knapp, S.; Scher, D.; Khati, N.; Brindle, K.; Amdur, R.; Messing, J.; Dunne, J.; Sarani, B. Inferior vena cava size is not associated with shock following injury. J. Trauma Acute Care Surg. 2014, 77, 34–39. [Google Scholar] [CrossRef]
- Anand, T.; Vansonnenberg, E.; Gadani, K.; Skinner, R. A snapshot of circulation failure following acute traumatic injury: The expansion of computed tomography beyond injury diagnosis. Injury 2016, 47, 50–52. [Google Scholar] [CrossRef]
- Barber, J.; Touska, P.; Negus, A. Inferior vena cava calibre on paediatric trauma CT may be a useful predictor for the development of shock. Clin. Radiol. 2016, 71, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Pommerening, M.J.; Goodman, M.D.; Holcomb, J.B.; Wade, C.E.; Fox, E.E.; Del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Cohen, M.J.; Alarcon, L.H.; et al. Clinical gestalt and the prediction of massive transfusion after trauma. Injury 2015, 46, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Cannon, J.W.; Khan, M.; Raja, A.; Cohen, M.J.; Como, J.J.; Cotton, B.A.; Dubose, J.J.; Fox, E.E.; Inaba, K.; Rodriguez, C.J.; et al. Damage Control Resuscitation in Patients with Severe Traumatic Hemorrhage: A Practice Management Guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2017, 82, 605–617. [Google Scholar] [CrossRef] [Green Version]
- Wachsberg, R.H.; Levine, C.D.; Baker, S.R. Flattened inferior vena cava: A normal finding on unenhanced abdominal computed tomographic scan. Emerg. Radiol. 1996, 3, 16–19. [Google Scholar] [CrossRef]
- Hatanaka, K.; Yanagawa, Y.; Sakamoto, T.; Okada, Y. Predicting the development of anemia by measuring the diameter of the inferior vena cava of patients with spinal cord injury. Am. J. Emerg. Med. 2008, 26, 446–449. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Sakamoto, T. Predicting the Development of Anemia by Measuring the Diameter of the Inferior Vena Cava for Children with Blunt Injuries. Pediatr. Emerg. Care 2008, 24, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Akasaki, Y.; Sugimori, H.; Momii, K.; Akahoshi, T.; Matsuura, S.; Iwamoto, Y.; Maehara, Y.; Hashizume, M. A simple predictive formula for the blood requirement in patients with high-energy blunt injuries transferred within one hour post-trauma. Acute Med. Surg. 2015, 2, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, C.-Y.; Yan, J.-L.; Han, S.T.; Chen, J.-T.; Huang, T.-S.; Chen, Y.-H.; Wang, C.-Y.; Lee, Y.-L.; Chen, K.-F. Inferior Vena Cava Volume Is an Independent Predictor of Massive Transfusion in Patients with Trauma. J. Intensiv. Care Med. 2021, 36, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Takada, H.; Hifumi, T.; Yoshioka, H.; Okada, I.; Kiriu, N.; Inoue, J.; Morimoto, K.; Matsumoto, J.; Koido, Y.; Kato, H. Initial inferior vena cava diameter predicts massive transfusion requirements in blunt trauma patients: A retrospective cohort study. Am. J. Emerg. Med. 2018, 36, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, W.Y.; Oh, J.; Kang, H.; Lim, T.H.; Ko, B.S. Association of inferior vena cava diameter ratio measured on computed tomography scans with the outcome of patients with septic shock. Medicine 2020, 99, e22880. [Google Scholar] [CrossRef] [PubMed]











| Author | Year | Study Type | Study Period | Location | Outcome | Patients | Index | Measure Site | CT Timing | Vitals during CT Scan | CT Slice | Threshold of Flat IVC Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jeffrey [15] | 1987 | retrospective | January–June 1987 | San Francisco, USA | hypotension (SBP < 100mmHg), mortality | 100 patients with abdominal trauma | flat IVC | infrahepatic level | NR | NR | 1 | NR |
| Wong [16] | 2003 | retrospective | 1996–2000 | Taiwan | early intervention, mortality | 32 BAT patients with contrast extravasation | flat IVC | renal vein level | within 3 h from admission | stable | 1 | T/AP ratio = 4 |
| Ames [17] | 2009 | retrospective | 2006–2008 | Pittsburgh, USA | mortality | 25 trauma patients | flat IVC | at least three contiguous sections | NR | NR | 4 to 64 MDCT | AP diameter = 9 mm |
| Matsumoto [18] | 2010 | retrospective | 2005—2007 | 3 hospitals, Japan | shock (SBP <90 mmHg or hear rate > 120 beats per min) | 114 adult patients with blunt torso trauma | flat IVC | renal vein level | within 30 min from admission | stable | 16 or 64 MDCT | T/AP ratio = 4 |
| Liao [19] | 2011 | retrospective | 2003–2006 | Taiwan | shock (SBP < 100 mmHg), mortality | 226 patients with blunt trauma (liver, spleen kidney) | flat IVC | infrahepatic level | within 1 h from admission | stable | 4 | TAP ratio = 3, AP diameter = 9 mm |
| Johnson [20] | 2013 | retrospective | Jan–Dec 2010 | Oklahoma, USA | shock (SBP <90 mmHg), mortality | 161 Trauma patients with ISS ≥ 9, ≥16 year | flat IVC | renal vein level | within 1 h from admission | stable | NR | T/AP ratio = 1.9 |
| Li [21] | 2013 | retrospective | 2008–2011 | China | hypovolemic shock (SBP < 90 mmHg and HR > 120 beats/min with urine output <30mL/h or lactate level < 2mmol/L within 24 hrs. | 63 adult trauma patients with multiple injuries | flat IVC | infrahepatic level | within 2 h from admission | stable | 64 MDCT | T/AP ratio = 3.02 |
| Milia [22] | 2013 | retrospective | 2006–2011 | Wisconsin, USA | shock (adjusted shock index > 50) | 307 elderly (≥55 years) patients with ISS ≥ 15 | flat IVC | renal vein level | within 1 h from admission | NR | 64 MDCT | T/AP ratio = 3 |
| Nguyen [23] | 2014 | retrospective | 2012–2013 | San Diego, USA | ED hypotension (≤90 mmHg), mortality | 264 adult patients with major trauma activation | flat IVC | renal vein level | within 1 h from admission | NR | 64 MDCT | T/AP ratio = 2.5 |
| Radomski [24] | 2014 | retrospective | January–December 2012 | Washington, USA | shock (shock index > 0.7), mortality | 272 adult who met highest-level trauma activation criteria | flat IVC | renal vein level | within 30 min from admission | stable | 64 MDCT | T/AP ratio = 3 (shock), 1.9 (mortality) |
| Anand [25] | 2016 | retrospective | 2010–2011 | Bakersfield, USA | mortality, MT | 90 trauma patients | flat IVC | renal vein level | within 1 h from admission | NR | NR | AP diameter = 9 mm |
| Barber [26] | 2016 | retrospective | 2012–2013 | London, UK | development of shock (hypotension and tachycardia using the PALS reference range) | 52 pediatric trauma patients (<16 years) | flat IVC | infrahepatic level | NR | NR | 128 MDCT | T/AP ratio = 2 |
| Subgroup | Pooled Sens (95% CrI) | Pooled Spec (95% CrI) | Pooled LRpos (95% CrI) | Pooled LRneg (95% CrI) | Pooled DOR (95% CrI) | AUC (95% CrI) | I2 | Cochran’s Q (p) |
|---|---|---|---|---|---|---|---|---|
| Flat IVC for Shock (overall) (k = 9) [15,18,19,20,21,22,23,24,26] | 0.46 (0.32–0.63) | 0.87 (0.78–0.94) | 4.24 (1.51–10.51) | 0.62 (0.37–0.86) | 7.70 (1.77–24.04) | 0.78 (0.58–0.93) | 41.7% | 13.71 (p = 0.090) |
| for Shock (threshold, T/AP ratio, 3 or more) (k = 5) [18,19,21,22,24] | 0.43 (0.24–0.64) | 0.88 (0.68–0.98) | 7.32 (0.86–33.31) | 0.67 (0.36–1.07) | 14.73 (0.79–72.55) | 0.80 (0.47–0.98) | 56.1% | 9.11 (p = 0.058) |
| for Shock (threshold, T/AP ratio between 1.9 and 2.5) (k = 3) [20,23,26] | 0.44 (0.18–0.75) | 0.81 (0.73–0.87) | 2.47 (0.76–5.04) | 0.68 (0.24–1.07) | 5.11 (0.71–19.37) | 0.58 (0.19–0.87) | 0% | 1.81 (p = 0.404) |
| for Shock (high risk of bias) (k = 3) [15,22,26] | 0.65 (0.27–0.98) | 0.85 (0.66–0.96) | 6.88 (0.77–25.06) | 0.44 (0.01–1.12) | 430.02 (0.66–1420.98) | 0.79 (0.33–0.99) | 9.8% | 2.22 (p = 0.330) |
| for Shock (low or unclear risk of bias) (k = 6) [18,19,20,21,23,24] | 0.42 (0.25–0.61) | 0.88 (0.73–0.97) | 5.08 (1.06–18.31) | 0.67 (0.40–0.98) | 9.01 (1.09–37.91) | 0.79 (0.52–0.97) | 32.5% | 7.41 (p = 0.192) |
| for Shock (measuring site = infrahepatic) (k = 4) [15,19,21,26] | 0.62 (0.27–0.93) | 0.91 (0.81–0.96) | 7.85 (1.75–20.71) | 0.43 (0.05–0.88) | 53.17 (2.12–282.45) | 0.78 (0.36–0.98) | 6.5% | 3.21 (p = 0.36) |
| for Shock (measuring site = renal) (k = 5) [18,20,22,23,24] | 0.41 (0.30–0.51) | 0.84 (0.63–0.97) | 5.14 (0.75–24.37) | 0.73 (0.50–1.20) | 8.7 (0.63–45.13) | 0.80 (0.49–0.98) | 56.80% | 9.27 (p = 0.055) |
| Flat IVC for Mortality (overall) (k = 8) [15,16,17,19,20,23,24,25] | 0.45 (0.21–0.72) | 0.70 (0.47–0.88) | 1.96 (0.38–6.04) | 0.85 (0.29–1.75) | 3.96 (0.22–19.78) | 0.60 (0.26–0.89) | 0% | 6.624 (p = 0.469) |
| for Mortality (threshold, T/AP ratio, 3 or more) (k = 3) [16,19,24] | 0.30 (0.05–0.72) | 0.61 (0.23–0.91) | 1.77 (0.05–9.54) | 1.59 (0.25–5.46) | 8.51 (0.01–36.63) | 0.43 (0.03–0.93) | 0% | 1.552 (p = 0.460) |
| for Mortality (threshold, T/AP ratio between 1.9 and 2.5 or IVC diameter under 9mm) (k = 5) [15,17,20,23,25] | 0.52 (0.32–0.78) | 0.74 (0.45–0.92) | 2.84 (0.68–9.24) | 0.69 (0.23–1.45) | 6.41 (0.46–31.17) | 0.71 (0.34–0.94) | 38.1% | 6.463 (p = 0.167) |
| for Mortality (low or unclear risk of bias) (k = 6) [17,19,20,23,24,25] | 0.36 (0.14–0.65) | 0.61 (0.32–0.84) | 1.24 (0.19–4.25) | 1.19 (0.41–2.91) | 1.89 (0.07–9.81) | 0.48 (0.13–0.83) | 0% | 4.343 (p = 0.501) |
| for Mortality (measuring site = renal) (k = 5) [16,20,23,24,25] | 0.28 (0.09–0.56) | 0.64 (0.36–0.85) | 1.12 (0.14–4.37) | 1.24 (0.45–2.68) | 1.71 (0.05–9.54) | 0.44 (0.11–0.83) | 0% | 3.428 (p = 0.489) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.W.; Yoo, H.S.; Kang, W.S. Flat Inferior Vena Cava on Computed Tomography for Predicting Shock and Mortality in Trauma: A Meta-Analysis. Diagnostics 2022, 12, 2972. https://doi.org/10.3390/diagnostics12122972
Kim DW, Yoo HS, Kang WS. Flat Inferior Vena Cava on Computed Tomography for Predicting Shock and Mortality in Trauma: A Meta-Analysis. Diagnostics. 2022; 12(12):2972. https://doi.org/10.3390/diagnostics12122972
Chicago/Turabian StyleKim, Do Wan, Hee Seon Yoo, and Wu Seong Kang. 2022. "Flat Inferior Vena Cava on Computed Tomography for Predicting Shock and Mortality in Trauma: A Meta-Analysis" Diagnostics 12, no. 12: 2972. https://doi.org/10.3390/diagnostics12122972
APA StyleKim, D. W., Yoo, H. S., & Kang, W. S. (2022). Flat Inferior Vena Cava on Computed Tomography for Predicting Shock and Mortality in Trauma: A Meta-Analysis. Diagnostics, 12(12), 2972. https://doi.org/10.3390/diagnostics12122972





