Macrophage-like Cells Are Increased in Patients with Vision-Threatening Diabetic Retinopathy and Correlate with Macular Edema
Abstract
1. Introduction
2. Methods
2.1. OCT-A Imaging
2.2. MLC Quantification
2.3. Superficial Vascular Plexus Perifoveal Vessel Length Density
2.4. Statistics
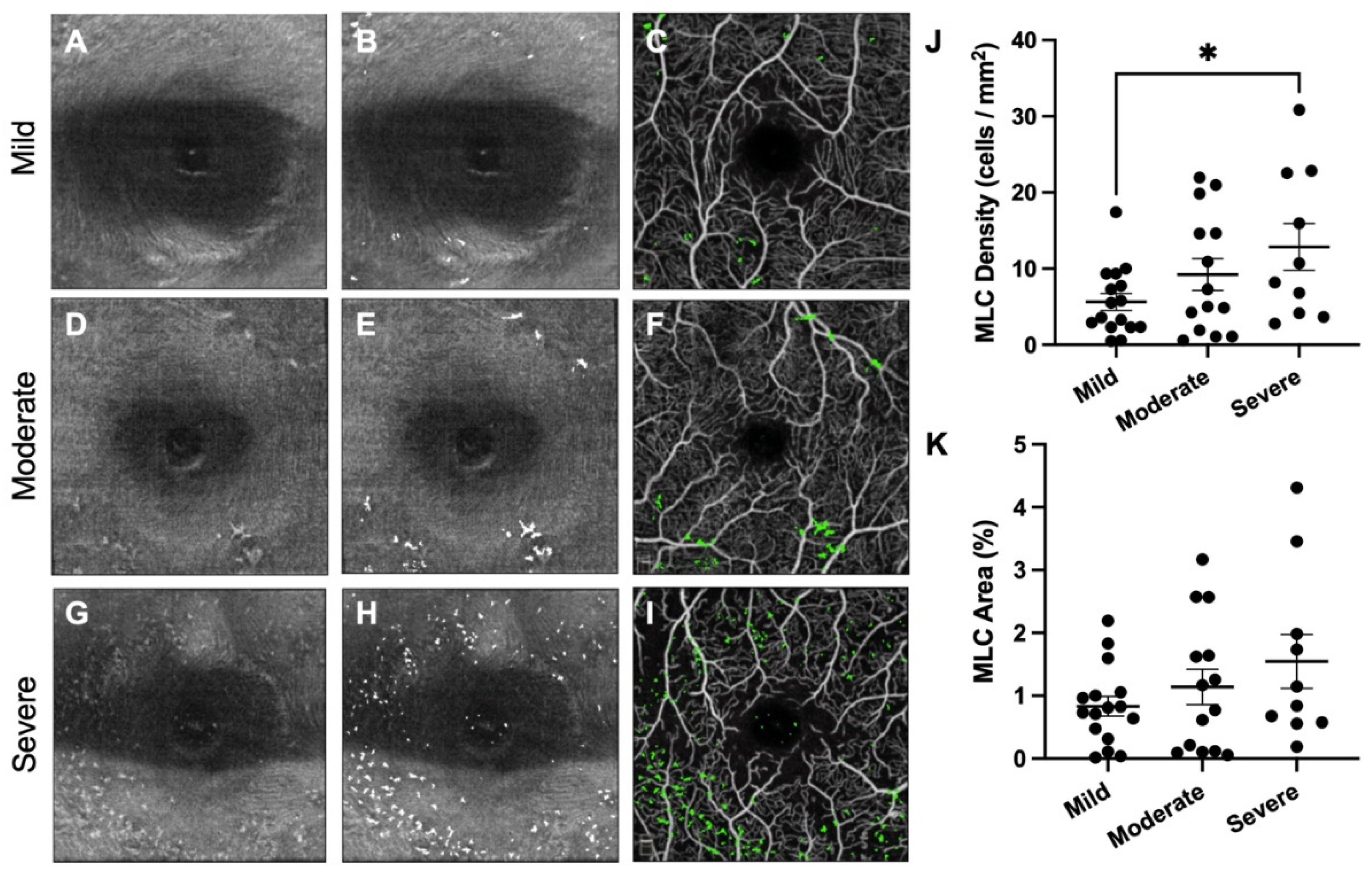
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Network, D.R.C.R.; Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef]
- Bressler, N.M.; Beaulieu, W.T.; Glassman, A.R.; Blinder, K.J.; Bressler, S.B.; Jampol, L.M.; Melia, M.; Wells, J.A.; Network, D.R.C.R. Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Network, D.R.C.R.; Elman, M.J.; Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Bressler, S.B.; Edwards, A.R.; Ferris, F.L.; Friedman, S.M.; Glassman, A.R.; et al. Randomized Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology 2010, 117, 1064–1077.e35. [Google Scholar] [CrossRef]
- Maturi, R.K.; Glassman, A.R.; Liu, D.; Beck, R.W.; Bhavsar, A.R.; Bressler, N.M.; Jampol, L.M.; Melia, M.; Punjabi, O.S.; Salehi-Had, H.; et al. Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 29–38. [Google Scholar] [CrossRef]
- Rangasamy, S.; McGuire, P.G.; Nitta, C.F.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine Mediated Monocyte Trafficking into the Retina: Role of Inflammation in Alteration of the Blood-Retinal Barrier in Diabetic Retinopathy. PLoS ONE 2014, 9, e108508-10. [Google Scholar] [CrossRef]
- Beli, E.; Dominguez, J.M.; Hu, P.; Thinschmidt, J.S.; Caballero, S.; Calzi, S.L.; Luo, D.; Shanmugam, S.; Salazar, T.E.; Duan, Y.; et al. CX3CR1 deficiency accelerates the development of retinopathy in a rodent model of type 1 diabetes. J. Mol. Med. 2016, 94, 1255–1265. [Google Scholar] [CrossRef]
- Castanos, M.V.; Zhou, D.B.; Linderman, R.E.; Allison, R.; Milman, T.; Carroll, J.; Migacz, J.; Rosen, R.B.; Chui, T.Y. Imaging of Macrophage-Like Cells in Living Human Retina Using Clinical OCT. Investig. Opthalmol. Vis. Sci. 2020, 61, 48. [Google Scholar] [CrossRef]
- Hammer, D.X.; Agrawal, A.; Villanueva, R.; Saeedi, O.; Liu, Z. Label-free adaptive optics imaging of human retinal macrophage distribution and dynamics. Proc. Natl. Acad. Sci. USA 2020, 42, 202010943–202010949. [Google Scholar] [CrossRef]
- Migacz, J.V.; Otero-Marquez, O.; Zhou, R.; Rickford, K.; Murillo, B.; Zhou, D.B.; Castanos, M.V.; Sredar, N.; Dubra, A.; Rosen, R.B.; et al. Imaging of vitreous cortex hyalocyte dynamics using non-confocal quadrant-detection adaptive optics scanning light ophthalmoscopy in human subjects. Biomed. Opt. Express 2022, 13, 1755. [Google Scholar] [CrossRef]
- Joseph, A.; Chu, C.J.; Feng, G.; Dholakia, K.; Schallek, J. Label-free imaging of immune cell dynamics in the living retina using adaptive optics. eLife 2020, 9, e60547. [Google Scholar] [CrossRef]
- Rajesh, A.; Droho, S.; Lavine, J.A. Macrophages in close proximity to the vitreoretinal interface are potential biomarkers of inflammation during retinal vascular disease. J. Neuroinflamm. 2022, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, X.; Mi, L.; Ji, Y.; Zhuang, X.; He, G.; Chen, X.; Wen, F. Macrophage-like Cells Characterized by En Face Optical Coherence Tomography was Associated with Fluorescein Vascular Leakage in Behçet’s Uveitis. Ocul. Immunol. Inflamm. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.X.; Nesper, P.L.; Fawzi, A.A.; Wang, J.M.; Lavine, J.A. Macrophage-Like Cell Density Is Increased in Proliferative Diabetic Retinopathy Characterized by Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2021, 62, 2. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, X.; Mi, L.; Gan, Y.; Su, Y.; Li, M.; Yang, R.; Zhang, Y.; Wen, F. Characterization of Macrophage-Like Cells in Retinal Vein Occlusion Using En Face Optical Coherence Tomography. Front. Immunol. 2022, 13, 855466. [Google Scholar] [CrossRef]
- Kempen, J.H.; O’Colmain, B.J.; Leske, M.C.; Haffner, S.M.; Klein, R.; Moss, S.E.; Taylor, H.R.; Hamman, R.F.; Group, E.D.P.R. The prevalence of diabetic retinopathy among adults in the United States. Arch. Ophthalmol. 2004, 122, 552–563. [Google Scholar] [CrossRef]
- Chalam, K.V.; Bressler, S.B.; Edwards, A.R.; Berger, B.B.; Bressler, N.M.; Glassman, A.R.; Grover, S.; Gupta, S.K.; Nielsen, J.S.; Network, D.R.C.R. Retinal Thickness in People with Diabetes and Minimal or No Diabetic Retinopathy: Heidelberg Spectralis Optical Coherence Tomography. Investig. Opthalmol. Vis. Sci. 2012, 53, 8154. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef]
- Nesper, P.L.; Ong, J.X.; Fawzi, A.A. Deep Capillary Geometric Perfusion Deficits on OCT Angiography Detect Clinically Referable Eyes with Diabetic Retinopathy. Ophthalmol. Retin. 2022. [Google Scholar] [CrossRef]
- Samara, W.A.; Shahlaee, A.; Adam, M.K.; Khan, M.A.; Chiang, A.; Maguire, J.I.; Hsu, J.; Ho, A.C. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology 2017, 124, 235–244. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, Y.; Yue, S.; Yang, K.; Zhang, G.; Chen, L.; Liu, L. Aqueous Humor Mediator and Cytokine Aberrations in Diabetic Retinopathy and Diabetic Macular Edema: A Systematic Review and Meta-Analysis. Dis. Markers 2019, 2019, 6928524. [Google Scholar] [CrossRef]
- Abraham, J.R.; Wykoff, C.C.; Arepalli, S.; Lunasco, L.; Yu, H.J.; Hu, M.; Reese, J.; Srivastava, S.K.; Brown, D.M.; Ehlers, J.P. Aqueous Cytokine Expression and Higher Order OCT Biomarkers: Assessment of the Anatomic-Biologic Bridge in the IMAGINE DME Study. Am. J. Ophthalmol. 2020, 222, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Boneva, S.K.; Wolf, J.; Rosmus, D.-D.; Schlecht, A.; Prinz, G.; Laich, Y.; Boeck, M.; Zhang, P.; Hilgendorf, I.; Stahl, A.; et al. Transcriptional Profiling Uncovers Human Hyalocytes as a Unique Innate Immune Cell Population. Front. Immunol. 2020, 11, 567274. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Boneva, S.; Rosmus, D.-D.; Agostini, H.; Schlunck, G.; Wieghofer, P.; Schlecht, A.; Lange, C. Deciphering the Molecular Signature of Human Hyalocytes in Relation to Other Innate Immune Cell Populations. Investig. Opthalmol. Vis. Sci. 2022, 63, 9. [Google Scholar] [CrossRef] [PubMed]
- O’Koren, E.G.; Mathew, R.; Saban, D.R. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Nat. Publ. Group 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- O’Koren, E.G.; Yu, C.; Klingeborn, M.; Wong, A.Y.; Prigge, C.L.; Mathew, R.; Kalnitsky, J.; Msallam, R.A.; Silvin, A.; Kay, J.N.; et al. Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration. Immunity 2019, 50, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Kerkhofs, D.; Mizuno, T.; Steinbusch, H.W.M.; Foulquier, S. Vessel-Associated Immune Cells in Cerebrovascular Diseases: From Perivascular Macrophages to Vessel-Associated Microglia. Front. Neurosci. 2019, 13, 1291. [Google Scholar] [CrossRef]
- Mendes-Jorge, L.; Ramos, D.; Luppo, M.; Llombart, C.; Alexandre-Pires, G.; Nacher, V.; Melgarejo, V.; Correia, M.; Navarro, M.; Carretero, A.; et al. Scavenger Function of Resident Autofluorescent Perivascular Macrophages and Their Contribution to the Maintenance of the Blood–Retinal Barrier. Investig. Opthalmol. Vis. Sci. 2009, 50, 5997–6005. [Google Scholar] [CrossRef]


| Groups | p-Value | ||
|---|---|---|---|
| Non-Vision Threatening Diabetic Retinopathy | Vision Threatening Diabetic Retinopathy | ||
| Number of Eyes | 18 | 22 | |
| NPDR Stage | Mild 12 (67%) Moderate 6 (33%) Severe (0%) | Mild 3 (14%) Moderate 9 (41%) Severe 10 (45%) | |
| DME | 0 (0%) | 16 (73%) | |
| Age (mean ± SD) | 49.5 ± 15.4 | 59.2 ± 12.8 | 0.041 |
| Sex, n Female (%) | 9 (50%) | 10 (45%) | 0.775 |
| Refractive Error (D mean ± SD) | −2.20 ± 2.21 | −1.39 ± 3.22 | 0.413 |
| Missing, n (%) | 5 (28%) | 2 (9%) | |
| DM Type, n Type 1 (%) | 12 (67%) | 2 (9%) | 0.014 |
| DM Duration (mean ± SD) | 21.9 ± 11.6 | 14.5 ± 10.3 | 0.046 |
| HbA1c (mean ± SD) | 7.1 ± 0.7 | 8.1 ± 3.0 | 0.043 |
| LogMAR BCVA | 0.10 ± 0.10 | 0.11 ± 0.14 | 0.242 |
| CST | 258 ± 23 | 322 ± 88 | 0.003 |
| Number of IVI | 0 ± 0 | 7.6 ± 12.5 | 0.017 |
| MLC Density (Univariate) | MLC Density (Multivariate) | MLC Percent Area (Univariate) | MLC Percent Area (Multivariate) | |
|---|---|---|---|---|
| MLC Percent Area | 0.930 (p < 0.001) | -- | 0.930 (p < 0.001) | -- |
| Age | 0.333 (p = 0.036) | p = 0.848 | 0.340 (p = 0.032) | p = 0.997 |
| Sex * | 0.037 (p = 0.821) | -- | 0.037 (p = 0.821) | -- |
| HbA1c | 0.126 (p = 0.437) | -- | 0.083 (p = 0.613) | -- |
| DM Duration | −0.151 (p = 0.359) | -- | −0.120 (p = 0.468) | -- |
| DM Type * | 0.527 (p < 0.001) | p = 0.429 | 0.499 (p = 0.001) | p = 0.352 |
| NPDR Stage * | 0.331 (p = 0.037) | p = 0.023 | 0.203 (p = 0.209) | p = 0.115 |
| DME Presence * | 0.318 (p = 0.045) | p = 0.214 | 0.332 (p = 0.037) | p = 0.300 |
| CST | 0.414 (p = 0.008) | p = 0.010 | 0.483 (p = 0.002) | p = 0.006 |
| LogMAR BCVA | 0.064 (p = 0.731) | -- | 0.150 (p = 0.422) | -- |
| Number of IVI | 0.171 (p = 0.303) | -- | 0.133 (p = 0.427) | -- |
| SVP VLD | −0.171 (p = 0.290) | -- | −0.168 (p = 0.301) | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.T.; Nesper, P.L.; Ong, J.X.; Wang, J.M.; Fawzi, A.A.; Lavine, J.A. Macrophage-like Cells Are Increased in Patients with Vision-Threatening Diabetic Retinopathy and Correlate with Macular Edema. Diagnostics 2022, 12, 2793. https://doi.org/10.3390/diagnostics12112793
Zhang NT, Nesper PL, Ong JX, Wang JM, Fawzi AA, Lavine JA. Macrophage-like Cells Are Increased in Patients with Vision-Threatening Diabetic Retinopathy and Correlate with Macular Edema. Diagnostics. 2022; 12(11):2793. https://doi.org/10.3390/diagnostics12112793
Chicago/Turabian StyleZhang, Nigel T., Peter L. Nesper, Janice X. Ong, Jacob M. Wang, Amani A. Fawzi, and Jeremy A. Lavine. 2022. "Macrophage-like Cells Are Increased in Patients with Vision-Threatening Diabetic Retinopathy and Correlate with Macular Edema" Diagnostics 12, no. 11: 2793. https://doi.org/10.3390/diagnostics12112793
APA StyleZhang, N. T., Nesper, P. L., Ong, J. X., Wang, J. M., Fawzi, A. A., & Lavine, J. A. (2022). Macrophage-like Cells Are Increased in Patients with Vision-Threatening Diabetic Retinopathy and Correlate with Macular Edema. Diagnostics, 12(11), 2793. https://doi.org/10.3390/diagnostics12112793







