Biochemical and Mechanical Analysis of Occlusal and Proximal Carious Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation for Raman and Hardness Measurements
2.2. Raman Spectroscopy
2.3. Vickers Microhardness
2.4. Statistical Analysis and Spectral Correlation Processing with Hardness Measurements
3. Results
3.1. Biochemical Analysis of Sound and Demineralized Zones in Occlusal and Proximal Lesions
3.2. Vickers Microhardness
3.3. Raman Spectral Correlation with Vickers Microhardness (VHN)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paula, J.S.; Leite, I.C.; Almeida, A.B.; Ambrosano, G.; Pereira, A.C.; Mialhe, F.L. The influence of oral health conditions, socioeconomic status and home environment factors on schoolchildren’s self-perception of quality of life. Health Qual. Life Out. 2012, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidd, E.A.; Fejerskov, O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J. Dent. Res. 2004, 83 (Suppl. S1), 35–38. [Google Scholar] [CrossRef]
- Li, S.H.; Kingman, A.; Forthofer, R.; Swango, P. Comparison of tooth surface-specific dental caries attack patterns in US schoolchildren from two national surveys. J. Dent. Res. 1993, 72, 1398–1405. [Google Scholar] [CrossRef]
- Nakagawa, H.; Sadr, A.; Shimada, Y.; Tagami, J.; Sumi, Y. Validation of swept source optical coherence tomography (SS-OCT) for the diagnosis of smooth surface caries in vitro. J. Dent. 2013, 41, 80–89. [Google Scholar] [CrossRef]
- Baelum, V.; Hintze, H.; Wenzel, A.; Danielsen, B.; Nyvad, B. Implications of caries diagnostic strategies for clinical management decisions. Community Dent. Oral Epidemiol. 2012, 40, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Frencken, J.; Innes, N. Caries Excavation, Evolution of Treating Cavitated Carious Lesions; Karger Medical and Scientific Publishers: Basel, Switzerland, 2018. [Google Scholar]
- Banerjee, A.; Pickard, H.M.; Watson, T.F. Pickard’s Manual of Operative Dentistry, 9th ed.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Schwendicke, F.; Dörfer, C.; Paris, S. Incomplete caries removal, a systematic review and meta-analysis. J. Dent. Res. 2013, 92, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Cook, R.; Kellow, S.; Shah, K.; Festy, F.; Sherriff, M.; Watson, T.F. Confocal micro-endoscopic investigation of the relationship between the microhardness of carious dentine and its autofluorescence. Eur. J. Oral Sci. 2010, 118, 75–79. [Google Scholar] [CrossRef]
- Wachsmann-Hogiu, S.; Weeks, T.; Huser, T. Chemical analysis in vivo and in vitro by Raman spectroscopy-from single cells to humans. Curr. Opin. Biotechnol. 2009, 20, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yao, X.; Liu, Y.W.; Wang, Y. A Fourier transform infrared spectroscopy analysis of carious dentin from transparent zone to normal zone. Caries Res. 2014, 48, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Osorio, E.; Aguilera, F.S.; Cabello, I.; Toledano-Osorio, M.; Osorio, R. Ex vivo detection and characterization of remineralised carious dentin, by nanoindentation and single point Raman spectroscopy, after amalgam restoration. J. Raman Spectrosc. 2017, 48, 384–392. [Google Scholar] [CrossRef]
- Alturki, M.; Koller, G.; Almhöjd, U.; Banerjee, A. Chemo-mechanical characterization of carious dentine using Raman microscopy and Knoop microhardness. R. Soc. Open Sci. 2020, 7, 200404. [Google Scholar] [CrossRef]
- Alturki, M.; Koller, G.; Warburton, F.; Almhöjd, U.; Banerjee, A. Biochemical characterisation of carious dentine zones using Raman spectroscopy. J. Dent. 2021, 105, 103558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P.; Walker, M.P. Chemical profile of adhesive/caries-affected dentin interfaces using Raman microspectroscopy. J. Biomed. Mater. Res. A 2007, 81, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, C.D.; Limirio, P.H.; Novais, V.R.; Dechichi, P. Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Almhöjd, U.S.; Norén, J.G.; Arvidsson, A.; Nilsson, A.; Lingström, P. Analysis of carious dentine using FTIR and ToF-SIMS. Oral Health Dent Manag. 2014, 13, 735–744. [Google Scholar] [PubMed]
- El-Sharkawyi, Y.H. Detection and characterization of human teeth caries using 2D correlation raman spectroscopy. J. Biomed. Phys. Eng. 2019, 9, 167. [Google Scholar] [CrossRef]
- Júnior, Z.S.S.; Botta, S.B.; Ana, P.A.; França, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Deana, A.; Bussadori, S.K. Effect of papain-based gel on type I collagen-spectroscopy applied for microstructural analysis. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, M.F.; Yannas, I.V.; Salzman, E.W.; Forbes, M.J. Collagen banded fibril structure and the collagen-platelet reaction. Thromb Res. 1989, 55, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Karabekiroğlu, S.; Kahraman, F.B.; Ünlü, N. Effectiveness of different preventive agents on initial occlusal and proximal caries lesions, A follow-up study. J. Dent. Sci. 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Kassa, D.; Day, P.; High, A.; Duggal, M. Histological comparison of pulpal inflammation in primary teeth with occlusal or proximal caries. Int. J. Paediatr. Dent. 2009, 19, 26–33. [Google Scholar] [CrossRef]
- Pitts, N.B.; Ekstrand, K.; ICDAS Foundation. International Caries Detection and Assessment System (ICDAS) and its International Caries Classification and Management System (ICCMS)-methods for staging of the caries process and enabling dentists to manage caries. Community Dent. Oral. Epidemiol. 2013, 41, e41–e52. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, V.; Toledano, M.; Osorio, R.; Carvalho, R.M. Microhardness of superficial and deep sound human dentin. J. Biomed. Mater. Res. Part A 2003, 66, 850–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, H.; Miyoshi, N.; Fukunaga, Y.; Ogawa, T.; Ogasawara, T.; Sano, K. Functional mapping of carious enamel in human teeth with Raman microspectroscopy. J. Raman Spectrosc. 2008, 39, 655–660. [Google Scholar] [CrossRef]
- Almahdy, A.; Downey, F.C.; Sauro, S.; Cook, R.C.; Sherriff, M.; Richards, D.; Watson, T.F.; Banerjee, A.; Festy, F. Microbiochemical analysis of carious dentine using Raman and fluorescence spectroscopy. Caries Res. 2012, 46, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef]
- Linde, A. Dentin matrix proteins, composition and possible functions in calcification. Anat Rec. 1989, 224, 154–166. [Google Scholar] [CrossRef]
- Ramakrishnaiah, R.; Rehman, G.U.; Basavarajappa, S.; Al Khuraif, A.A.; Durgesh, B.H.; Khan, A.S.; Rehman, I.U. Applications of Raman spectroscopy in dentistry, analysis of tooth structure. Appl. Spectrosc. Rev. 2015, 50, 332–350. [Google Scholar] [CrossRef]
- Krafft, C.; Sergo, V. Biomedical applications of Raman and infrared spectroscopy to diagnose tissues. Spectrosc 2006, 20, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Maske, T.T.; Isolan, C.P.; van de Sande, F.H.; Peixoto, A.C.; Faria-e-Silva, A.L.; Cenci, M.S.; Moraes, R.R. A biofilm cariogenic challenge model for dentin demineralization and dentin bonding analysis. Clin. Oral Investig. 2015, 19, 1047–1053. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Verdelis, K.; Doty, S.B.; Boskey, A.L.; Mendelsohn, R.; Yamauch, M.I. Spectroscopic characterization of collagen cross-links in bone. J. Bone Miner. Res. 2001, 16, 1821–1828. [Google Scholar] [CrossRef]
- Dirksen, T.R. Lipid components of sound and carious dentine. J. Dent. Res. 1963, 42, 128–132. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Phase transformations in a human tooth tissue at the initial stage of caries. PLoS ONE 2015, 10, e0124008. [Google Scholar] [CrossRef]
- Gourion-Arsiquaud, S.; West, P.A.; Boskey, A.L. Fourier transform-infrared microspectroscopy and microscopic imaging. Osteoporosis 2008, 2, 293–303. [Google Scholar] [CrossRef]
- Pugach, M.; Strother, J.; Darling, C.; Fried, D.; Gansky, S.; Marshall, S.; Marshall, G. Dentin caries zones, mineral, structure, and properties. J. Dent. Res. 2009, 88, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ami, D.; Natalello, A.; Gatti-Lafranconi, P.; Schultz, T.; Lotti, M.; de Marco, A.; Doglia, S.M. FT-IR spectroscopy for the study of bacterial membrane stress induced by recombinant protein production. Microb. Cell Factories 2006, 5, 1–2. [Google Scholar] [CrossRef]
- Braga, M.M.; Floriano, I.; Ferreira, F.R.; Silveira, J.M.; Reyes, A.; Tedesco, T.K.; Raggio, D.P.; Imparato, J.C.; Mendes, F.M. Are the approximal caries lesions in primary teeth a challenge to deal with? A critical appraisal of recent evidences in this field. VIRDI, MC Emerging trends in Oral Health Sciences and Dentistry. Intech 2015, 11, 83–116. [Google Scholar] [CrossRef] [Green Version]
- de Barros Pinto, L.; Lira, M.L.; Cavalcanti, Y.W.; Dantas, E.L.; Vieira, M.L.; de Carvalho, G.G.; de Sousa, F.B. Natural enamel caries, dentine reactions, dentinal fluid and biofilm. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shellis, R.P. Transport Processes in Enamel and Dentine; Tooth wear and sensitivity; Martin Dunitz: London, UK, 2000; pp. 19–28. [Google Scholar]
- Banerjee, A.; Frencken, J.E.; Schwendicke, F.; Innes, N.P.T. Contemporary operative caries management, consensus recommendations on minimally invasive caries removal. Br. Dent. J. 2017, 223, 215–222. [Google Scholar] [CrossRef] [PubMed]

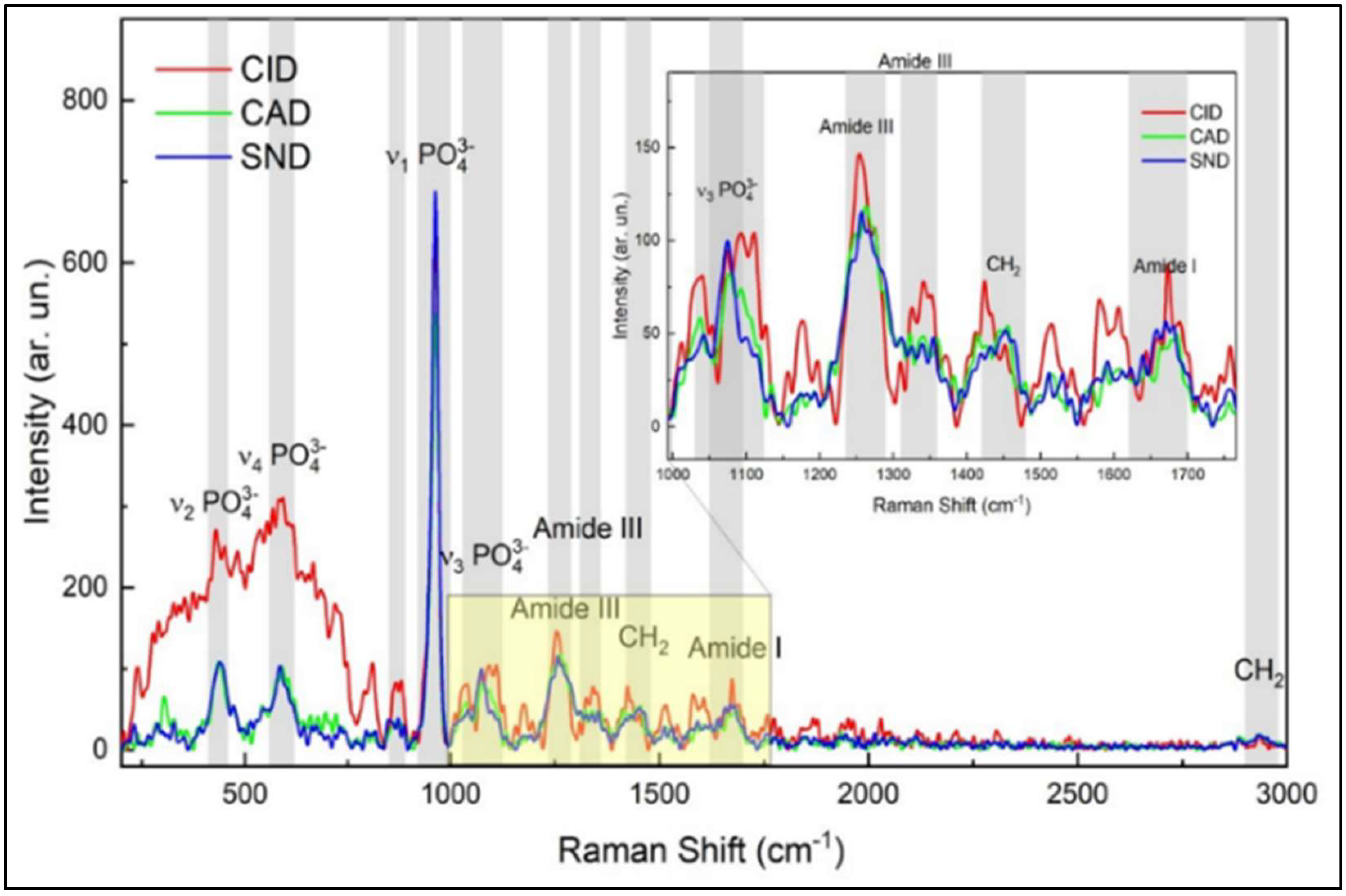

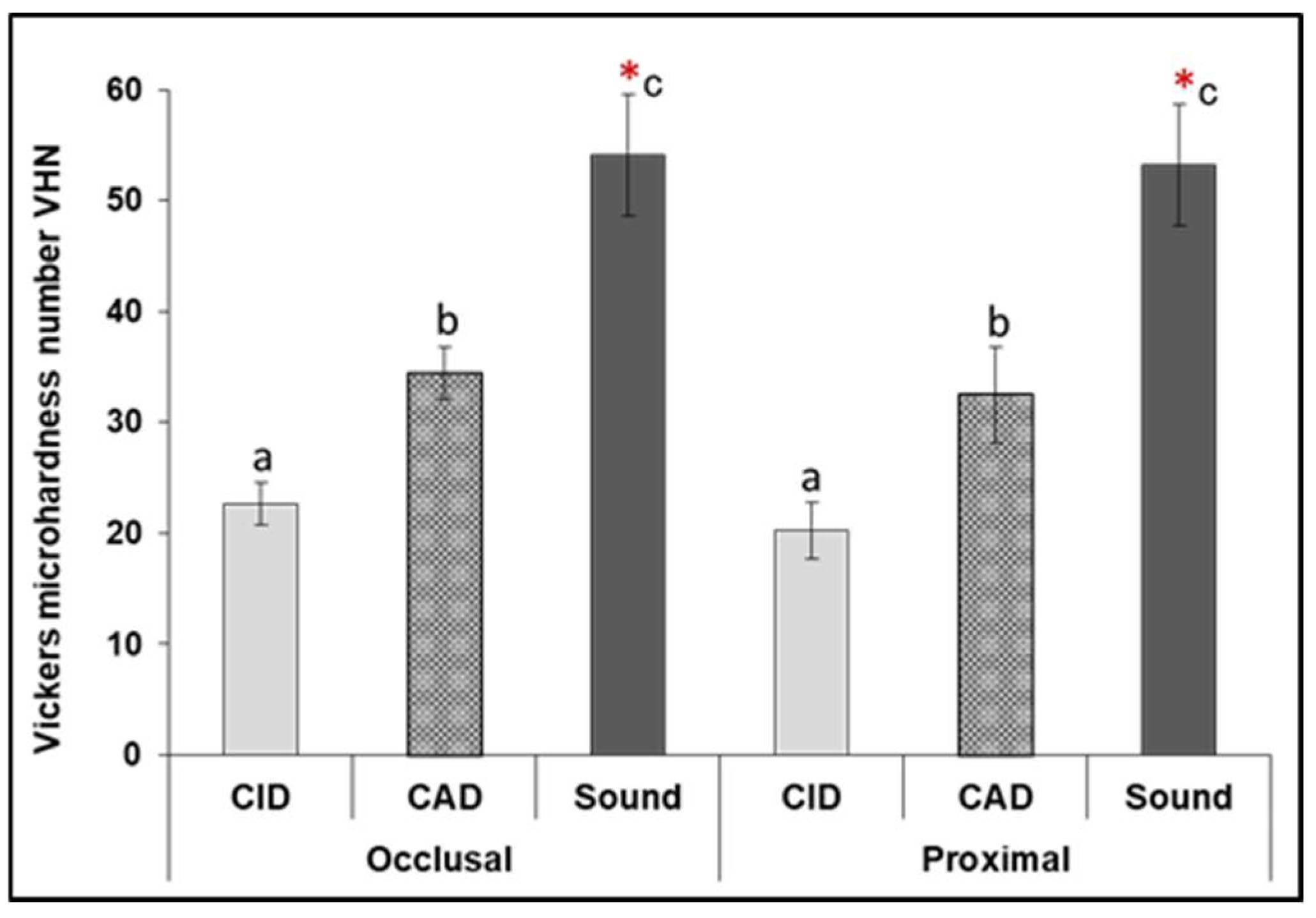
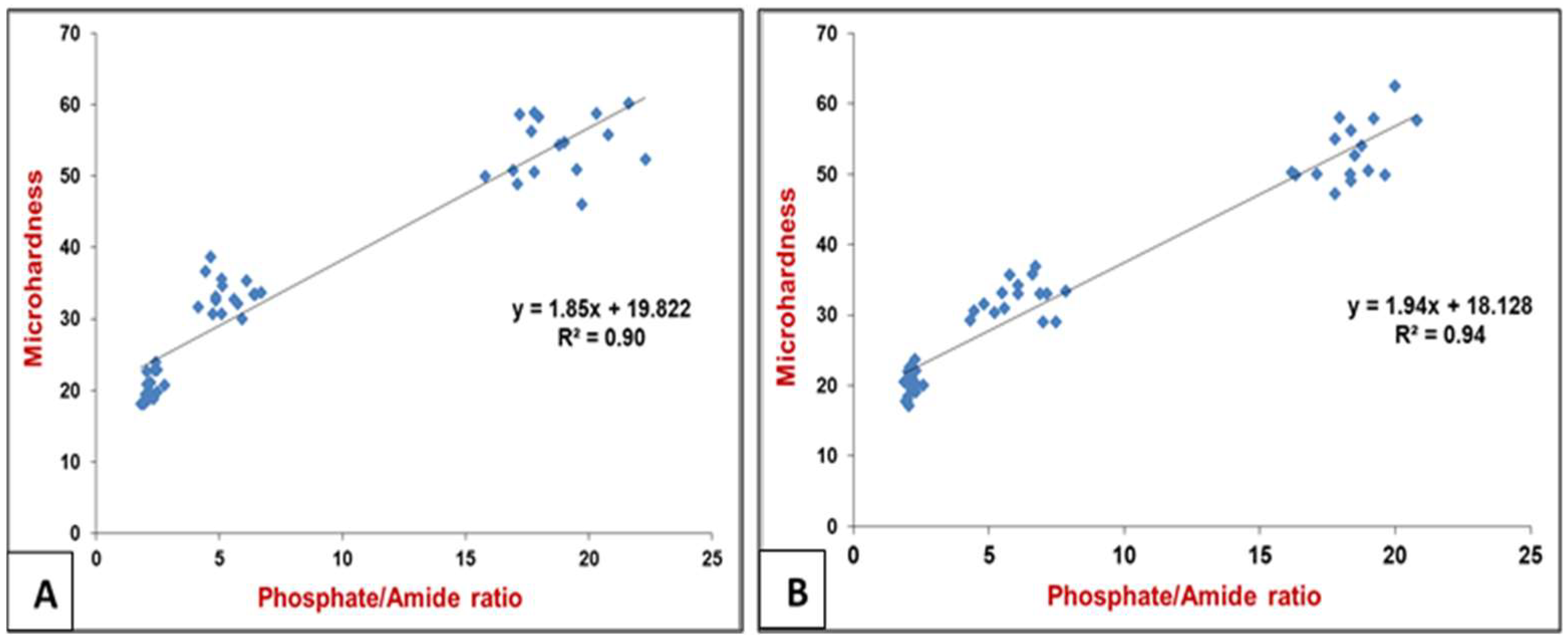
| Raman Peaks | Carious Zones (n = 10 per Zone) | Mean of Peaks Intensity (Occlusal Lesion) (Mean ± SD) | Mean of Peak Intensity (Proximal Lesion) (Mean ± SD) |
|---|---|---|---|
| Phosphate peak v1-PO (960 cm−1) | CID | 89.0 ± 11.3 a | 79.6 ± 7.7 a |
| CAD | 344.4 ± 95.1 b | 369.6 ± 84.6 b | |
| Sound | 2105.3 ± 112.1 *c | 2156.4 ± 122.7 *c | |
| Amide I (1650 cm−1) | CID | 84.0 ± 7.1 * | 68.5 ± 7.2 *^ |
| CAD | 62.0 ± 9.6 d | 60.5 ± 10.9 d | |
| Sound | 60.6 ± 9.9 e | 51.81 ± 10.2 e | |
| Amide III (1235 cm−1) | CID | 112.1 ± 5.3 * | 69.8 ± 3.7 *^ |
| CAD | 80.4 ± 5.7 | 60.4 ± 6.0 ^ | |
| Sound | 78.5 ± 11.5 | 56.7 ± 7.9 ^ | |
| C-H bond of pyrrolidine ring (1450 cm−1) | CID | 88.9 ± 4.9 *f | 85.3 ± 3.9 *f |
| CAD | 74.9 ± 5.5 g | 80.0 ± 5.7 g | |
| Sound | 70.7 ± 8.6 h | 68.1 ± 9.8 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shareefi, S.; Addie, A.; Al-Taee, L. Biochemical and Mechanical Analysis of Occlusal and Proximal Carious Lesions. Diagnostics 2022, 12, 2944. https://doi.org/10.3390/diagnostics12122944
Al-Shareefi S, Addie A, Al-Taee L. Biochemical and Mechanical Analysis of Occlusal and Proximal Carious Lesions. Diagnostics. 2022; 12(12):2944. https://doi.org/10.3390/diagnostics12122944
Chicago/Turabian StyleAl-Shareefi, Sahar, Ali Addie, and Lamis Al-Taee. 2022. "Biochemical and Mechanical Analysis of Occlusal and Proximal Carious Lesions" Diagnostics 12, no. 12: 2944. https://doi.org/10.3390/diagnostics12122944
APA StyleAl-Shareefi, S., Addie, A., & Al-Taee, L. (2022). Biochemical and Mechanical Analysis of Occlusal and Proximal Carious Lesions. Diagnostics, 12(12), 2944. https://doi.org/10.3390/diagnostics12122944







