Artificial Intelligence, Wearables and Remote Monitoring for Heart Failure: Current and Future Applications
Abstract
1. Introduction
2. Introduction to AI/ML and Time-Series Forecasting
3. The Current State of Telemonitoring, Wearable, and Implantable Device Technology in HF
4. Current Applications of AI/ML in Remote Monitoring via Wearables and Implantable Cardiac Devices
5. Limitations and Future Prospects
5.1. Data Extraction and Storage
5.2. Data Quality Challenges
5.3. Challenges to Digital Technology Adoption
5.4. Challenges Inherent to AI/ML Model Development and Processing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.U.; Khan, M.Z.; Alkhouli, M. Trends of Clinical Outcomes and Health Care Resource Use in Heart Failure in the United States. J. Am. Heart Assoc. 2020, 9, e016782. [Google Scholar] [CrossRef] [PubMed]
- Urbich, M.; Globe, G.; Pantiri, K.; Heisen, M.; Bennison, C.; Wirtz, H.S.; Di Tanna, G.L. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014–2020). PharmacoEconomics 2020, 38, 1219–1236. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the Future of Cardiovascular Disease in the United States. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Shah, N.D.; Shi, Q.; Morlan, B.; VanHouten, H.; Hall Long, K.; Roger, V.L. Lifetime costs of medical care after heart failure diagnosis. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bergethon, K.E.; Ju, C.; DeVore, A.D.; Hardy, N.C.; Fonarow, G.C.; Yancy, C.W.; Heidenreich, P.A.; Bhatt, D.L.; Peterson, E.D.; Hernandez, A.F. Trends in 30-Day Readmission Rates for Patients Hospitalized With Heart Failure. Circ. Heart Fail. 2016, 9, e002594. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Sreenivasan, J.; Lateef, N.; Abougergi, M.S.; Greene, S.J.; Ahmad, T.; Anker, S.D.; Fonarow, G.C.; Butler, J. Trends in 30- and 90-Day Readmission Rates for Heart Failure. Circ. Heart Fail. 2021, 14, e008335. [Google Scholar] [CrossRef]
- Shahar, E.; Lee, S.; Kim, J.; Duval, S.; Barber, C.; Luepker, R.V. Hospitalized heart failure: Rates and long-term mortality. J. Card. Fail. 2004, 10, 374–379. [Google Scholar] [CrossRef]
- McAlister, F.A.; Youngson, E.; Kaul, P.; Ezekowitz, J.A. Early Follow-Up After a Heart Failure Exacerbation. Circ. Heart Fail. 2016, 9, e003194. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Angermann, C.E.; Assmus, B.; Anker, S.D.; Asselbergs, F.W.; Brachmann, J.; Brett, M.E.; Brugts, J.J.; Ertl, G.; Ginn, G.; Hilker, L.; et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: The CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur. J. Heart Fail. 2020, 22, 1891–1901. [Google Scholar] [CrossRef]
- Gautam, N.; Saluja, P.; Malkawi, A.; Rabbat, M.G.; Al-Mallah, M.H.; Pontone, G.; Zhang, Y.; Lee, B.C.; Al’Aref, S.J. Current and Future Applications of Artificial Intelligence in Coronary Artery Disease. Healthcare 2022, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.L. Some Studies in Machine Learning Using the Game of Checkers. IBM J. Res. Dev. 1959, 3, 210–229. [Google Scholar] [CrossRef]
- Gautam, N.; Ghanta, S.N.; Clausen, A.; Saluja, P.; Sivakumar, K.; Dhar, G.; Chang, Q.; DeMazumder, D.; Rabbat, M.G.; Greene, S.J.; et al. Contemporary Applications of Machine Learning for Device Therapy in Heart Failure. JACC Heart Fail. 2022, 10, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.W.; Mohamed, A.; Yap, B.W. Supervised and unsupervised learning for data science; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial intelligence in cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; He, B.; Ghorbani, A.; Yuan, N.; Ebinger, J.; Langlotz, C.P.; Heidenreich, P.A.; Harrington, R.A.; Liang, D.H.; Ashley, E.A.; et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature 2020, 580, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Alsharef, A.; Aggarwal, K.; Sonia; Kumar, M.; Mishra, A. Review of ML and AutoML Solutions to Forecast Time-Series Data. Arch. Comput. Methods Eng. 2022, 29, 5297–5311. [Google Scholar] [CrossRef]
- Kim, T.Y.; Oh, K.J.; Kim, C.; Do, J.D. Artificial neural networks for non-stationary time series. Neurocomputing 2004, 61, 439–447. [Google Scholar] [CrossRef]
- Marhon, S.A.; Cameron, C.J.F.; Kremer, S.C. Recurrent Neural Networks. In Handbook on Neural Information Processing, Bianchini, M., Maggini, M., Jain, L.C., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 29–65. [Google Scholar]
- Van Houdt, G.; Mosquera, C.; Nápoles, G. A review on the long short-term memory model. Artif. Intell. Rev. 2020, 53, 5929–5955. [Google Scholar] [CrossRef]
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; De Marco, T.; Escarce, J.J.; Evangelista, L.S.; Hanna, B.; et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition—Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 310–318. [Google Scholar] [CrossRef]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Böhm, M.; Boll, H.; Baumann, G.; Honold, M.; Koehler, K.; et al. Impact of Remote Telemedical Management on Mortality and Hospitalizations in Ambulatory Patients With Chronic Heart Failure. Circulation 2011, 123, 1873–1880. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Dontje, M.L.; van der Wal, M.H.; Stolk, R.P.; Brügemann, J.; Jaarsma, T.; Wijtvliet, P.E.; van der Schans, C.P.; de Greef, M.H. Daily physical activity in stable heart failure patients. J. Cardiovasc. Nurs. 2014, 29, 218–226. [Google Scholar] [CrossRef]
- Redfield, M.M.; Anstrom, K.J.; Levine, J.A.; Koepp, G.A.; Borlaug, B.A.; Chen, H.H.; LeWinter, M.M.; Joseph, S.M.; Shah, S.J.; Semigran, M.J.; et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. New Engl. J. Med. 2015, 373, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Compton, S.; Haas, G.; Foreman, B.; Canby, R.C.; Fishel, R.; McRae, S.; Toledo, G.B.; Sarkar, S.; Hettrick, D.A. Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: Results of the Fluid Accumulation Status Trial (FAST). Congest. Heart Fail. 2011, 17, 51–55. [Google Scholar] [CrossRef]
- Catanzariti, D.; Lunati, M.; Landolina, M.; Zanotto, G.; Lonardi, G.; Iacopino, S.; Oliva, F.; Perego, G.B.; Varbaro, A.; Denaro, A. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Pacing Clin. Electrophysiol. 2009, 32, 363–370. [Google Scholar] [CrossRef]
- Conraads, V.M.; Tavazzi, L.; Santini, M.; Oliva, F.; Gerritse, B.; Yu, C.-M.; Cowie, M.R. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: The SENSE-HF trial. Eur. Heart J. 2011, 32, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Domenichini, G.; Rahneva, T.; Diab, I.G.; Dhillon, O.S.; Campbell, N.G.; Finlay, M.C.; Baker, V.; Hunter, R.J.; Earley, M.J.; Schilling, R.J. The lung impedance monitoring in treatment of chronic heart failure (the LIMIT-CHF study). Europace 2016, 18, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-W.; Hua, W.; Ding, L.-G.; Wang, J.; Zheng, L.-H.; Li, C.-Q.; Liu, Z.-M.; Chen, K.-P.; Zhang, S. OptiVol fluid index predicts acute decompensation of heart failure with a high rate of unexplained events. J. Geriatr. Cardiol. JGC 2013, 10, 253. [Google Scholar]
- Amir, O.; Ben-Gal, T.; Weinstein, J.M.; Schliamser, J.; Burkhoff, D.; Abbo, A.; Abraham, W.T. Evaluation of remote dielectric sensing (ReDS) technology-guided therapy for decreasing heart failure re-hospitalizations. Int. J. Cardiol. 2017, 240, 279–284. [Google Scholar] [CrossRef]
- Shavelle, D.M.; Desai, A.S.; Abraham, W.T.; Bourge, R.C.; Raval, N.; Rathman, L.D.; Heywood, J.T.; Jermyn, R.A.; Pelzel, J.; Jonsson, O.T.; et al. Lower Rates of Heart Failure and All-Cause Hospitalizations During Pulmonary Artery Pressure-Guided Therapy for Ambulatory Heart Failure: One-Year Outcomes From the CardioMEMS Post-Approval Study. Circ. Heart Fail. 2020, 13, e006863. [Google Scholar] [CrossRef]
- Perl, L.; Meerkin, D.; D’Amario, D.; Avraham, B.B.; Gal, T.B.; Weitsman, T.; Hasin, T.; Ince, H.; Feickert, S.; D’Ancona, G.; et al. The V-LAP System for Remote Left Atrial Pressure Monitoring of Patients with Heart Failure: Remote Left Atrial Pressure Monitoring. J. Card. Fail. 2022, 28, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, E.; Pooyan, M. Early detection of sudden cardiac death by using classical linear techniques and time-frequency methods on electrocardiogram signals. J. Biomed. Sci. Eng. 2011, 4, 699. [Google Scholar] [CrossRef]
- Inan, O.T.; Baran Pouyan, M.; Javaid, A.Q.; Dowling, S.; Etemadi, M.; Dorier, A.; Heller, J.A.; Bicen, A.O.; Roy, S.; De Marco, T.; et al. Novel Wearable Seismocardiography and Machine Learning Algorithms Can Assess Clinical Status of Heart Failure Patients. Circ. Heart Fail. 2018, 11, e004313. [Google Scholar] [CrossRef]
- Shandhi, M.M.H.; Fan, J.; Heller, J.; Etemadi, M.; Klein, L.; Inan, O. Estimation of Changes in Intracardiac Hemodynamics Using Wearable Seismocardiography and Machine Learning in Patients with Heart Failure: A Feasibility Study. IEEE Trans. Biomed. Eng. 2022, 69, 2443–2455. [Google Scholar] [CrossRef]
- Voss, A.; Witt, K.; Fischer, C.; Reulecke, S.; Poitz, W.; Kechagias, V.; Surber, R.; Figulla, H.R. Smelling heart failure from human skin odor with an electronic nose. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2012, 2012, 4034–4037. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Fujita, H.; Sudarshan, V.K.; Sree, V.S.; Eugene, L.W.J.; Ghista, D.N.; San Tan, R. An integrated index for detection of sudden cardiac death using discrete wavelet transform and nonlinear features. Knowl.-Based Syst. 2015, 83, 149–158. [Google Scholar] [CrossRef]
- Stehlik, J.; Schmalfuss, C.; Bozkurt, B.; Nativi-Nicolau, J.; Wohlfahrt, P.; Wegerich, S.; Rose, K.; Ray, R.; Schofield, R.; Deswal, A. Continuous wearable monitoring analytics predict heart failure hospitalization: The LINK-HF multicenter study. Circ. Heart Fail. 2020, 13, e006513. [Google Scholar] [CrossRef]
- Lee, H.; Shin, S.-Y.; Seo, M.; Nam, G.-B.; Joo, S. Prediction of ventricular tachycardia one hour before occurrence using artificial neural networks. Sci. Rep. 2016, 6, 32390. [Google Scholar] [CrossRef]
- Taye, G.T.; Shim, E.B.; Hwang, H.-J.; Lim, K.M. Machine Learning Approach to Predict Ventricular Fibrillation Based on QRS Complex Shape. Front. Physiol. 2019, 10, 1193. [Google Scholar] [CrossRef]
- Chowdhury, M.E.; Alzoubi, K.; Khandakar, A.; Khallifa, R.; Abouhasera, R.; Koubaa, S.; Ahmed, R.; Hasan, A. Wearable real-time heart attack detection and warning system to reduce road accidents. Sensors 2019, 19, 2780. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, W.-T.M.; Reinhall, P.G.; Bardy, G.H.; Brunton, S.L. Development and validation of warning system of ventricular tachyarrhythmia in patients with heart failure with heart rate variability data. PLoS ONE 2018, 13, e0207215. [Google Scholar] [CrossRef]
- Joo, S.; Choi, K.-J.; Huh, S.-J. Prediction of spontaneous ventricular tachyarrhythmia by an artificial neural network using parameters gleaned from short-term heart rate variability. Expert Syst. Appl. 2012, 39, 3862–3866. [Google Scholar] [CrossRef]
- Kim, M.; Kang, Y.; You, S.C.; Park, H.-D.; Lee, S.-S.; Kim, T.-H.; Yu, H.T.; Choi, E.-K.; Park, H.-S.; Park, J.; et al. Artificial intelligence predicts clinically relevant atrial high-rate episodes in patients with cardiac implantable electronic devices. Sci. Rep. 2022, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Burton, T.; Ramchandani, S.; Bhavnani, S.P.; Khedraki, R.; Cohoon, T.J.; Stuckey, T.D.; Steuter, J.A.; Meine, F.J.; Bennett, B.A.; Carroll, W.S.; et al. Identifying novel phenotypes of elevated left ventricular end diastolic pressure using hierarchical clustering of features derived from electromechanical waveform data. Front. Cardiovasc. Med. 2022, 9, 980625. [Google Scholar] [CrossRef]
- DeMazumder, D.; Limpitikul, W.B.; Dorante, M.; Dey, S.; Mukhopadhyay, B.; Zhang, Y.; Moorman, J.R.; Cheng, A.; Berger, R.D.; Guallar, E.; et al. Entropy of cardiac repolarization predicts ventricular arrhythmias and mortality in patients receiving an implantable cardioverter-defibrillator for primary prevention of sudden death. Europace 2016, 18, 1818–1828. [Google Scholar] [CrossRef]
- Dey, S.; DeMazumder, D.; Sidor, A.; Foster, D.B.; O’Rourke, B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ. Res. 2018, 123, 356–371. [Google Scholar] [CrossRef]
- DeMazumder, D.; Lake, D.E.; Cheng, A.; Moss, T.J.; Guallar, E.; Weiss, R.G.; Jones, S.R.; Tomaselli, G.F.; Moorman, J.R. Dynamic analysis of cardiac rhythms for discriminating atrial fibrillation from lethal ventricular arrhythmias. Circ. Arrhythm. Electrophysiol. 2013, 6, 555–561. [Google Scholar] [CrossRef]
- Thirumal, R.; Vaughan, B.; Dey, S.; Vogel, A.; Limpitikul, W.B.; Dorante, M.; Guallar, E.; Punjabi, N.M.; Jones, S.R.; Demazumder, D. Abstract 11604: The Cincinnati Atrial Fibrillation Score (CAFS): Multi-Center Derivation of the First Polysomnography-Based Risk Scoring System for Predicting Incident Atrial Fibrillation in Asymptomatic Community Adults. Circulation 2021, 144, A11604. [Google Scholar] [CrossRef]
- Thirumal, R.; Dey, S.; Vaughan, B.; Chen, G.; Kappagantula, S.; Guallar, E.; Punjabi, N.M.; Tomaselli, G.F.; Blumenthal, R.S.; Redline, S.; et al. The Cincinnati Atrial Fibrillation Score (Cafs): Multicenter Validation of the First Polysomnography-Based Risk Score for Predicting Incident Atrial Fibrillation in Asymptomatic Ambulatory Community Adults. J. Am. Coll. Cardiol. 2022, 79, 27. [Google Scholar] [CrossRef]
- Artificial Intelligence Mobile Health Trial of a Digital Platform to Optimize GDMT Using Wearable Sensors. Available online: https://ClinicalTrials.gov/show/NCT04191330 (accessed on 30 September 2022).
- Activity-Aware Prompting to Improve Medication Adherence in Heart Failure Patients. Available online: https://ClinicalTrials.gov/show/NCT04152031 (accessed on 30 September 2022).
- Heart Failure Monitoring with Eko Electronic Stethoscopes (CardioMEMS). Available online: https://ClinicalTrials.gov/show/NCT05080504 (accessed on 30 September 2022).
- Interactive Patient’s Assistant—LUCY. Available online: https://ClinicalTrials.gov/show/NCT03474315 (accessed on 30 September 2022).
- LINK-HF2—Remote Monitoring Analytics in Heart Failure. Available online: https://ClinicalTrials.gov/show/NCT04502563 (accessed on 30 September 2022).
- Validation of Ejection Fraction and Cardiac Output Using Biostrap Wristband. Available online: https://ClinicalTrials.gov/show/NCT05279066 (accessed on 30 September 2022).
- Implementing Digital Health in a Learning Health System. Available online: https://ClinicalTrials.gov/show/NCT03713333 (accessed on 30 September 2022).
- International Multi-center Study to Validate an Early Warning Algorithm for Worsening Heart Failure. Available online: https://ClinicalTrials.gov/show/NCT04758429 (accessed on 30 September 2022).
- Weintraub, W.S. Role of Big Data in Cardiovascular Research. J. Am. Heart Assoc. 2019, 8, e012791. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.F.; Sabanci, K.; Durdu, A. A CNN-based novel solution for determining the survival status of heart failure patients with clinical record data: Numeric to image. Biomed. Signal Process. Control. 2021, 68, 102716. [Google Scholar] [CrossRef]
- Pandey, M.; Xu, Z.; Sholle, E.; Maliakal, G.; Singh, G.; Fatima, Z.; Larine, D.; Lee, B.C.; Wang, J.; van Rosendael, A.R.; et al. Extraction of radiographic findings from unstructured thoracoabdominal computed tomography reports using convolutional neural network based natural language processing. PLoS ONE 2020, 15, e0236827. [Google Scholar] [CrossRef]
- Hasselgren, A.; Kralevska, K.; Gligoroski, D.; Pedersen, S.A.; Faxvaag, A. Blockchain in healthcare and health sciences—A scoping review. Int. J. Med. Inform. 2020, 134, 104040. [Google Scholar] [CrossRef]
- Nugent, T.; Upton, D.; Cimpoesu, M. Improving data transparency in clinical trials using blockchain smart contracts. F1000Res 2016, 5, 2541. [Google Scholar] [CrossRef] [PubMed]
- O’Herrin, J.K.; Fost, N.; Kudsk, K.A. Health Insurance Portability Accountability Act (HIPAA) regulations: Effect on medical record research. Ann. Surg. 2004, 239, 772–776; discussion 776–778. [Google Scholar] [CrossRef] [PubMed]
- Papandrea, P. Addressing the HIPAA-potamus sized gap in wearable technology regulation. Minn. L. Rev. 2019, 104, 1095. [Google Scholar]
- Hawkins, N.M.; Jhund, P.S.; McMurray, J.J.V.; Capewell, S. Heart failure and socioeconomic status: Accumulating evidence of inequality. Eur. J. Heart Fail. 2012, 14, 138–146. [Google Scholar] [CrossRef]
- Potter, E.L.; Hopper, I.; Sen, J.; Salim, A.; Marwick, T.H. Impact of socioeconomic status on incident heart failure and left ventricular dysfunction: Systematic review and meta-analysis. Eur. Heart J.—Qual. Care Clin. Outcomes 2018, 5, 169–179. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Katthula, V.; Moustakas, E. Patterns of Use and Key Predictors for the Use of Wearable Health Care Devices by US Adults: Insights from a National Survey. J. Med. Internet Res. 2020, 22, e22443. [Google Scholar] [CrossRef]
- Yang, W.E.; Spaulding, E.M.; Lumelsky, D.; Hung, G.; Huynh, P.P.; Knowles, K.; Marvel, F.A.; Vilarino, V.; Wang, J.; Shah, L.M.; et al. Strategies for the Successful Implementation of a Novel iPhone Loaner System (iShare) in mHealth Interventions: Prospective Study. JMIR Mhealth Uhealth 2019, 7, e16391. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Khurshid, S.; Singer, D.E.; Atlas, S.J.; Ashburner, J.M.; Ellinor, P.T.; McManus, D.D.; Lubitz, S.A.; Chhatwal, J. Cost-effectiveness of Screening for Atrial Fibrillation Using Wearable Devices. JAMA Health Forum 2022, 3, e222419. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.T.; Goldhaber-Fiebert, J.D.; Owens, D.K.; Turakhia, M.P.; Kaiser, D.W.; Heidenreich, P.A. Cost-Effectiveness of Implantable Pulmonary Artery Pressure Monitoring in Chronic Heart Failure. JACC Heart Fail. 2016, 4, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Simon, M.; Klein, L.; Thokala, P. The cost-effectiveness of real-time pulmonary artery pressure monitoring in heart failure patients: A European perspective. Eur. J. Heart Fail. 2017, 19, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Tat, E.; Bhatt, D.L.; Rabbat, M.G. Addressing bias: Artificial intelligence in cardiovascular medicine. Lancet Digit. Health 2020, 2, e635–e636. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Powers, B.; Vogeli, C.; Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 2019, 366, 447–453. [Google Scholar] [CrossRef]
- Hughes, G. On the mean accuracy of statistical pattern recognizers. IEEE Trans. Inf. Theory 1968, 14, 55–63. [Google Scholar] [CrossRef]
- Shin, S.; Austin, P.C.; Ross, H.J.; Abdel-Qadir, H.; Freitas, C.; Tomlinson, G.; Chicco, D.; Mahendiran, M.; Lawler, P.R.; Billia, F. Machine learning vs. conventional statistical models for predicting heart failure readmission and mortality. ESC Heart Fail. 2021, 8, 106–115. [Google Scholar] [CrossRef]
- Dey, P.; Ross, J.S.; Ritchie, J.D.; Desai, N.R.; Bhavnani, S.P.; Krumholz, H.M. Data Sharing and Cardiology: Platforms and Possibilities. J. Am. Coll. Cardiol. 2017, 70, 3018–3025. [Google Scholar] [CrossRef]
- Studer, R.; Sartini, C.; Suzart-Woischnik, K.; Agrawal, R.; Natani, H.; Gill, S.K.; Wirta, S.B.; Asselbergs, F.W.; Dobson, R.; Denaxas, S.; et al. Identification and Mapping Real-World Data Sources for Heart Failure, Acute Coronary Syndrome, and Atrial Fibrillation. Cardiology 2022, 147, 98–106. [Google Scholar] [CrossRef]
- Jernberg, T.; Attebring, M.F.; Hambraeus, K.; Ivert, T.; James, S.; Jeppsson, A.; Lagerqvist, B.; Lindahl, B.; Stenestrand, U.; Wallentin, L. The Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART). Heart 2010, 96, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Petsiuk, V.; Das, A.; Saenko, K. Rise: Randomized input sampling for explanation of black-box models. arXiv 2018, arXiv:1806.07421. [Google Scholar]
- Makridakis, S.; Spiliotis, E.; Assimakopoulos, V. Statistical and Machine Learning forecasting methods: Concerns and ways forward. PLoS ONE 2018, 13, e0194889. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cruz Rivera, S.; Moher, D.; Calvert, M.J.; Denniston, A.K.; Chan, A.-W.; Darzi, A.; Holmes, C.; Yau, C.; Ashrafian, H.; et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Nat. Med. 2020, 26, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
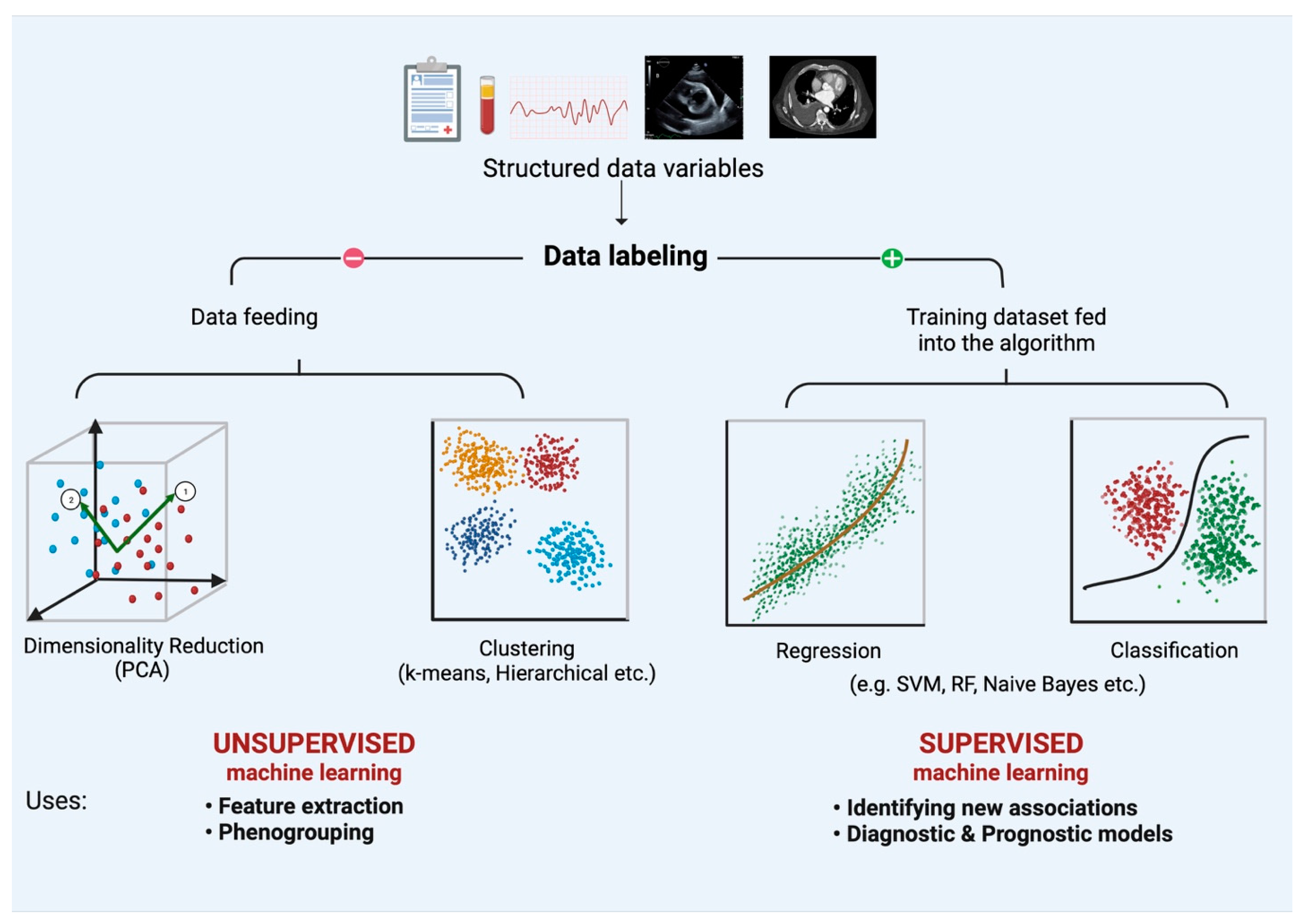
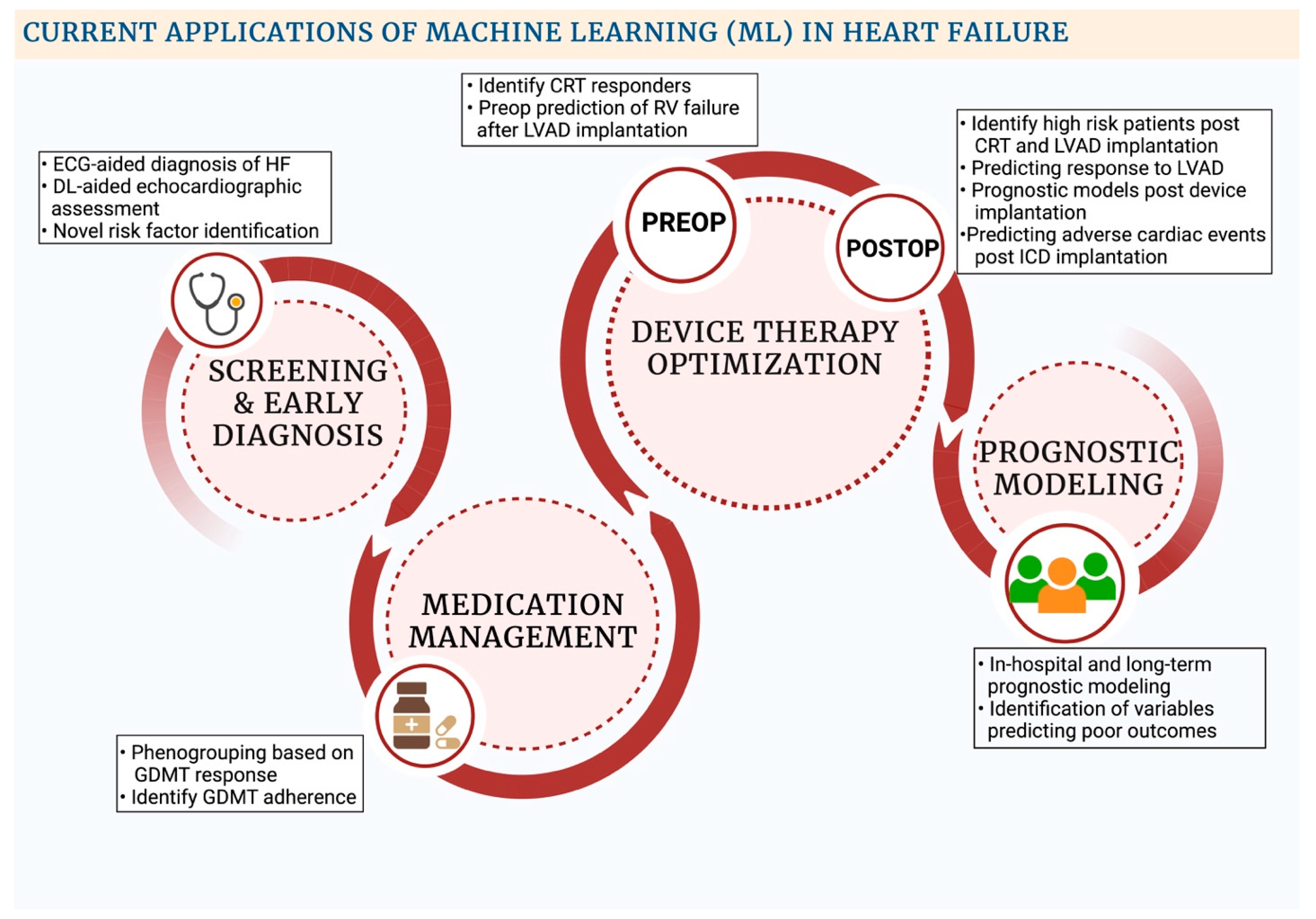
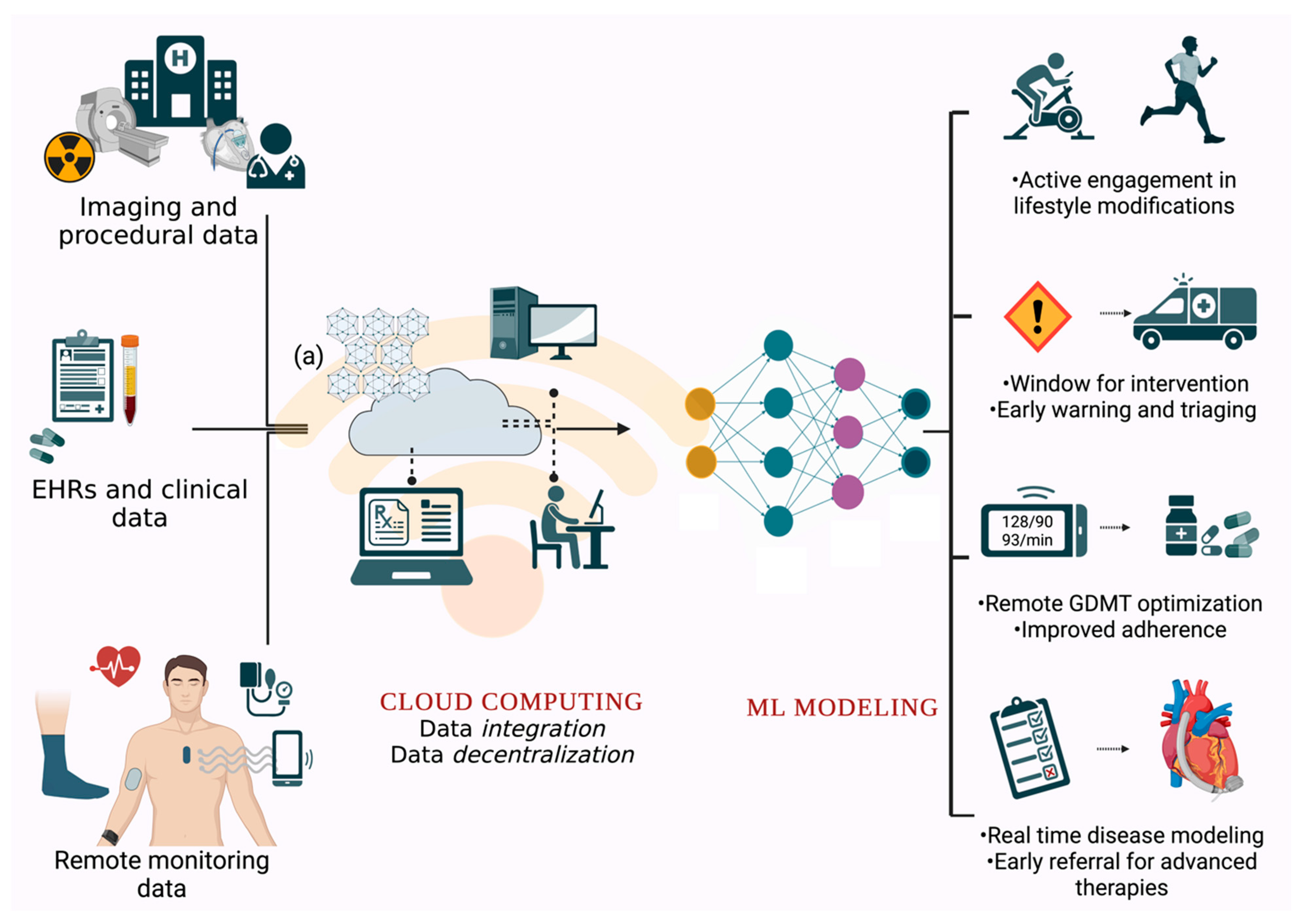
| Algorithms | Description |
|---|---|
| Support Vector Regression | Supervised ML algorithm based on support vector machines, whereby creation of hyperplane is done in order to minimize the error and maximizes the margin. |
| k-Nearest Neighbors | Supervised classifier or regression ML algorithm whereby the test variable is classified based on its proximity to k number of data points. |
| Recurrent Neural network (RNN) | DL algorithm whereby data is passed via multiple layers of neurons (consisting of input, hidden and output layers), with the output from one layer being looped back into the hidden layer, in order to predict sequential data. Associated with the vanishing and exploding gradient problem due to exponential decrease or increase of the gradient during backpropagation, leading to minimal change in adjusted weights in the earlier layers, thereby translating into a short-term memory which can limit its role in larger datasets. |
| Long short-term memory | Special form of RNN’s (DL algorithms) useful for larger datasets, consisting of a memory unit, comprised of three gates. The forget gate is responsible for screening out irrelevant data, and the output gate is responsible for generation of the new cell state and the hidden state, and the process is repeated over again to yield the final model. Complex model, requiring higher computational power. |
| Gated recurrent units | Another form of RNN’s (DL algorithms) consisting of two gates-update and the reset gate. The update gate decides the information to be omitted and added and the reset gate is to decide on how much past information can be omitted. Simpler model than LSTM, requiring lesser computational power, while retaining the long-term memory. |
| Author | Study Design and Sample Size (n) | ML Model | Results | Limitations |
|---|---|---|---|---|
| Inan et al. [35] 2018 | n = 45; single center. SCG signals and ECG signals were analyzed at rest, during 6MWT and 5 min of recovery. GSS was developed using SCG and ECG signals to assess HF state. | GSS developed with the help of K-means clustering | GSS can significantly differentiate between compensated and decompensated HF (p < 0.0001). GSS can longitudinally assess improvement in HF status and cardiovascular reserve from admission to discharge (p < 0.05). | Differentiation between decompensated and compensated HF groups is subjective and future work is needed to enhance this classification. Investigators were not blinded to the HF state of each patient. Small single center study in a controlled setting. |
| Shandhi et al. [36] 2022 | n = 20; measuring changes in PAP and PCWP via SCG signals after vasodilator infusion during RHC. | Globalized (population) regression model developed using logistic regression | Regression model estimated changes in PAP and PCWP in both validation and training sets with good accuracy. SCG signals can be used to track changes in intracardiac pressures non-invasively. | Single center study design with small population size, needs future research to extrapolate results. |
| Stehlik et al. [39] 2020 | n = 100; multicenter observational study. Subjects were fitted with a wearable sensor that collected continuous ECG waveform, skin impedance, continuous 3-axis accelerometry, temperature and patient activity/posture data. | Multivariate change index was developed using Cloud-based analytics derived from similarity-based modelling | Multivariate chain index platform was able to detect risk of HF hospitalization with 76% to 87.5% sensitivity and 85% specificity. Clinical alert triggered by personalized machine learning algorithm preceded hospitalization by a median time of 6.5 to 8.5 days. Predictive accuracy to detect impending HF hospitalization was similar to implanted devices. | Non-compliant subjects were excluded from the analysis. Lack of formal testing and validation sets. Observation study mainly on male patients with reduced ejection fraction. |
| Au-Yeung et al. [44] 2018 | n = 788; ICD data of patients enrolled in Sudden Cardiac Death-Heart Failure Trial (SCD-HF) was used to automatically predict ventricular arrythmias via heart rate variability (HRV). | RF and SVM | The accuracy of 5-min prediction using RF and SVM was about 0.81 (AUC) whereas 10-s prediction of ventricular arrhythmia was higher with an accuracy of 0.87–0.88. | Real time continuous monitoring requires significant computational resources and would rapidly drain device battery. HRV data employed can be influenced by multiple non-cardiac factors like exercise, anxiety, etc. Rarity of life-threatening ventricular arrythmia increases the difficulty of accurate arrythmia prediction with many false positives. |
| Joo et al. [45] 2012 | n = 78 patients; >1000 EKGs from the Spontaneous Ventricular Arrythmia (SVM) database 1.0 from Medtronic ICDs were used to predict VT/VF using HRV analysis. | ANN | ANN models were able to detect VT, VF, VT + VF events with an accuracy of 76.6%, 92.2% and 75.6%, respectively. The normalized areas under the ROC curve of each ANN were 0.75, 0.93 and 0.76, respectively. | Small sample size in the training set was insufficient to ensure statistical classification. Database used had limited pre-VF data, leading to sampling bias. ECGs from single manufacturers were studies; which limits generalizability. ANN require devices with high computational power. |
| Kim et al. [46] 2022 | n = 721; A prospective multicenter study aimed at predicting clinically relevant atrial high-rate episodes (AHREs) after pacemaker implantation. | RF, SVMs and extreme gradient boosting | Predictive accuracy of ML models was higher compared to logistic regression-based models (AUC for RF: 0.742, SVM: 0.675 and XgBoost 0.745, vs. logistic regression: 0.669). | Data sets used to develop the validation set were relatively small and contained limited features. |
| Acharya et al. [38] 2015 | n = 41 patients; ECG signals from an open access Holter database and normal sinus rhythm database were used to develop a novel integrated index for prediction of SCD. | Decision trees; K-Nearest Neighbor, and SVMs | 1. SCD Index had a predictive ability of 92.11%, 98.68%, 93.42% and 92.11% for first, second, third and fourth minutes before the occurrence of SCD, respectively. | 1. Small sample size. |
| Taye et al. [41] 2019 | n = 55; ECG data from multiple freely available databases was analyzed to predict VF using QRS complex morphology. | ANN, SVM, KNN, RF | Prediction accuracy for VF was significantly higher using QRS complex features compared to HRV features: 98.6% vs. 72% (p < 0.05). In addition, sensitivity, and specificity of VF prediction 30 s before occurrence was higher using QRS complex features compared to HRV (AUC 0.99 and 0.71 for QRS complex shape and HRV, respectively). | Small sample size and shorter length of signals before occurrence of VF. |
| Lee et al. [40] 2019 | n = 82; early VT prediction ML model was developed using HRV and RRV data from monitors of patients admitted to cardiovascular ICU. | ANN | ML model predicted VT with a sensitivity of 88%, specificity of 82% (AUC: 0.93). | Single center study with small sample size. |
| Clinical Trial | Wearable/Implantable | Description | Current Stage |
|---|---|---|---|
| Activity-Aware Prompting to Improve Medication Adherence in Heart Failure Patients (NCT04152031) [54] | Smartphone | Designing ML-based software algorithms aimed at analyzing daily behavior and to utilize it to improve medication compliance among patients with HF. | Recruitment complete |
| AIM-POWER study (NCT04191330) [53] | BiovitalsHF | To study the effectiveness of a cloud-based platform (BiovitalsHF) collecting data using remote wearable sensors in improving GDMT adoption among patients with HF. | Recruiting |
| ASE-INNOVATE study (NCT03713333) [59] | Multiple digital health devices | To study the effectiveness of technology-based visitations (outpatient visit supplemented by focused echocardiography, ECG, and vitals collected by digital devices) on long term cardiovascular outcomes. | Unclear |
| Heart Failure Monitoring With Eko Electronic Stethoscopes (CardioMEMS) (NCT05080504) [55] | Eko electronic stethoscopes (AI based) | Designing a ML-based algorithm which can correlate Eko stethoscope acoustic and ECG recordings with the pulmonary artery pressure measurements taken via the CardioMEMS device. | Recruiting |
| Interactive Patient’s Assistant-LUCY (NCT03474315) [56] | Implanted CRT and ICD devices | Designing a ML-based algorithm based on remotely monitored data to determine the parameters in CRT/ICD requiring an ambulatory device clinic visit, overall optimizing long term patient care. | Unclear |
| LINK-HF2 study (NCT04502563) [57] | Continuous remote monitoring via wearable sensors | To study the impact of remote monitoring on 90-day HF hospitalizations rate in patients with HF. | Recruiting |
| Validation of Ejection Fraction and Cardiac Output Using Biostrap Wristband (NCT05279066) [58] | Wristband with photoplethysmgraphy sensor | Correlation of ejection fraction and cardiac output measured via AI-powered translation of wristband PPG recordings with echocardiogram and pulmonary artery catheter measurements. | Recruiting |
| VESTA study (NCT04758429) [60] | Wearable sensor data | Validation of ML algorithm for early detection of HF events via multi-parametric sensor data. | Not yet recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, N.; Ghanta, S.N.; Mueller, J.; Mansour, M.; Chen, Z.; Puente, C.; Ha, Y.M.; Tarun, T.; Dhar, G.; Sivakumar, K.; et al. Artificial Intelligence, Wearables and Remote Monitoring for Heart Failure: Current and Future Applications. Diagnostics 2022, 12, 2964. https://doi.org/10.3390/diagnostics12122964
Gautam N, Ghanta SN, Mueller J, Mansour M, Chen Z, Puente C, Ha YM, Tarun T, Dhar G, Sivakumar K, et al. Artificial Intelligence, Wearables and Remote Monitoring for Heart Failure: Current and Future Applications. Diagnostics. 2022; 12(12):2964. https://doi.org/10.3390/diagnostics12122964
Chicago/Turabian StyleGautam, Nitesh, Sai Nikhila Ghanta, Joshua Mueller, Munthir Mansour, Zhongning Chen, Clara Puente, Yu Mi Ha, Tushar Tarun, Gaurav Dhar, Kalai Sivakumar, and et al. 2022. "Artificial Intelligence, Wearables and Remote Monitoring for Heart Failure: Current and Future Applications" Diagnostics 12, no. 12: 2964. https://doi.org/10.3390/diagnostics12122964
APA StyleGautam, N., Ghanta, S. N., Mueller, J., Mansour, M., Chen, Z., Puente, C., Ha, Y. M., Tarun, T., Dhar, G., Sivakumar, K., Zhang, Y., Halimeh, A. A., Nakarmi, U., Al-Kindi, S., DeMazumder, D., & Al’Aref, S. J. (2022). Artificial Intelligence, Wearables and Remote Monitoring for Heart Failure: Current and Future Applications. Diagnostics, 12(12), 2964. https://doi.org/10.3390/diagnostics12122964






