Shear Wave Dispersion Slope Measured with Shear Wave Dispersion Imaging Is Associated with Variceal Hemorrhage in Cirrhotic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ultrasound Examination
2.3. Laboratory Test
2.4. Statistical Analysis
3. Results
3.1. Comparisons of Variables According to the Presence of VH
3.2. Factors Associated with VH
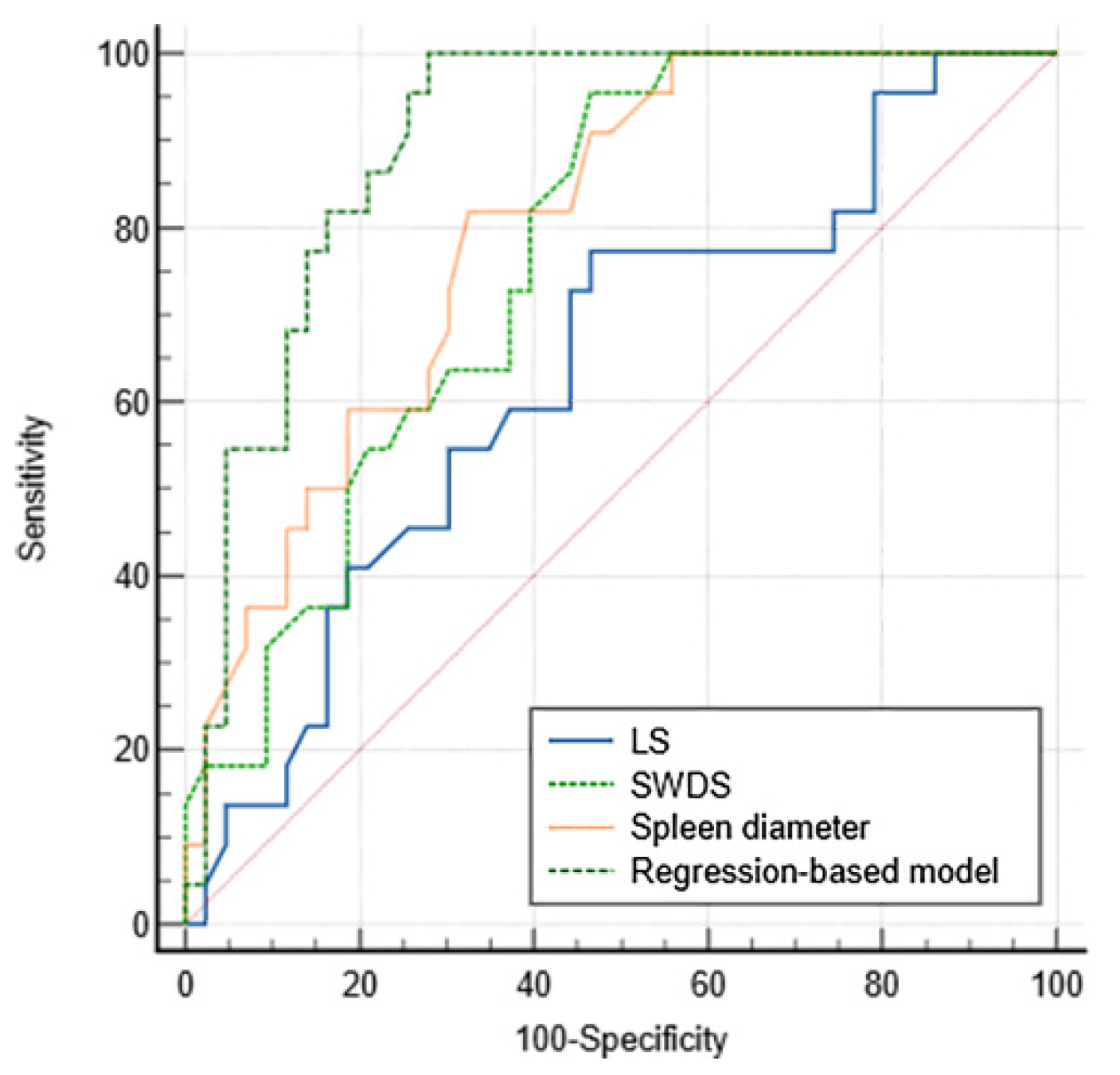
3.3. Diagnostic Performance for VH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovalak, M.; Lake, J.; Mattek, N.; Eisen, G.; Lieberman, D.; Zaman, A. Endoscopic screening for varices in cirrhotic patients: Data from a national endoscopic database. Gastrointest. Endosc. 2007, 65, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Stanley, A.J.; Hayes, P.C.; Patch, D.; Millson, C.; Mehrzad, H.; Austin, A.; Ferguson, J.W.; Olliff, S.P.; Hudson, M.; et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015, 64, 1680–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, K.; Moriyasu, F.; Oshiro, H.; Takeuchi, H.; Yoshimasu, Y.; Kasai, Y.; Itoi, T. Clinical utilization of shear wave dispersion imaging in diffuse liver disease. Ultrasonography 2020, 39, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, K.; Moriyasu, F.; Oshiro, H.; Takeuchi, H.; Yoshimasu, Y.; Kasai, Y.; Furuichi, Y.; Itoi, T. Viscoelasticity Measurement in Rat Livers Using Shear-Wave US Elastography. Ultrasound Med. Biol. 2018, 44, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, T.O.G.; Jensen, D.M. Varices: Esophageal, Gastric, and Rectal. Clin. Liver Dis. 2019, 23, 625–642. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; Clinical Practice Guideline Panel; Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis-2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Barr, R.G.; Wilson, S.R.; Rubens, D.; Garcia-Tsao, G.; Ferraioli, G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology 2020, 296, 263–274. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef] [Green Version]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
- Berzigotti, A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J. Hepatol. 2017, 67, 399–411. [Google Scholar] [CrossRef]
- Fofiu, R.; Bende, F.; Popescu, A.; Şirli, R.; Lupușoru, R.; Ghiuchici, A.M.; Sporea, I. Spleen and Liver Stiffness for Predicting High-Risk Varices in Patients with Compensated Liver Cirrhosis. Ultrasound Med. Biol. 2021, 47, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, T.Y.; Kim, Y.; Lim, S.; Jeong, W.K.; Sohn, J.H. Diagnostic Performance of Shear Wave Elastography for Predicting Esophageal Varices in Patients With Compensated Liver Cirrhosis. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2016, 35, 1373–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassinotto, C.; Charrie, A.; Mouries, A.; Lapuyade, B.; Hiriart, J.B.; Vergniol, J.; Gaye, D.; Hocquelet, A.; Charbonnier, M.; Foucher, J.; et al. Liver and spleen elastography using supersonic shear imaging for the non-invasive diagnosis of cirrhosis severity and oesophageal varices. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2015, 47, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.W.; Kim, Y.S.; Kim, S.G.; Yoo, J.J.; Jeong, S.W.; Jang, J.Y.; Lee, S.H.; Kim, H.S.; Kim, Y.D.; Cheon, G.J.; et al. Usefulness of noninvasive methods including assessment of liver stiffness by 2-dimensional shear wave elastography for predicting esophageal varices. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2019, 51, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xing, X.; Lu, Q.; Wang, X.; Luo, X.; Yang, L. Two-dimensional shear wave elastography for sparing endoscopy screening in patients with HBV-related compensated advanced chronic liver disease. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Gennisson, J.L.; Deffieux, T.; Tanter, M.; Fink, M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: Preliminary in vivo feasibility study. Ultrasound Med. Biol. 2009, 35, 219–229. [Google Scholar] [CrossRef]
- Deffieux, T.; Montaldo, G.; Tanter, M.; Fink, M. Shear wave spectroscopy for in vivo quantification of human soft tissues visco-elasticity. IEEE Trans. Med. Imaging 2009, 28, 313–322. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.Y.; Bae, J.S.; Yi, N.J.; Lee, K.W.; Suh, K.S.; Kim, H.; Lee, K.B.; Han, J.K. Shear-Wave Dispersion Slope from US Shear-Wave Elastography: Detection of Allograft Damage after Liver Transplantation. Radiology 2019, 293, 327–333. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zheng, R.Q.; Jin, J.Y.; Wang, J.F.; Zhang, T.; Zeng, J. US Shear-Wave Elastography Dispersion for Characterization of Chronic Liver Disease. Radiology 2022. online ahead of print. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Zhou, W.; Wen, L.; Zhang, S.; Yu, D. Clinical Application of Shear Wave Elastography with Shear Wave Dispersion Imaging in the Preoperative Evaluation of Hepatic Parenchyma in Patients with Liver Tumors. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Vizzutti, F.; Arena, U.; Romanelli, R.G.; Rega, L.; Foschi, M.; Colagrande, S.; Petrarca, A.; Moscarella, S.; Belli, G.; Zignego, A.L.; et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 2007, 45, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Marrone, G.; Fernández-Iglesias, A. Hepatic microcirculation and mechanisms of portal hypertension. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Thabut, D.; Kamath, P.S.; Shah, V.H. Oesophageal varices in cirrhotic patients: From variceal screening to primary prophylaxis of the first oesophageal variceal bleeding. Liver Int. Off. J. Int. Assoc. Study Liver 2011, 31, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Buechter, M.; Kahraman, A.; Manka, P.; Gerken, G.; Jochum, C.; Canbay, A.; Dechêne, A. Spleen and Liver Stiffness Is Positively Correlated with the Risk of Esophageal Variceal Bleeding. Digestion 2016, 94, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Lim, S.; Kim, Y.; Sohn, J.H.; Jeong, J.Y. Spleen Stiffness Measurement Using 2-Dimensional Shear Wave Elastography: The Predictors of Measurability and the Normal Spleen Stiffness Value. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2019, 38, 423–431. [Google Scholar] [CrossRef] [PubMed]
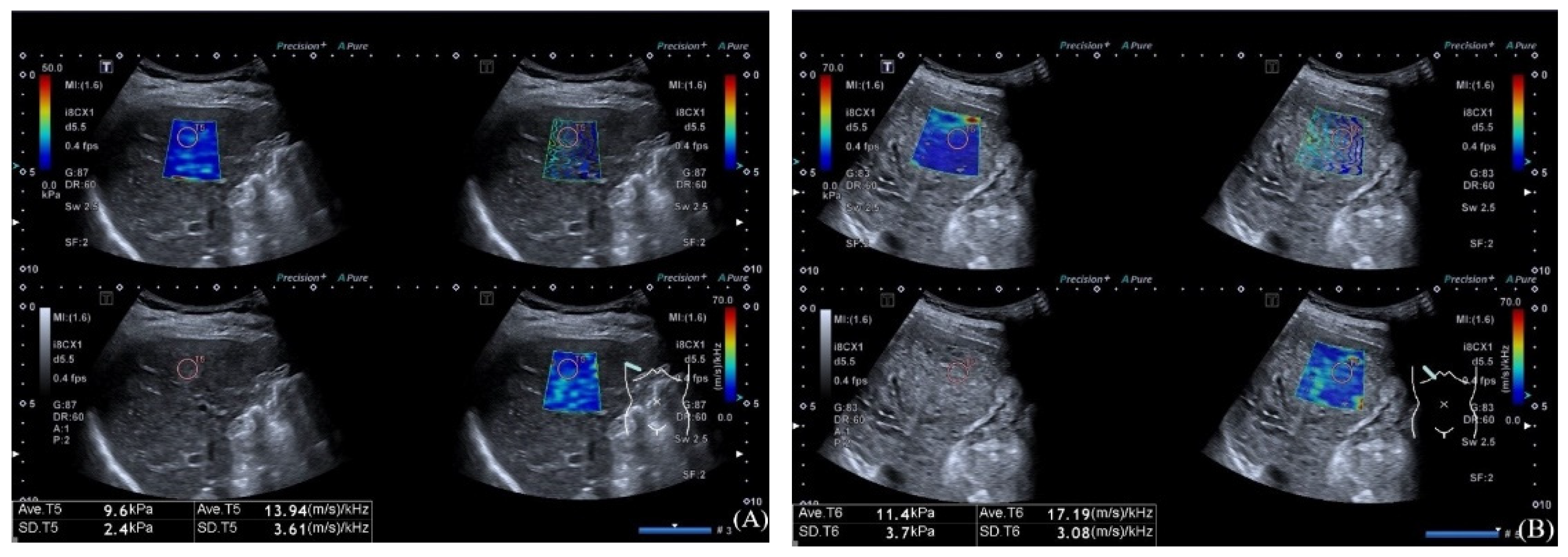
| Variables | Non VH (n = 43) | VH (n = 22) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| t/z/χ² | p Value | OR (95% CI) | p Value | |||
| Age (years) | 55.65 ± 11.97 | 58.41 ± 11.73 | −0.885 | 0.380 | — | — |
| Sex | 0.210 | 0.647 | — | — | ||
| Male | 26 (60.5%) | 12 (54.5%) | ||||
| Female | 17 (39.5%) | 10 (45.5%) | ||||
| BMI (kg/m2) | 23.69 ± 3.53 | 22.47 ± 2.36 | 1.457 | 0.150 | — | — |
| Etiology | 7.434 | 0.055 | — | — | ||
| Chronic viral hepatitis | 24 (55.8%) | 6 (27.3%) | ||||
| PBC | 11 (25.6%) | 5 (22.7%) | ||||
| others | 4 (9.3%) | 5 (22.7%) | ||||
| Cryptogenic cirrhosis | 4 (9.3%) | 6 (27.3%) | ||||
| PLT (×109/L) | 145.44 ± 59.61 | 92.59 ± 38.11 | 3.755 | <0.001 | — | 0.286 |
| ALT (IU/L) | 24.80 (17.00–36.20) | 20.50 (14.75–43.18) | −0.284 | 0.776 | — | — |
| AST (IU/L) | 28.00 (22.50–43.00) | 34.00 (24.00–49.28) | −1.096 | 0.273 | — | — |
| TBIL (μmol/L) | 17.80 (13.00–27.80) | 23.95 (16.88–44.45) | −1.879 | 0.060 | — | — |
| ALB (g/L) | 39.96 ± 6.53 | 36.06 ± 4.90 | 2.463 | 0.017 | — | 0.227 |
| Ascites | 9.449 | 0.002 | 6.066 (1.287–28.585) | 0.023 | ||
| No | 29 (67.4%) | 6 (27.3%) | ||||
| Yes | 14 (32.6%) | 16 (72.7%) | ||||
| Portal vein diameter (cm) | 1.10 (1.00-1.30) | 1.30 (1.28-1.40) | −3.578 | <0.001 | — | 0.105 |
| Spleen diameter (cm) | 11.48 ± 2.71 | 14.36 ± 2.00 | −4.414 | <0.001 | 1.567 (1.126–2.181) | 0.008 |
| LS (kPa) | 9.30 (7.40–11.70) | 10.60 (9.10–12.68) | −1.796 | 0.073 | — | — |
| SWDS (m/s/kHz) | 15.17 ± 1.88 | 17.04 ± 1.45 | −4.062 | <0.001 | 2.234 (1.267–3.941) | 0.005 |
| Criteria | AUC | p Value | Cutoff Value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| LS | 0.637 (0.508–0.753) | 0.062 | 9.5 | 77.3 (54.6–92.2) | 53.5 (37.7–68.8) | 45.9 (36.5–55.7) | 82.1 (67.0–91.3) |
| SWDS | 0.768 (0.647–0.864) | <0.001 | 15.2 | 95.5 (77.2–99.9) | 53.5 (37.7–68.8) | 51.2 (42.9–59.4) | 95.8 (76.9–99.4) |
| Spleen diameter | 0.798 (0.680–0.887) | <0.001 | 12.6 | 81.8 (59.7–94.8) | 67.4 (51.5–80.9) | 56.2 (44.5–67.4) | 87.9 (74.5–94.7) |
| Regression-based model | 0.900 (0.800–0.961) | <0.001 | 0.19 | 100.0 (84.6–100) | 72.1 (56.3–84.7) | 64.7 (53.1–74.8) | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhang, L.; Jiang, L.; Cui, L.; Li, X. Shear Wave Dispersion Slope Measured with Shear Wave Dispersion Imaging Is Associated with Variceal Hemorrhage in Cirrhotic Patients. Diagnostics 2022, 12, 2909. https://doi.org/10.3390/diagnostics12122909
Sun X, Zhang L, Jiang L, Cui L, Li X. Shear Wave Dispersion Slope Measured with Shear Wave Dispersion Imaging Is Associated with Variceal Hemorrhage in Cirrhotic Patients. Diagnostics. 2022; 12(12):2909. https://doi.org/10.3390/diagnostics12122909
Chicago/Turabian StyleSun, Xiaohui, Li Zhang, Ling Jiang, Ligang Cui, and Xiaoguang Li. 2022. "Shear Wave Dispersion Slope Measured with Shear Wave Dispersion Imaging Is Associated with Variceal Hemorrhage in Cirrhotic Patients" Diagnostics 12, no. 12: 2909. https://doi.org/10.3390/diagnostics12122909
APA StyleSun, X., Zhang, L., Jiang, L., Cui, L., & Li, X. (2022). Shear Wave Dispersion Slope Measured with Shear Wave Dispersion Imaging Is Associated with Variceal Hemorrhage in Cirrhotic Patients. Diagnostics, 12(12), 2909. https://doi.org/10.3390/diagnostics12122909






