ECMO in Cardiogenic Shock: Time Course of Blood Biomarkers and Associated Mortality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Acquisition
2.2. ECMO-Support Management and Anticoagulation
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
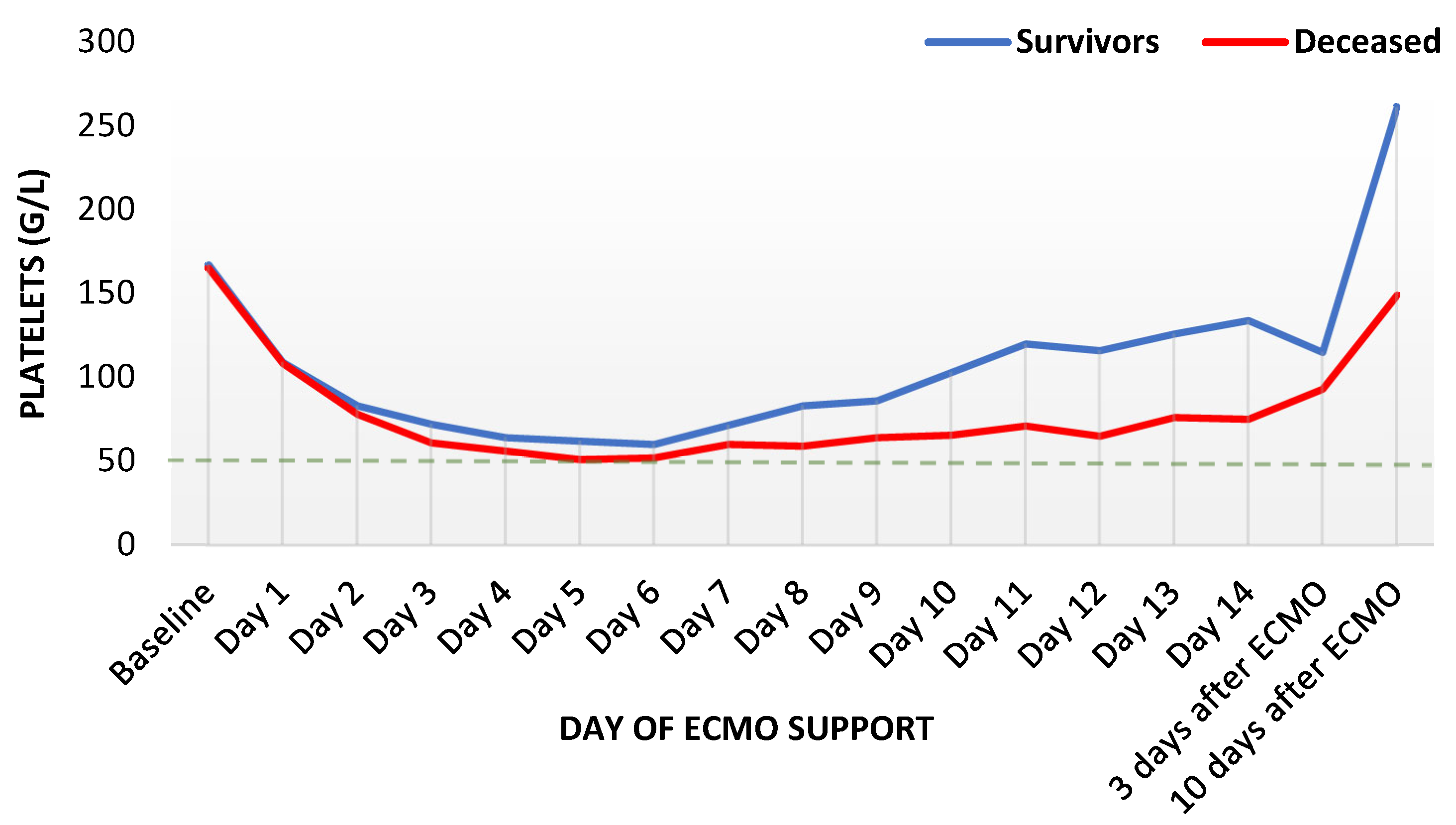
3.2. Laboratory Parameters during ECMO Support
3.3. Adverse Events during ECMO Support
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brogan, T.V.; Lequier, L.; Lorusso, R.; MacLaren, G.; Peek, G.J. Extracorporeal Life Support: The ELSO Red Book, 5th ed.; Extracorporeal Life Support Organization: Ann Arbor, MI, USA, 2017; p. 831. [Google Scholar]
- Édes, I.F.; Németh, B.T.; Hartyánszky, I.; Szilveszter, B.; Kulyassa, P.; Fazekas, L.; Pólos, M.; Németh, E.; Becker, D.; Merkely, B. Predictors of mortality following extracorporeal membrane oxygenation support in an unselected, critically ill patient population. Postep. W Kardiol. Interwencyjnej Adv. Interv. Cardiol. 2021, 17, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, H.S.; Han, S.M.; Cho, H.J. Predictors of mortality in patients with VA-extracorporeal membrane oxygenation. Eur. Heart J. 2020, 41, ehaa946-1237. [Google Scholar] [CrossRef]
- Sahli, S.D.; Kaserer, A.; Braun, J.; Halbe, M.; Dahlem, Y.; Spahn, M.A.; Rössler, J.; Krüger, B.; Maisano, F.; Spahn, D.R.; et al. Predictors associated with mortality of extracorporeal life support therapy for acute heart failure: Single-center experience with 679 patients. J. Thorac. Dis. 2022, 14, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Sohail, S.; Fan, E.; Foroutan, F.; Ross, H.J.; Billia, F.; Alba, A.C. Predictors of Mortality in Patients Treated with Veno-Arterial ECMO for Cardiogenic Shock Complicating Acute Myocardial Infarction: A Systematic Review and Meta–Analysis. J. Cardiovasc. Transl. Res. 2022, 15, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Treml, B.; Breitkopf, R.; Bukumirić, Z.; Bachler, M.; Boesch, J.; Rajsic, S. ECMO Predictors of Mortality: A 10-Year Referral Centre Experience. J. Clin. Med. 2022, 11, 1224. [Google Scholar] [CrossRef]
- Vigneshwar, N.G.; Kohtz, P.D.; Lucas, M.T.; Bronsert, M.J.; Weyant, M.F.; Masood, M.; Itoh, A.; Rove, J.Y.; Reece, T.B.; Cleveland, J.C.; et al. Clinical predictors of in-hospital mortality in venoarterial extracorporeal membrane oxygenation. J. Card. Surg. 2020, 35, 2512–2521. [Google Scholar] [CrossRef]
- Zangrillo, A.; Landoni, G.; Biondi-Zoccai, G.; Greco, M.; Greco, T.; Frati, G.; Patroniti, N.; Antonelli, M.; Pesenti, A.; Pappalardo, F. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2013, 15, 172–178. [Google Scholar]
- Rajsic, S.; Treml, B.; Jadzic, D.; Breitkopf, R.; Oberleitner, C.; Krneta, M.P.; Bukumiric, Z. Extracorporeal membrane oxygenation for cardiogenic shock: A meta-analysis of mortality and complications. Ann. Intensive Care 2022, 12, 93. [Google Scholar] [CrossRef]
- Aubron, C.; DePuydt, J.; Belon, F.; Bailey, M.; Schmidt, M.; Sheldrake, J.; Murphy, D.; Scheinkestel, C.; Cooper, D.J.; Capellier, G.; et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann. Intensive Care 2016, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Lotz, C.; Streiber, N.; Roewer, N.; Lepper, P.M.; Muellenbach, R.M.; Kredel, M. Therapeutic Interventions and Risk Factors of Bleeding During Extracorporeal Membrane Oxygenation. ASAIO J. 2017, 63, 624–630. [Google Scholar] [CrossRef]
- Olson, S.R.; Murphree, C.R.; Zonies, D.; Meyer, A.D.; McCarty, O.J.T.; Deloughery, T.G.; Shatzel, J.J. Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. ASAIO J. 2021, 67, 290–296. [Google Scholar] [CrossRef]
- Lansink-Hartgring, A.O.; de Vries, A.J.; Droogh, J.M.; van den Bergh, W.M. Hemorrhagic complications during extracorporeal membrane oxygenation—The role of anticoagulation and platelets. J. Crit. Care 2019, 54, 239–243. [Google Scholar] [CrossRef]
- Rajsic, S.; Breitkopf, R.; Jadzic, D.; Krneta, M.P.; Tauber, H.; Treml, B. Anticoagulation Strategies during Extracorporeal Membrane Oxygenation: A Narrative Review. J. Clin. Med. 2022, 11, 5147. [Google Scholar] [CrossRef]
- Stokes, J.W.; Gannon, W.D.; Sherrill, W.H.; Armistead, L.B.; Bacchetta, M.; Rice, T.W.; Semler, M.W.; Casey, J.D. Bleeding, Thromboembolism, and Clinical Outcomes in Venovenous Extracorporeal Membrane Oxygenation. Crit. Care Explor. 2020, 2, e0267. [Google Scholar] [CrossRef]
- Arachchillage, D.J.; Rajakaruna, I.; Scott, I.; Gaspar, M.; Odho, Z.; Banya, W.; Vlachou, A.; Isgro, G.; Cagova, L.; Wade, J.; et al. Impact of major bleeding and thrombosis on 180-day survival in patients with severe COVID-19 supported with veno-venous extracorporeal membrane oxygenation in the United Kingdom: A multicentre observational study. Br. J. Haematol. 2021, 196, 566–576. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, H.R.; Hochman, J.S. Cardiogenic Shock. Circulation 2008, 117, 686–697. [Google Scholar] [CrossRef]
- Lequier, L.; Annich, G.; Al-Ibrahim, O.; Bembea, M.; Brodie, D.; Brogan, T.; Buckvold, S.; Chicoine, L.; Conrad, S.; Cooper, D.; et al. ELSO Anticoagulation Guideline; The Extracorporeal Life Support Organization (ELSO): Ann Arbor, MI, USA, 2014. [Google Scholar]
- McMichael, A.B.V.; Ryerson, L.M.; Ratano, D.; Fan, E.; Faraoni, D.; Annich, G.M. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J. 2022, 68, 303–310. [Google Scholar] [CrossRef]
- Mansour, A.; Flecher, E.; Schmidt, M.; Rozec, B.; Gouin-Thibault, I.; Esvan, M.; Fougerou, C.; Levy, B.; Porto, A.; Ross, J.T.; et al. Bleeding and thrombotic events in patients with severe COVID-19 supported with extracorporeal membrane oxygenation: A nationwide cohort study. Intensive Care Med. 2022, 48, 1039–1052. [Google Scholar] [CrossRef]
- Pieri, M.; Greco, T.; De Bonis, M.; Maj, G.; Fumagalli, L.; Zangrillo, A.; Pappalardo, F. Diagnosis of infection in patients undergoing extracorporeal membrane oxygenation: A case-control study. J. Thorac. Cardiovasc. Surg. 2012, 143, 1411–1416. [Google Scholar] [CrossRef] [Green Version]
- Arkader, R.; Troster, E.J.; Lopes, M.R.; Júnior, R.R.; Carcillo, J.A.; Leone, C.; Okay, T.S. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch. Dis. Child. 2006, 91, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Aouifi, A.; Piriou, V.; Blanc, P.; Bouvier, H.; Bastien, O.; Chiari, P.; Rousson, R.; Evans, R.; Lehot, J.J. Effect of cardiopulmonary bypass on serum procalcitonin and C-reactive protein concentrations. Br. J. Anaesth. 1999, 83, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.U.; Heslet, L.; Jensen, T.H.; Espersen, K.; Steffensen, P.; Tvede, M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit. Care Med. 2006, 34, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhydkov, A.; Christ-Crain, M.; Thomann, R.; Hoess, C.; Henzen, C.; Zimmerli, W.; Mueller, B.; Schuetz, P.; ProHOSP Study Group. Utility of procalcitonin, C-reactive protein and white blood cells alone and in combination for the prediction of clinical outcomes in community-acquired pneumonia. Clin. Chem. Lab. Med. 2015, 53, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet. Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef] [Green Version]
- de Werra, I.; Jaccard, C.; Corradin, S.B.; Chioléro, R.; Yersin, B.; Gallati, H.; Assicot, M.; Bohuon, C.; Baumgartner, J.D.; Glauser, M.P.; et al. Cytokines, nitrite/nitrate, soluble tumor necrosis factor receptors, and procalcitonin concentrations: Comparisons in patients with septic shock, cardiogenic shock, and bacterial pneumonia. Crit. Care Med. 1997, 25, 607–613. [Google Scholar] [CrossRef]
- Brunkhorst, F.M.; Clark, A.L.; Forycki, Z.F.; Anker, S.D. Pyrexia, procalcitonin, immune activation and survival in cardiogenic shock: The potential importance of bacterial translocation. Int. J. Cardiol. 1999, 72, 3–10. [Google Scholar] [CrossRef]
- Griffith, D.M.; Lewis, S.; Rossi, A.G.; Rennie, J.; Salisbury, L.; Merriweather, J.L.; Templeton, K.; Walsh, T.S. Systemic inflammation after critical illness: Relationship with physical recovery and exploration of potential mechanisms. Thorax 2016, 71, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Doo, I.; Staub, L.P.; Mattke, A.; Haisz, E.; Seidler, A.L.; Alphonso, N.; Schlapbach, L.J. Diagnostic Accuracy of Infection Markers to Diagnose Infections in Neonates and Children Receiving Extracorporeal Membrane Oxygenation. Front. Pediatr. 2022, 9, 1633. [Google Scholar] [CrossRef]
- Rajsic, S.; Breitkopf, R.; Oezpeker, U.C.; Bukumirić, Z.; Dobesberger, M.; Treml, B. The Role of Excessive Anticoagulation and Missing Hyperinflammation in ECMO-Associated Bleeding. J. Clin. Med. 2022, 11, 2314. [Google Scholar] [CrossRef]
- Ranucci, M.; Ballotta, A.; Di Dedda, U.; Baryshnikova, E.; Dei Poli, M.; Resta, M.; Falco, M.; Albano, G.; Menicanti, L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020, 18, 1747–1751. [Google Scholar] [CrossRef]
- Day, J.R.S.; Taylor, K.M. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int. J. Surg. 2005, 3, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Tatsumi, E.; Nakamura, F.; Oite, T. PaO2 greater than 300 mmHg promotes an inflammatory response during extracorporeal circulation in a rat extracorporeal membrane oxygenation model. J. Thorac. Dis. 2020, 12, 749–757. [Google Scholar] [CrossRef]
- Caprarola, S.D.; Ng, D.K.; Carroll, M.K.; Tekes, A.; Felling, R.J.; Salorio, C.F.; Almuqati, R.; Schwartz, J.M.; Everett, A.D.; Bembea, M.M. Pediatric ECMO: Unfavorable outcomes are associated with inflammation and endothelial activation. Pediatr. Res. 2022, 92, 549–556. [Google Scholar] [CrossRef]
- Levin, B.; Ortoleva, J.; Tagliavia, A.; Colon, K.; Crowley, J.; Shelton, K.; Dalia, A.A. One-Year Survival for Adult Venoarterial Extracorporeal Membrane Oxygenation Patients Requiring Renal-Replacement Therapy. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1942–1948. [Google Scholar] [CrossRef]
- Antonucci, E.; Lamanna, I.; Fagnoul, D.; Vincent, J.-L.; De Backer, D.; Taccone, F.F.S. The Impact of Renal Failure and Renal Replacement Therapy on Outcome during Extracorporeal Membrane Oxygenation Therapy. Artif. Organs 2016, 40, 746–754. [Google Scholar] [CrossRef]



| Patient Characteristics | All Patients (n = 435) | Survivors (n = 268) | Deceased (n = 167) | p-Value | Missing Data (n/total) | |
|---|---|---|---|---|---|---|
| Age (years) | 60.8 ± 14.3 | 60.0 ± 14.2 | 62.1 ± 14.4 | 0.145 | 0/435 | |
| Male sex | 303 (69.7) | 190 (70.9) | 113 (67.7) | 0.520 | 0/435 | |
| Body mass index (kg/m2) | 26.6 ± 4.6 | 26.4 ± 4.8 | 26.8 ± 4.4 | 0.438 | 3/435 | |
| SAPS III score | 62 (15–104) | 58 (15–99) | 70 (31–104) | <0.001 | 1/435 | |
| SOFA score | 11 (1–21) | 11 (2–21) | 11 (1–20) | 0.021 | 1/435 | |
| SOFA respiratory | 2 (0–4) | 2 (0–4) | 2 (0–4) | 0.760 | 1/435 | |
| SOFA coagulation | 1 (0–4) | 1 (0–3) | 1 (0–4) | 0.464 | 1/435 | |
| SOFA liver | 1 (0–4) | 1 (0–3) | 1 (0–4) | 0.630 | 1/435 | |
| SOFA cardiovascular | 4 (0–4) | 4 (0–4), mean 3.5 | 4 (0–4), mean 3.8 | <0.001 | 1/435 | |
| SOFA neurology | 4 (0–4) | 4 (0–4), mean 2.1 | 4 (0–4), mean 2.5 | 0.035 | 1/435 | |
| SOFA renal | 1 (0–4) | 1 (0–4), mean 0.9 | 1 (0–4), mean 1.1 | 0.045 | 1/435 | |
| CPR before ECMO initiation | 118 (27.1) | 62 (23.1) | 56 (33.5) | 0.020 | 0/435 | |
| Length of ICU stay (days) | 18 (2–170) | 21 (4–98) | 11 (2–170) | <0.001 | 0/435 | |
| Cardiogenic shock etiology | 0/435 | |||||
| No cardiotomy | ||||||
| Acute heart failure | 121 (27.8) | 63 (23.5) | 58 (34.7) | 0.009 | ||
| Right heart failure | 29 (6.7) | 15 (5.6) | 14 (8.4) | |||
| Postcardiotomy | ||||||
| Coronary artery bypass surgery (CABG) | 65 (14.9) | 35 (13.1) | 30 (18.0) | |||
| Heart valve surgery (HVS) | 158 (36.3) | 112 (41.8) | 46 (27.5) | |||
| Combined (CABG and HVS, including aortic aneurysm) | 38 (8.7) | 25 (9.3) | 13 (7.8) | |||
| Chronic heart failure | 24 (5.5) | 18 (6.7) | 6 (3.6) | |||
| Mortality-related outcomes | 0/435 | |||||
| Death during ECMO support | 75 (17.2) | - | - | |||
| Death during ICU | 154 (35.4) | - | - | |||
| Death within 90 days | 166 (38.2) | - | - | |||
| Death within 365 days | 173 (39.8) | - | - | |||
| Survived beyond one year | 262 (60.2) | - | - | |||
| Cause of death | 4/167 | |||||
| Cardiac | 68 (41.7) | - | - | |||
| Respiratory | 5 (3.1) | - | - | |||
| Brain death | 19 (11.7) | - | - | |||
| Sepsis | 20 (12.3) | - | - | |||
| MODS | 51 (31.3) | - | - | |||
| Clinical Characteristics | All Patients (n = 435) | Survivors (n = 268) | Deceased (n = 167) | p-Value | Missing Data (n/total) | |
|---|---|---|---|---|---|---|
| ECMO-related clinical course | 0/435 | |||||
| ECMO-support duration (days) | 6 (2–22) | 6 (2–20) | 6 (2–22) | 0.923 | ||
| ECMO-support duration < 7 days | 308 (70.8) | 198 (73.9) | 110 (65.9) | 0.083 | ||
| Anticoagulation during ECMO support | 0/435 | |||||
| Unfractionated heparin | 312 (71.7) | 197 (73.5) | 115 (68.9) | 0.006 | ||
| Argatroban | 71 (16.3) | 46 (17.2) | 25 (15.0) | |||
| Epoprostenol | 2 (0.5) | 0 (0.0) | 2 (1.2) | |||
| Switch from unfractionated heparin to argatroban | 12 (2.8) | 10 (3.7) | 2 (1.2) | |||
| None | 38 (8.7) | 15 (5.6) | 23 (13.8) | |||
| Complications | ||||||
| Hemorrhage | 199 (45.7) | 106 (39.6) | 93 (55.7) | 0.001 | 0/435 | |
| Major hemorrhage | 114 (26.2) | 55 (20.5) | 59 (35.2) | 0.001 | 0/435 | |
| Minor hemorrhage | 85 (19.5) | 51 (19.0) | 34 (20.4) | 0.804 | 0/435 | |
| Thromboembolic events | 110 (25.3) | 71 (26.5) | 39 (23.4) | 0.497 | 0/435 | |
| Thrombosis venous | 66 (15.2) | 52 (19.4) | 14 (8.4) | 0.002 | 0/435 | |
| Thrombosis arterial | 65 (14.9) | 31 (11.6) | 34 (20.4) | 0.018 | 0/435 | |
| Sepsis | 80 (18.4) | 40 (14.9) | 40 (24.1) | 0.022 | 0/435 | |
| Variable | B-Coefficient | p-Value | HR | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Procalcitonin on day three | 0.008 | 0.001 | 1.01 a | 1.00 | 2.25 |
| Resuscitation before ECMO | 0.120 | 0.596 | 1.13 | 0.72 | 1.75 |
| Surgical intervention | 0.399 | 0.057 | 1.49 | 0.99 | 2.25 |
| Hemorrhage | 0.548 | 0.005 | 1.73 | 1.18 | 2.53 |
| Arterial thrombosis | 0.327 | 0.184 | 1.39 | 0.86 | 2.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajsic, S.; Breitkopf, R.; Oezpeker, U.C.; Treml, B. ECMO in Cardiogenic Shock: Time Course of Blood Biomarkers and Associated Mortality. Diagnostics 2022, 12, 2963. https://doi.org/10.3390/diagnostics12122963
Rajsic S, Breitkopf R, Oezpeker UC, Treml B. ECMO in Cardiogenic Shock: Time Course of Blood Biomarkers and Associated Mortality. Diagnostics. 2022; 12(12):2963. https://doi.org/10.3390/diagnostics12122963
Chicago/Turabian StyleRajsic, Sasa, Robert Breitkopf, Ulvi Cenk Oezpeker, and Benedikt Treml. 2022. "ECMO in Cardiogenic Shock: Time Course of Blood Biomarkers and Associated Mortality" Diagnostics 12, no. 12: 2963. https://doi.org/10.3390/diagnostics12122963
APA StyleRajsic, S., Breitkopf, R., Oezpeker, U. C., & Treml, B. (2022). ECMO in Cardiogenic Shock: Time Course of Blood Biomarkers and Associated Mortality. Diagnostics, 12(12), 2963. https://doi.org/10.3390/diagnostics12122963







