Label-Free Optical Spectroscopy for Early Detection of Oral Cancer
Abstract
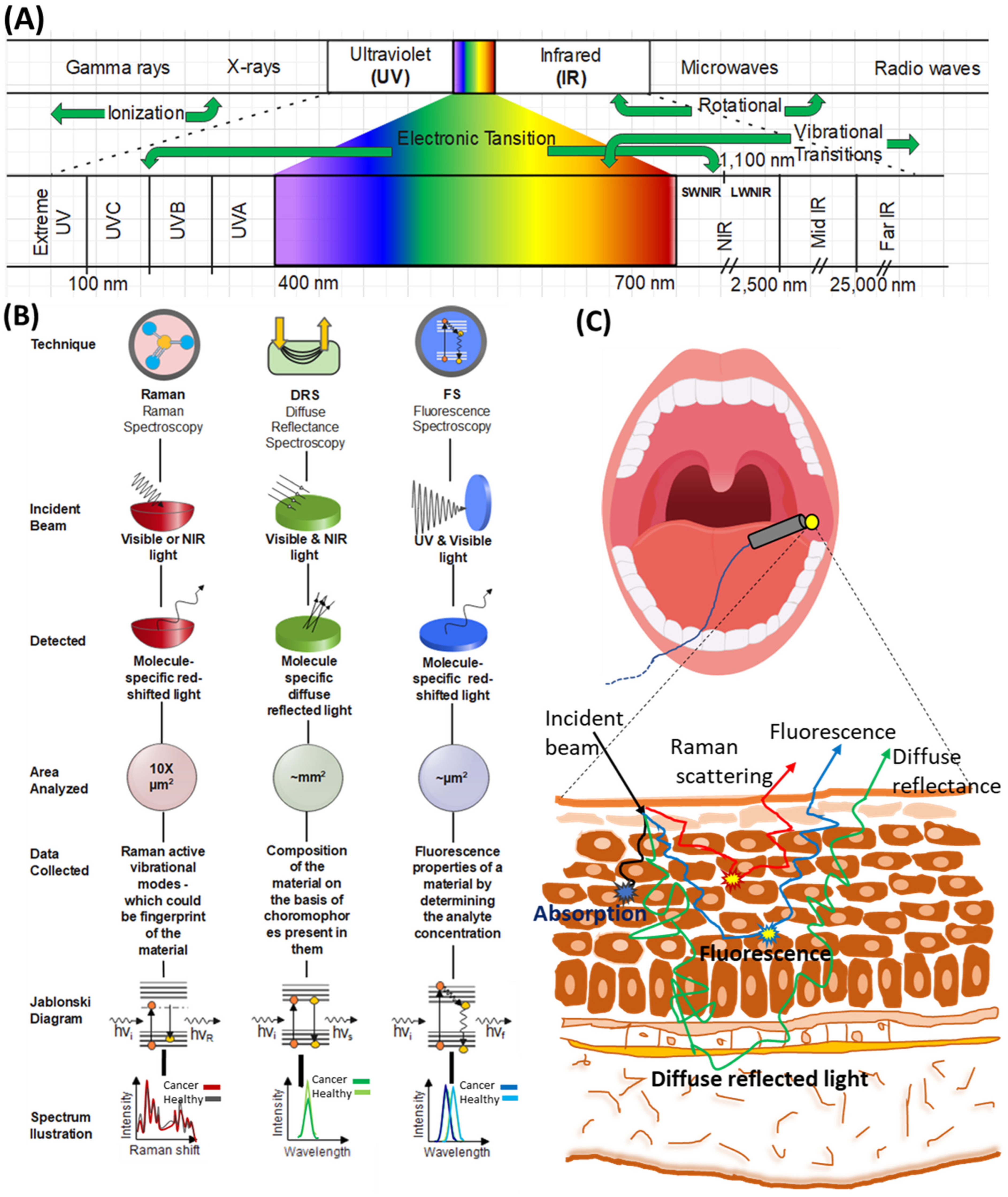
:1. Introduction
2. Diagnosis of Oral Cancer
3. Optical Spectroscopy for Oral Cancer Screening Ex Vivo
4. Optical Spectroscopy for In Vivo Diagnosis
5. Multimodal Spectroscopy Approach
6. Discussion
7. Limitations and Future Outlook
7.1. Instrumentation
7.2. Analysis Approach/Data Analysis
7.3. Requirement of Standardization
8. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Bray, F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020, 102, 104551. [Google Scholar] [CrossRef]
- Markopoulos, A.K. Current aspects on oral squamous cell carcinoma. Open Dent. J. 2012, 6, 126. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-J.; Zhang, X.-Q.; Liu, Q.; Zhang, J.; Zhou, G. Nanotechnology: A promising method for oral cancer detection and diagnosis. J. Nanobiotechnol. 2018, 16, 52. [Google Scholar] [CrossRef]
- Koch, F.P.; Kunkel, M.; Biesterfeld, S.; Wagner, W. Diagnostic efficiency of differentiating small cancerous and precancerous lesions using mucosal brush smears of the oral cavity—A prospective and blinded study. Clin. Oral Investig. 2011, 15, 763–769. [Google Scholar] [CrossRef]
- Weinberg, M.A.; Estefan, D.J. Assessing oral malignancies. Am. Fam. Physician 2002, 65, 1379. [Google Scholar]
- Oliver, R.; Sloan, P.; Pemberton, M. Verifiable CPD paper: Oral biopsies: Methods and applications. Br. Dent. J. 2004, 196, 329. [Google Scholar] [CrossRef] [Green Version]
- Rethman, M.P.; Carpenter, W.; Cohen, E.E.; Epstein, J.; Evans, C.A.; Flaitz, C.M.; Graham, F.J.; Hujoel, P.P.; Kalmar, J.R.; Koch, W.M. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. J. Am. Dent. Assoc. 2010, 141, 509–520. [Google Scholar] [CrossRef]
- Mian, S.A.; Yorucu, C.; Ullah, M.S.; Rehman, I.U.; Colley, H.E. Raman spectroscopy can discriminate between normal, dysplastic and cancerous oral mucosa: A tissue-engineering approach. J. Tissue Eng. Regen. Med. 2017, 11, 3253–3262. [Google Scholar] [CrossRef]
- Bouaoud, J.; Bossi, P.; Elkabets, M.; Schmitz, S.; van Kempen, L.C.; Martinez, P.; Jagadeeshan, S.; Breuskin, I.; Puppels, G.J.; Hoffmann, C.; et al. Unmet needs and perspectives in oral cancer prevention. Cancers 2022, 14, 1815. [Google Scholar] [CrossRef]
- Singh, S.; Ibrahim, O.; Byrne, H.J.; Mikkonen, J.W.; Koistinen, A.P.; Kullaa, A.M.; Lyng, F.M. Recent advances in optical diagnosis of oral cancers: Review and future perspectives. Head Neck 2016, 38, E2403–E2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanthi. Difference Between Histopathology and Cytology. Available online: https://www.differencebetween.com/difference-between-histopathology-and-cytology/ (accessed on 21 April 2022).
- Nitya, K.; Amberkar, V.S.; Nadar, B.G. Vital Staining-Pivotal Role in the Field of Pathology. Ann. Cytol. Pathol. 2020, 5, 058–063. [Google Scholar] [CrossRef]
- Wilson, B.C.; Jermyn, M.; Leblond, F. Challenges and opportunities in clinical translation of biomedical optical spectroscopy and imaging. J. Biomed. Opt. 2018, 23, 030901. [Google Scholar] [CrossRef] [PubMed]
- Lecchi, M.; Fossati, P.; Elisei, F.; Orecchia, R.; Lucignani, G. Current concepts on imaging in radiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 821–837. [Google Scholar] [CrossRef]
- Muzic, R.F., Jr.; DiFilippo, F.P. PET/MRI—Technical review. Semin. Roentgenol. 2014, 49, 242–254. [Google Scholar] [CrossRef] [Green Version]
- Baron, J.A. Screening for cancer with molecular markers: Progress comes with potential problems. Nat. Rev. Cancer 2012, 12, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Furrer, D.; Sanschagrin, F.; Jacob, S.; Diorio, C. Advantages and disadvantages of technologies for HER2 testing in breast cancer specimens. Am. J. Clin. Pathol. 2015, 144, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C. Pros and Cons of Genetic Testing and Cancer. Available online: https://www.cancer.net/blog/2016-09/pros-and-cons-genetic-testing-and-cancer (accessed on 21 April 2022).
- Lee, J.; Kim, B.; Park, B.; Won, Y.; Kim, S.-Y.; Lee, S. Real-time cancer diagnosis of breast cancer using fluorescence lifetime endoscopy based on the pH. Sci. Rep. 2021, 11, 16864. [Google Scholar] [CrossRef]
- Jermyn, M.; Desroches, J.; Aubertin, K.; St-Arnaud, K.; Madore, W.-J.; De Montigny, E.; Guiot, M.-C.; Trudel, D.; Wilson, B.C.; Petrecca, K.; et al. A review of Raman spectroscopy advances with an emphasis on clinical translation challenges in oncology. Phys. Med. Biol. 2016, 61, R370. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, W.; Lin, D.; Wu, S.; Chen, M.; Ge, X.; Lin, X.; Sun, L. Discrimination of nasopharyngeal carcinoma from noncancerous ex vivo tissue using reflectance spectroscopy. J. Spectrosc. 2016, 2016, 1596845. [Google Scholar] [CrossRef]
- Marcu, L. Fluorescence lifetime techniques in medical applications. Ann. Biomed. Eng. 2012, 40, 304–331. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.; Johnson-Obaseki, S.; Lumingu, J.; Corsten, M. Oncologic, functional and surgical outcomes of primary Transoral Robotic Surgery for early squamous cell cancer of the oropharynx: A systematic review. Oral Oncol. 2014, 50, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Jermyn, M.; Mercier, J.; Aubertin, K.; Desroches, J.; Urmey, K.; Karamchandiani, J.; Marple, E.; Guiot, M.-C.; Leblond, F.; Petrecca, K. Highly accurate detection of cancer in situ with intraoperative, label-free, multimodal optical spectroscopy. Cancer Res. 2017, 77, 3942–3950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Pan, J.; Huang, H.; Chen, G.; Qiu, S.; Shi, H.; Chen, W.; Yu, Y.; Feng, S.; Chen, R. Label-free blood plasma test based on surface-enhanced Raman scattering for tumor stages detection in nasopharyngeal cancer. Sci. Rep. 2014, 4, 4751. [Google Scholar] [CrossRef] [Green Version]
- Jeng, M.-J.; Sharma, M.; Sharma, L.; Chao, T.-Y.; Huang, S.-F.; Chang, L.-B.; Wu, S.-L.; Chow, L. Raman spectroscopy analysis for optical diagnosis of oral cancer detection. J. Clin. Med. 2019, 8, 1313. [Google Scholar] [CrossRef]
- Gorpas, D.; Davari, P.; Bec, J.; Fung, M.A.; Marcu, L.; Farwell, D.G.; Fazel, N. Time-resolved fluorescence spectroscopy for the diagnosis of oral lichen planus. Clin. Exp. Dermatol. 2018, 43, 546–552. [Google Scholar] [CrossRef] [Green Version]
- De Koning, S.G.B.; Baltussen, E.J.; Karakullukcu, M.B.; Dashtbozorg, B.; Smit, L.A.; Dirven, R.; Hendriks, B.H.; Sterenborg, H.J.; Ruers, T.J. Toward complete oral cavity cancer resection using a handheld diffuse reflectance spectroscopy probe. J. Biomed. Opt. 2018, 23, 121611. [Google Scholar]
- Eliasson, C.; Matousek, P. A Noninvasive Method for Deep Raman Spectroscopy of Living Tissue and Powders. Posted on August 2007. Available online: https://www.americanlaboratory.com/914-Application-Notes/1389-A-Noninvasive-Method-for-Deep-Raman-Spectroscopy-of-Living-Tissue-and-Powders/ (accessed on 21 October 2022).
- Matousek, P.; Stone, N. Recent advances in the development of Raman spectroscopy for deep non-invasive medical diagnosis. J. Biophotonics 2013, 6, 7–19. [Google Scholar] [CrossRef]
- Greening, G.; Mundo, A.; Rajaram, N.; Muldoon, T.J. Sampling depth of a diffuse reflectance spectroscopy probe for in-vivo physiological quantification of murine subcutaneous tumor allografts. J. Biomed. Opt. 2018, 23, 085006. [Google Scholar] [CrossRef] [Green Version]
- Swartling, J.; Svensson, J.; Bengtsson, D.; Terike, K.; Andersson-Engels, S. Fluorescence spectra provide information on the depth of fluorescent lesions in tissue. Appl. Opt. 2005, 44, 1934–1941. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.; Nogueira, M.; Canevari, R.; Carvalho, L. Vibration spectroscopy and body biofluids: Literature review for clinical applications. Photodiagnosis Photodyn. Ther. 2018, 24, 237–244. [Google Scholar] [CrossRef]
- Kah, J.C.Y.; Kho, K.W.; Lee, C.G.L.; James, C.; Sheppard, R.; Shen, Z.X.; Soo, K.C.; Olivo, M.C. Early diagnosis of oral cancer based on the surface plasmon resonance of gold nanoparticles. Int. J. Nanomed. 2007, 2, 785–798. [Google Scholar]
- Connolly, J.M.; Davies, K.; Kazakeviciute, A.; Wheatley, A.M.; Dockery, P.; Keogh, I.; Olivo, M. Non-invasive and label-free detection of oral squamous cell carcinoma using saliva surface-enhanced Raman spectroscopy and multivariate analysis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1593–1601. [Google Scholar] [CrossRef]
- Elumalai, B.; Prakasarao, A.; Ganesan, B.; Dornadula, K.; Ganesan, S. Raman spectroscopic characterization of urine of normal and oral cancer subjects. J. Raman Spectrosc. 2015, 46, 84–93. [Google Scholar] [CrossRef]
- Choi, S.; Park, H.K.; Min, G.E.; Kim, Y.H. Biochemical investigations of human papillomavirus-infected cervical fluids. Microsc. Res. Tech. 2015, 78, 200–206. [Google Scholar] [CrossRef]
- Harris, A.T.; Lungari, A.; Needham, C.J.; Smith, S.L.; Lones, M.A.; Fisher, S.E.; Yang, X.B.; Cooper, N.; Kirkham, J.; Smith, D.A.; et al. Potential for Raman spectroscopy to provide cancer screening using a peripheral blood sample. Head Neck Oncol. 2009, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mi, X.; Tan, X.; Xiang, R. Recent Progress on Liquid Biopsy Analysis using Surface-Enhanced Raman Spectroscopy. Theranostics 2019, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, P.; Wang, X.; Duan, Y. Investigation and identification of potential biomarkers in human saliva for the early diagnosis of oral squamous cell carcinoma. Clin. Chim. Acta 2014, 427, 79–85. [Google Scholar] [CrossRef]
- Kumar, P. Detection of inaccessible head and neck lesions using human saliva and fluorescence spectroscopy. Lasers Med. Sci. 2022, 37, 1821–1827. [Google Scholar] [CrossRef]
- Lee, L.; Wong, Y.; Hsiao, H.; Wang, Y.; Chan, M.; Chang, K. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2018, 47, 699–707. [Google Scholar] [CrossRef]
- Hole, A.; Jadhav, P.; Pansare, K.; Noothalapati, H.; Deshmukh, A.; Gota, V.; Chaturvedi, P.; Krishna, C.M. Salivary Raman spectroscopy: Understanding alterations in saliva of tobacco habitués and oral cancer subjects. Vib. Spectrosc. 2022, 122, 103414. [Google Scholar] [CrossRef]
- Koster, H.J.; Guillen-Perez, A.; Gomez-Diaz, J.S.; Navas-Moreno, M.; Birkeland, A.C.; Carney, R.P. Fused Raman spectroscopic analysis of blood and saliva delivers high accuracy for head and neck cancer diagnostics. Sci. Rep. 2022, 12, 18464. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.; Sherlin, H.J.; Ramani, P.; Premkumar, P.; Natesan, A.; Chandrasekar, T. Amino acid profile of saliva from patients with oral squamous cell carcinoma using high performance liquid chromatography. J. Oral Sci. 2012, 54, 279–283. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Gao, P.; Cheng, F.; Wang, X.; Duan, Y. Measurement of salivary metabolite biomarkers for early monitoring of oral cancer with ultra performance liquid chromatography-mass spectrometry. Talanta 2014, 119, 299–305. [Google Scholar] [CrossRef]
- Tankiewicz, A.; Dziemiańczyk, D.; Buczko, P.; Szarmach, I.; Grabowska, S.; Pawlak, D. Tryptophan and its metabolites in patients with oral squamous cell carcinoma: Preliminary study. Adv. Med. Sci. 2006, 51, 221–224. [Google Scholar]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V.; Kosenok, V.K. Diagnostic and Prognostic Value of Salivary Biochemical Markers in Oral Squamous Cell Carcinoma. Diagnostics 2020, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Chiappin, S.; Antonelli, G.; Gatti, R.; Elio, F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- Koduru, M.R.; Ramesh, A.; Adapa, S.; Shetty, J. Salivary albumin as a biomarker for oral squamous cell carcinoma and chronic periodontitis. Ann. Med. Health Sci. Res. 2017, 7, 337–340. [Google Scholar]
- Ramya, A.S.; Uppala, D.; Majumdar, S.; Surekha, C.; Deepak, K. Are salivary amylase and pH–Prognostic indicators of cancers? J. Oral Biol. Craniofacial Res. 2015, 5, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-T.; Darvishi, S.; Preet, A.; Huang, T.-Y.; Lin, S.-H.; Girault, H.H.; Wang, L.; Lin, T.-E. A review: Electrochemical biosensors for oral cancer. Chemosensors 2020, 8, 54. [Google Scholar] [CrossRef]
- Katakura, A.; Kamiyama, I.; Takano, N.; Shibahara, T.; Muramatsu, T.; Ishihara, K.; Takagi, R.; Shouno, T. Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull. Tokyo Dent. Coll. 2007, 48, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Brailo, V.; Vucicevic-Boras, V.; Lukac, J.; Biocina-Lukenda, D.; Zilic-Alajbeg, I.; Milenovic, A.; Balija, M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med. Oral Patol. Oral Cirugía Bucal 2012, 17, e10. [Google Scholar] [CrossRef]
- Gustafsson, A.; Ajeti, V.; Ljunggren, L. Detection of suPAR in the saliva of healthy young adults: Comparison with plasma levels. Biomark. Insights 2011, 6, 119–125. [Google Scholar] [CrossRef]
- Santarelli, A.; Mascitti, M.; Lo Russo, L.; Colella, G.; Giannatempo, G.; Bambini, F.; Emanuelli, M.; Procaccini, M.; Lo Muzio, L. Detection level of salivary survivin in patients with OSCC. J. Carcinog. Mutagen. 2013, 5, 29–85. [Google Scholar]
- Feng, Y.; Li, Q.; Chen, J.; Yi, P.; Xu, X.; Fan, Y.; Cui, B.; Yu, Y.; Li, X.; Du, Y.; et al. Salivary protease spectrum biomarkers of oral cancer. Int. J. Oral Sci. 2019, 11, 7. [Google Scholar] [CrossRef]
- Mirzaii-Dizgah, I.; Riahi, E. Serum and saliva levels of cathepsin L in patients with acute coronary syndrome. J. Contemp. Dent. Pract. 2011, 12, 114–119. [Google Scholar] [CrossRef]
- Gholizadeh, N.; Ramandi, M.A.; Motiee-Langroudi, M.; Jafari, M.; Sharouny, H.; Sheykhbahaei, N. Serum and salivary levels of lactate dehydrogenase in oral squamous cell carcinoma, oral lichen planus and oral lichenoid reaction. BMC Oral Health 2020, 20, 314. [Google Scholar] [CrossRef]
- Rakesh Yadav, S.; Deherkar, J.; Sangle, A.; Chandorkar, S. Utility of Salivary CA-125 And LDH As Tumor Markers in Oral Malignancy. Int. J. Clin. Biomed. Res. 2018, 4, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, R.R.; Yurgel, L.S.; Campos, M.M. Evaluation of salivary endothelin-1 levels in oral squamous cell carcinoma and oral leukoplakia. Regul. Pept. 2011, 166, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-S.L.; Rees, T.; Jordan, L.; Oxford, L.; O’Brien, J.; Chen, H.-S.; Wong, D. Salivary endothelin-1 potential for detecting oral cancer in patients with oral lichen planus or oral cancer in remission. Oral Oncol. 2011, 47, 1122–1126. [Google Scholar] [CrossRef] [Green Version]
- Pateel, D.G.S.; Gunjal, S.; Math, S.Y.; Murugeshappa, D.G.; Nair, S.M. Correlation of salivary statherin and calcium levels with dental calculus formation: A preliminary study. Int. J. Dent. 2017, 2017, 2857629. [Google Scholar] [CrossRef] [Green Version]
- Contucci, A.; Inzitari, R.; Agostino, S.; Vitali, A.; Fiorita, A.; Cabras, T.; Scarano, E.; Messana, I. Statherin levels in saliva of patients with precancerous and cancerous lesions of the oral cavity: A preliminary report. Oral Dis. 2005, 11, 95–99. [Google Scholar] [CrossRef]
- Geng, X.-F.; Du, M.; Han, J.-X.; Zhang, M.; Tang, X.-F.; Xing, R.-D. Saliva CA125 and TPS levels in patients with oral squamous cell carcinoma. Int. J. Biol. Markers 2013, 28, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, E.J.; Reategui, E.P.; Carraway, K.L.; Hamilton, K.L.; Weed, D.T.; Goodwin, W.J. Salivary soluble CD44: A potential molecular marker for head and neck cancer. Cancer Epidemiol. Prev. Biomark. 2005, 14, 735–739. [Google Scholar] [CrossRef]
- Shivashankara, A. Salivary total protein, sialic acid, lipid peroxidation and glutathione in oral squamous cell carcinoma. Biomed. Res. 2011, 22, 355–359. [Google Scholar]
- Bhat, S.; Babu, S.G.; Bhat, S.K.; Castelino, R.L.; Rao, K.; Madi, M. Status of serum and salivary ascorbic acid in oral potentially malignant disorders and oral cancer. Indian J. Med. Paediatr. Oncol. Off. J. Indian Soc. Med. Paediatr. Oncol. 2017, 38, 306. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, E.; Prabhu, V.; Pai, V.R.; D’souza, J.M.; Harish, S.; Jose, M. Salivary L-fucose as a biomarker for oral potentially malignant disorders and oral cancer. J. Cancer Res. Ther. 2020, 16, 546. [Google Scholar] [CrossRef]
- Arthisri, A.S.; Sathiyamoorthy, A.; Meenakshi, B.; Chandran, C.R. Ratio of salivary sialic acid to fucose as tumor markers in potentially malignant disorders and oral cancer. Contemp. Clin. Dent. 2020, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.V.; Ramesh, M.; Murali, S.; Ramesh, K.; Sanjay, P.; Abraham, P. A non-invasive study to estimate and compare salivary sialic acid level as tumor marker in patients with pre-cancer and oral cancer. J. Cancer Res. Ther. 2016, 12, 634. [Google Scholar] [PubMed]
- Metzger, K.; Angres, G.; Maier, H.; Lehmann, W.D. Lipoxygenase products in human saliva: Patients with oral cancer compared to controls. Free Radic. Biol. Med. 1995, 18, 185–194. [Google Scholar] [CrossRef]
- Bhat, M.A.; Prasad, K.; Trivedi, D.; Rajeev, B.; Battur, H. Pyruvic acid levels in serum and saliva: A new course for oral cancer screening? J. Oral Maxillofac. Pathol. JOMFP 2016, 20, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Arellano, M.; Boontheung, P.; Wang, J.; Zhou, H.; Jiang, J.; Elashoff, D.; Wei, R.; Loo, J.A.; Wong, D.T. Salivary proteomics for oral cancer biomarker discovery. Clin. Cancer Res. 2008, 14, 6246–6252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, K.; Ramya, R.; Nandhini, G.; Rajashree, P.; Ramesh Kumar, A.; Nirmala Anandan, S. Salivary and serum level of CYFRA 21-1 in oral precancer and oral squamous cell carcinoma. Oral Dis. 2015, 21, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Tripathi, A.; Patil, R.; Kumar, V.; Khanna, V.; Singh, V. Estimation of salivary and serum basic fibroblast growth factor in treated and untreated patients with oral squamous cell carcinoma. J. Oral Biol. Craniofacial Res. 2019, 9, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Pyykkö, I.; van Setten, G.; Norlander, T.; Nastri, A.; Westermark, A. Basic fibroblast growth factor (bFGF) in saliva and oral mucosa in patients with oral lichen planus: Preliminary observations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2004, 98, 324–332. [Google Scholar] [CrossRef]
- Lin, D.; Qiu, S.; Huang, W.; Pan, J.; Xu, Z.; Chen, R.; Feng, S.; Chen, G.; Li, Y.; Short, M.; et al. Autofluorescence and white light imaging-guided endoscopic Raman and diffuse reflectance spectroscopy for in vivo nasopharyngeal cancer detection. J. Biophotonics 2018, 11, e201700251. [Google Scholar] [CrossRef]
- Behl, I.; Calado, G.; Vishwakarma, A.; Traynor, D.; Flint, S.; Galvin, S.; Healy, C.M.; Pimentel, M.L.; Malkin, A.; Byrne, H.J.; et al. Classification of cytological samples from oral potentially malignant lesions through Raman spectroscopy: A pilot study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120437. [Google Scholar] [CrossRef]
- Larion, M.; Dowdy, T.; Ruiz-Rodado, V.; Meyer, M.W.; Song, H.; Zhang, W.; Davis, D.; Gilbert, M.R.; Lita, A. Detection of metabolic changes induced via drug treatments in live cancer cells and tissue using Raman imaging microscopy. Biosensors 2018, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, O.; Toner, M.; Flint, S.; Byrne, H.J.; Lyng, F.M. The Potential of Raman Spectroscopy in the Diagnosis of Dysplastic and Malignant Oral Lesions. Cancers 2021, 13, 619. [Google Scholar] [CrossRef]
- Kuzmin, A.N.; Pliss, A.; Rzhevskii, A.; Lita, A.; Larion, M. BCAbox algorithm expands capabilities of Raman microscope for single organelles assessment. Biosensors 2018, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Kuzmin, A.N.; Levchenko, S.M.; Pliss, A.; Qu, J.; Prasad, P.N. Molecular profiling of single organelles for quantitative analysis of cellular heterogeneity. Sci. Rep. 2017, 7, 6512. [Google Scholar] [CrossRef] [Green Version]
- Malini, R.; Venkatakrishna, K.; Kurien, J.; Pai, K.M.; Rao, L.; Kartha, V.; Krishna, C.M. Discrimination of normal, inflammatory, premalignant, and malignant oral tissue: A Raman spectroscopy study. Biopolym. Orig. Res. Biomol. 2006, 81, 179–193. [Google Scholar] [CrossRef]
- Li, B.; Gu, Z.-Y.; Yan, K.-X.; Wen, Z.-N.; Zhao, Z.-H.; Li, L.-J.; Li, Y. Evaluating oral epithelial dysplasia classification system by near-infrared Raman spectroscopy. Oncotarget 2017, 8, 76257. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Rodado, V.; Lita, A.; Larion, M. Advances in measuring cancer cell metabolism with subcellular resolution. Nat. Methods 2022, 19, 1048–1063. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Udayakumar, K.; Jayanth, V.; Rao, A.P.; Bharanidharan, G.; Koteeswaran, D.; Munusamy, B.D.; Ganesan, S. Fluorescence spectroscopic characterization of salivary metabolites of oral cancer patients. J. Photochem. Photobiol. B Biol. 2014, 130, 153–160. [Google Scholar] [CrossRef]
- Venugopal, C.; Nazeer, S.S.; Balan, A.; Jayasree, R. Autofluorescence spectroscopy augmented by multivariate analysis as a potential noninvasive tool for early diagnosis of oral cavity disorders. Photomed. Laser Surg. 2013, 31, 605–612. [Google Scholar] [CrossRef]
- Wang, J. Real-time calibrating polarization-sensitive diffuse reflectance handheld probe characterizes clinically relevant anatomical locations of oral tissue in vivo. Biomed. Opt. Express 2022, 13, 105–116. [Google Scholar] [CrossRef]
- Greening, G.J.; James, H.M.; Dierks, M.K.; Vongkittiargorn, N.; Osterholm, S.M.; Rajaram, N.; Muldoon, T.J. Towards monitoring dysplastic progression in the oral cavity using a hybrid fiber-bundle imaging and spectroscopy probe. Sci. Rep. 2016, 6, 26734. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; McWilliams, A.; Lam, S. Optical spectroscopy and imaging for early lung cancer detection: A review. Photodiagnosis Photodyn. Ther. 2004, 1, 111–122. [Google Scholar] [CrossRef]
- Ibrahim, O. The Potential of Raman Spectroscopy in the Diagnosis of Premalignant Oral Lesions. Ph.D. Thesis, Technological University Dublin, Dublin, Ireland, December 2017. [Google Scholar]
- Jaychandran, S.; Meenapriya, P.; Ganesan, S. Raman spectroscopic analysis of blood, urine, saliva and tissue of oral potentially malignant disorders and malignancy-A diagnostic study. Int. J. Oral Craniofacial Sci. 2016, 2, 011–014. [Google Scholar]
- Nour, M.; Hamdy, O.; Faid, A.H.; Eltayeb, E.A.; Zaky, A.A. Utilization of gold nanoparticles for the detection of squamous cell carcinoma of the tongue based on laser-induced fluorescence and diffuse reflectance characteristics: An in vitro study. Lasers Med. Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Nazeer, S.S.; Asish, R.; Venugopal, C.; Anita, B.; Gupta, A.K.; Jayasree, R.S. Noninvasive assessment of the risk of tobacco abuse in oral mucosa using fluorescence spectroscopy: A clinical approach. J. Biomed. Opt. 2014, 19, 057013. [Google Scholar] [CrossRef]
- Francisco, A.L.N.; Correr, W.R.; Azevedo, L.H.; Kern, V.G.; Pinto, C.A.L.; Kowalski, L.P.; Kurachi, C. Fluorescence spectroscopy for the detection of potentially malignant disorders and squamous cell carcinoma of the oral cavity. Photodiagnosis Photodyn. Ther. 2014, 11, 82–90. [Google Scholar] [CrossRef]
- Francisco, A.L.N.; Correr, W.R.; Pinto, C.A.L.; Gonçalves Filho, J.; Chulam, T.C.; Kurachi, C.; Kowalski, L.P. Analysis of surgical margins in oral cancer using in situ fluorescence spectroscopy. Oral Oncol. 2014, 50, 593–599. [Google Scholar] [CrossRef]
- Scepanovic, O.R.; Fitzmaurice, M.; Miller, A.; Kong, C.-R.; Volynskaya, Z.I.; Dasari, R.R.; Kramer, J.R., Jr.; Feld, M.S. Multimodal spectroscopy detects features of vulnerable atherosclerotic plaque. J. Biomed. Opt. 2011, 16, 011009. [Google Scholar] [CrossRef]
- Summers, K.L.; Fimognari, N.; Hollings, A.; Kiernan, M.; Lam, V.; Tidy, R.J.; Paterson, D.; Tobin, M.J.; Takechi, R.; George, G.N.; et al. A multimodal spectroscopic imaging method to characterize the metal and macromolecular content of proteinaceous aggregates (“amyloid plaques”). Biochemistry 2017, 56, 4107–4116. [Google Scholar] [CrossRef]
- Volynskaya, Z.I. Multimodal Spectroscopy: Real-Time Diagnosis of Breast Cancer during Core Needle Biopsy. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2010. [Google Scholar]
- Salomatina, E.; Muzikansky, A.; Neel, V.; Yaroslavsky, A.N. Multimodal optical imaging and spectroscopy for the intraoperative mapping of nonmelanoma skin cancer. J. Appl. Phys. 2009, 105, 102010. [Google Scholar] [CrossRef]
- Kowalski, L.P.; Bagietto, R.; Lara, J.R.; Santos, R.L.; Silva, J.F., Jr.; Magrin, J. Prognostic significance of the distribution of neck node metastasis from oral carcinoma. Head Neck J. Sci. Spec. Head Neck 2000, 22, 207–214. [Google Scholar] [CrossRef]
- Brennan, J.A.; Mao, L.; Hruban, R.H.; Boyle, J.O.; Eby, Y.J.; Koch, W.M.; Goodman, S.N.; Sidransky, D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 1995, 332, 429–435. [Google Scholar] [CrossRef]
- Wikner, J.; Gröbe, A.; Pantel, K.; Riethdorf, S. Squamous cell carcinoma of the oral cavity and circulating tumour cells. World J. Clin. Oncol. 2014, 5, 114. [Google Scholar] [CrossRef]
- Moros, J.; Garrigues, S.; de la Guardia, M. Vibrational spectroscopy provides a green tool for multi-component analysis. TrAC Trends Anal. Chem. 2010, 29, 578–591. [Google Scholar] [CrossRef]
- Barroso, E.; Smits, R.; Bakker Schut, T.; Ten Hove, I.; Hardillo, J.; Wolvius, E.; Baatenburg de Jong, R.; Koljenovic, S.; Puppels, G. Discrimination between oral cancer and healthy tissue based on water content determined by Raman spectroscopy. Anal. Chem. 2015, 87, 2419–2426. [Google Scholar] [CrossRef]
- Huck, C.W. Advances of vibrational spectroscopic technologies in life sciences. Molecules 2017, 22, 278. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Tech. Instrum. 2015, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, M.S.; Maryam, S.; Amissah, M.; Lu, H.; Lynch, N.; Killeen, S.; O’Riordain, M.; Andersson-Engels, S. Evaluation of wavelength ranges and tissue depth probed by diffuse reflectance spectroscopy for colorectal cancer detection. Sci. Rep. 2021, 11, 798. [Google Scholar] [CrossRef]
- Wabnitz, H.; Taubert, D.R.; Mazurenka, M.; Steinkellner, O.; Jelzow, A.; Macdonald, R.; Milej, D.; Sawosz, P.; Kacprzak, M.; Liebert, A. Performance assessment of time-domain optical brain imagers, part 1: Basic instrumental performance protocol. J. Biomed. Opt. 2014, 19, 086010. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, A.; Torricelli, A.; Bassi, A.; Taroni, P.; Cubeddu, R.; Wabnitz, H.; Grosenick, D.; Möller, M.; Macdonald, R.; Swartling, J.; et al. Performance assessment of photon migration instruments: The MEDPHOT protocol. Appl. Opt. 2005, 44, 2104–2114. [Google Scholar] [CrossRef]
| Diagnostic Techniques | In Vivo | Non-invasive | Real Time | Time Required * | Label-Free | Non-ionizing | Portability | Spatial Resolution | Detection Accuracy | Cost-Effectiveness ** | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histopathology | Excisional biopsy | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | [12,13] |
| Incisional biopsy | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | ||
| Brush biopsy | ✕ | ✓ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | ||
| Punch biopsy | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | ||
| Vital staining | Toluidine blue | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | [14] |
| Iodine staining | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | ||
| Methylene blue | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | ||
| Lugol’s iodine | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | ||
| Acetowhite staining | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | ||
| Double staining | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | High | Low cost | ||
| Imaging techniques | OCT (Optical coherence tomography) | ✓ | ✓ | ✓ | 10–20 min | ✓ | ✓ | ✕ | µm | Medium | Expensive | [15,16,17] |
| CT scan (computed tomography) | ✓ | ✓ | ✓ | 10–20 min | ✕ | ✕ | ✕ | Mm | Medium | Expensive | ||
| PET scan (positron emission tomography) | ✓ | ✓ | ✓ | 45–60 min | ✕ | ✕ | ✕ | Mm | Medium | Expensive | ||
| MRI (magnetic resonance imaging) | ✓ | ✓ | ✕ | 15–90 min | ✓ | ✓ | ✕ | Mm | Low | Expensive | ||
| Molecular analysis | Immunohistochemistry | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | Very high | Low cost | [18,19,20] |
| In situ hybridization | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | Very high | Low cost | ||
| Flow cytometry | ✕ | ✕ | ✕ | days | ✕ | ✓ | ✕ | µm | Very high | Low cost | ||
| Mass spectrometry | ✕ | ✕ | ✓ | s | ✓ | ✕ | ✕ | NA | Very high | Low cost | ||
| PCR (polymerase chain reaction) | ✕ | ✓ | ✓ | min | ✓ | ✓ | ✓ | NA | Very high | Low cost | ||
| Light-based detection | Tissue fluorescence (autofluorescence imaging, Velscope, Identafi) | ✓ | ✓ | ✓ | s | ✓ | ✓ | ✓ | Low | Low | Low cost | [12,15,21] |
| Chemiluminiscence (ViziLite, Microlux/DL, MCE) | ✓ | ✓ | ✓ | s | ✕ | ✓ | ✓ | Low | Low | Low cost | ||
| Optical spectroscopy | ✓ | ✓ | ✓ | s | ✓ | ✓ | ✓ | Low | Medium | Low cost | ||
| Raman Spectroscopy | Diffuse Reflectance Spectroscopy | Fluorescence Spectroscopy | |
|---|---|---|---|
| Basic Phenomenon | Scattering | Scattering and absorption | Photoluminescence event |
| Incident light | Ultraviolet, visible, NIR | Visible, NIR | Ultraviolet, visible |
| Detected light | Inelastically scattered Raman-shifted light | Diffusely scattered light | Emission from endogenous fluorophores |
| Spectral range | 10–4000 cm−1 | 200–3000 nm | 360–700 nm |
| Typical tissue depth probed | <0.1 mm [31,32] | <1.5 mm [33] | 0.1–10 mm [34] |
| Label-free/noninvasive/nondestructive | Yes | Yes | Yes |
| Real time data acquisition | Yes | Yes | Yes |
| Suitable for ex vivo/in vivo analysis | Yes | Yes | Yes |
| Photo-bleaching | No | No | Yes |
| Suitable for biological specimens | Yes | Yes | Yes |
| Suitable to incorporate with endoscopy | Yes | Yes | Yes |
| Suitable for water-based specimens, blood, and saliva | Yes | Only highly scattering specimens (selective wavelength regions) | Yes |
| Special sample preparation | No | No | No |
| Specificity (bandwidth) | High | Moderate | Moderate |
| Sensitivity | Low | High | High |
| Nanoparticles | Not required but can be used —SERS | Not required | Not required—can be used in SPR-enhanced fluorescence |
| Possible biomarkers | Lipids, protein, nucleic acid, circulating tumor cells | Water, lipid, collagen, deoxy and oxyhaemoglobin | Endogenous fluorophores—such as NADH, FAD, collagen, and porphyrins |
| Category | Biomarker | Healthy | Oral Cancer | Significance | Trend | Ref. |
|---|---|---|---|---|---|---|
| Proteins and amino acids | Phenylalanine | 0.011 µmol/mL | 0.105 µmol/mL | * | Increase | [48] |
| 4100 ng/mL | T1, T2 = 2500 ng/mL; T3, T4 = 1900 ng/mL | * | Decrease | [49] | ||
| Tyrosine | 0.112 µmol/mL | 0.343 µmol/mL | * | Increase | [48] | |
| Tryptophan | 3.81 ± 0.62 µM | [50] | ||||
| Leucin | 0.015 µmol/mL | 0.241 µmol/mL | * | Increase | [48] | |
| 2300 ng/mL | T1, T2 = 600 ng/mL; T3, T4 = 500 ng/mL | * | Decrease | [49] | ||
| Alanine | 0.096 µmol/mL | 0.178 µmol/mL | * | Increase | [48] | |
| Valine | 0.038 µmol/mL | 0.165 µmol/mL | NS | Increase | [48] | |
| Isoleucin | 0.033 µmol/mL | 0.236 µmol/mL | * | Increase | [48] | |
| Aspartic acid | 0.035 µmol/mL | 0.241 µmol/mL | * | Increase | [48] | |
| Serine | 0.050 µmol/mL | 0.187 µmol/mL | * | Increase | [48] | |
| Glycine | 0.065 µmol/mL | 0.288 µmol/mL | * | Increase | [48] | |
| Threonine | 0.157 µmol/mL | 0.435 µmol/mL | NS | Increase | [48] | |
| Arginine | 0.047 µmol/mL | 0.220 µmol/mL | * | Increase | [48] | |
| Isoleucine | 0.033 µmol/mL | 0.236 µmol/mL | * | Increase | [48] | |
| Methionine | 0.012 µmol/mL | 0.162 µmol/mL | * | Increase | [48] | |
| Albumin | 0.17–0.36 g/L; mean 0.24 g/L | 0.192–0.67; mean 0.36 g/L | * | Increase | [51] | |
| 0.2 ± 0.1 mg/mL | [52] | |||||
| 0.28 ± 0.19 g/dL | 0.82 ± 0.41 g/dL | * | Increase | [53] | ||
| 0.8–192 mg/dL | [52] | |||||
| α-amylase | 3257 ± 1682 U/mL | [52] | ||||
| 65.2 mg/mL | 68.07 mg/mL | Increase | [54] | |||
| 1080 ± 135.6 IU/L | [52] | |||||
| Interlukins (IL-8) | 250 pg/mL | OSCC 720 pg/mL | * | Increase | [55,56] | |
| Interlukins (IL-6) | 0 pg/mL | OC 86.5 pg/mL | * | Increase | [56] | |
| 16 ± 3.91 # pg/mL | 129 ± 66.59 # pg/mL | * | Increase | [57] | ||
| Osteopontin | 35.1 ng/mL | 39.23 ng/mL | Increase | [56] | ||
| CRP (inflamation marker) | 0.05–61 µg/L | [58] | ||||
| suPAR | 5.22–28.1 ng/mL | [58] | ||||
| Survivin | 2.44 ± 4.22 pg/mL | 8.69 ± 10.15 pg/mL | * | Increase | [59] | |
| Kallikrien 5 | ~6 pg/mL | ~12 pg/mL | * | Increase | [60] | |
| Cathepsin | 9–18 * ng/mL | [61] | ||||
| Cathepsin V | ~8 pg/mL | ~14 pg/mL | * | Increase | [60] | |
| lactate dehydrogenase (LDH) | 3.833 ± 1.1044 U/L | 99.83 ± 49.33 U/L | * | Increase | [62] | |
| 63.04 ± 47.4 mg/dL | 1515.17 ± 765.14 md/dL | Increase | [63] | |||
| (Endotheline-1) ET-1 | 0 to 9.629 fmol/m | 0 to 7.554 fmol/mL | NS | Increase | [64] | |
| 0.506–19.280 pg/mL; 4.5299 ± 3.7380 pg/mL | 2.140–52.229 pg/mL; 13.51 ± 14.15 pg/mL | * | Increase | [65] | ||
| Statherin | 0.5–4.0 μg/mL; mean 0.96 µg/mL | [66] | ||||
| 4.3–5.59 µM; mean 4.93 ± 0.61 µM | 0–6.45 µM; mean 2.28 ± 2.86 µM | * | Decrease | [67] | ||
| Carbohydrate antigen (CA 125) | 137.12 ± 124.58 U/mL | 498.10 # U/mL; 19.9–1312.32 U/mL | NS | [68] | ||
| 33.00 ± 24.37 mg/dL | 888.15 ± 306.1 mg/dL | * | Increase | [63] | ||
| Tissue polypeptide-specific antigen (TPS) | 96.20 ± 71.60 U/mL | 272.28 U/m #; 13.61–4706.17 U/mL | NS | [68] | ||
| CD 44 | 1.09 ng/mL | 7.85 ng/mL | * | Increase | [69] | |
| Antioxidants | Glutathione | 9.4 µmol/dL | 8.2 µmol/dL | Decrease | [70] | |
| vitamin c | 0.925 mg/dL | 0.4787 mg/dL | * | Decrease | [71] | |
| Carbohydrates | Fucose | 0.38–17.0 mg/dL mean 2.94 mg/dL | Pre-cancer 0.112–18.46 mg/dL; mean 7.02 mg/dL | * | Increase | [72] |
| OSCC 0.11–30.60 mg/dL; mean 11.66 mg/dL | ||||||
| 3.19 ±1.94 mg/dL | 6.14 + 2.16 mg/dL | * | Increase | [73] | ||
| 3.18 mg/dL | 11.66 mg/dL | * | Increase | [72] | ||
| Sialic acid | 0.134–0.311; mean 0.189 mmol/L | 0.140–0.336; mean 0.22 mmol/L | * | Increase | [73] | |
| 21.65 ± 5.71 mg/dL | 204.85 ± 60.38 mg/dL | * | Increase | [74] | ||
| 1.35 ± 1.53 mg/dL | 5.30 ±1.45 mg/dL | * | Increase | [73] | ||
| Lipids | Linoleic acid | 339.3 ± 267.9 ng/mL | 1092.3 ± 1927.8 ng/mL | Increase | [75] | |
| 15-HETE (Hydroxyeicosatetraenoic acids) | 0.4 ± 0.8 ng/mL | 5.4 ± 6.8 ng/mL | * | Increase | [75] | |
| Arachidonic acid | 32.6 ± 26.6 ng/mL | 606.9 ± 1695.7 ng/mL | Increase | [75] | ||
| Lipo per oxidation products (MDA) | 6.15–9.06 nmol/mL; mean 6.92 nmol/mL | 5.56–7.78 nmol/mL; mean 6.58 nmol/mL | NS | [51] | ||
| Other biomarkers | Pyruvic acid | 1.32 ± 0.10 | 3.49 ± 0.47 | * | Increase | [76] |
| Uric Acid | 25.2–161.2 nmol/mL; mean 76.8 | 43.2–182.9; mean 93 nmol/mL | * | Increase | [51] | |
| Urea | 4.35–8.78 mmol/L; mean 6.76 mmol/L | 4.62–11.29; mean 8.66 mmol/L | * | Increase | [51] | |
| Total protein | 0.78–1.53; mean 1.11 g/L | 0.47–1.59 g/L; mean 0.84 g/L | * | Decrease | [51] | |
| 0.47 ± 0.19 mg/mL | [52] | |||||
| 0.9 ± 0.2 mg/mL | [52] | |||||
| 43–710.0 mg/dL | [52] | |||||
| 1.07 ± 0.59 mg/mL | 1.01 ± 0.43 mg/mL | NS | [77] | |||
| 2.67 ± 0.54 mg/mL | [52] | |||||
| CYFRA-21-1 | 3.06 ng/mL | 17.46 ± 1.46 ng/mL | * | Increase | [78] | |
| Basic fibroblast growth factor (bFGF) | 3.17 ± 0.43 pg/mL | 8.80 ± 1.26 pg/mL | * | Increase | [79] | |
| 0.3 ± 0.3 pg/mL | OLP patients 5.9 ± 2.9 pg/mL | NS | increase | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maryam, S.; Nogueira, M.S.; Gautam, R.; Krishnamoorthy, S.; Venkata Sekar, S.K.; Kho, K.W.; Lu, H.; Ni Riordain, R.; Feeley, L.; Sheahan, P.; et al. Label-Free Optical Spectroscopy for Early Detection of Oral Cancer. Diagnostics 2022, 12, 2896. https://doi.org/10.3390/diagnostics12122896
Maryam S, Nogueira MS, Gautam R, Krishnamoorthy S, Venkata Sekar SK, Kho KW, Lu H, Ni Riordain R, Feeley L, Sheahan P, et al. Label-Free Optical Spectroscopy for Early Detection of Oral Cancer. Diagnostics. 2022; 12(12):2896. https://doi.org/10.3390/diagnostics12122896
Chicago/Turabian StyleMaryam, Siddra, Marcelo Saito Nogueira, Rekha Gautam, Shree Krishnamoorthy, Sanathana Konugolu Venkata Sekar, Kiang Wei Kho, Huihui Lu, Richeal Ni Riordain, Linda Feeley, Patrick Sheahan, and et al. 2022. "Label-Free Optical Spectroscopy for Early Detection of Oral Cancer" Diagnostics 12, no. 12: 2896. https://doi.org/10.3390/diagnostics12122896
APA StyleMaryam, S., Nogueira, M. S., Gautam, R., Krishnamoorthy, S., Venkata Sekar, S. K., Kho, K. W., Lu, H., Ni Riordain, R., Feeley, L., Sheahan, P., Burke, R., & Andersson-Engels, S. (2022). Label-Free Optical Spectroscopy for Early Detection of Oral Cancer. Diagnostics, 12(12), 2896. https://doi.org/10.3390/diagnostics12122896








