Distinctive Morphological Patterns of Complicated Coronary Plaques in Acute Coronary Syndromes: Insights from an Optical Coherence Tomography Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. OCT Acquisition Technique and Image Analysis
2.3. Statistical Analysis
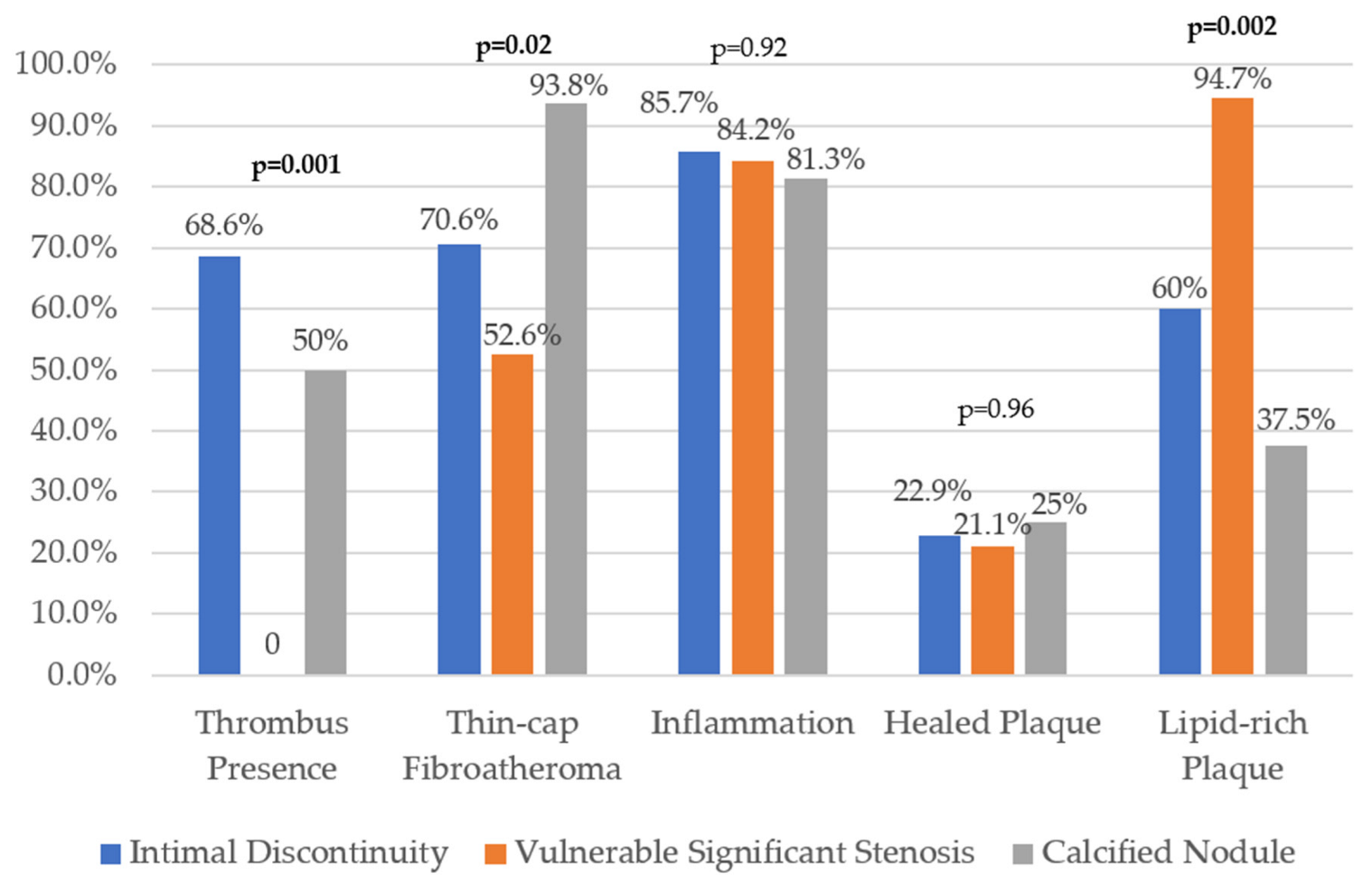
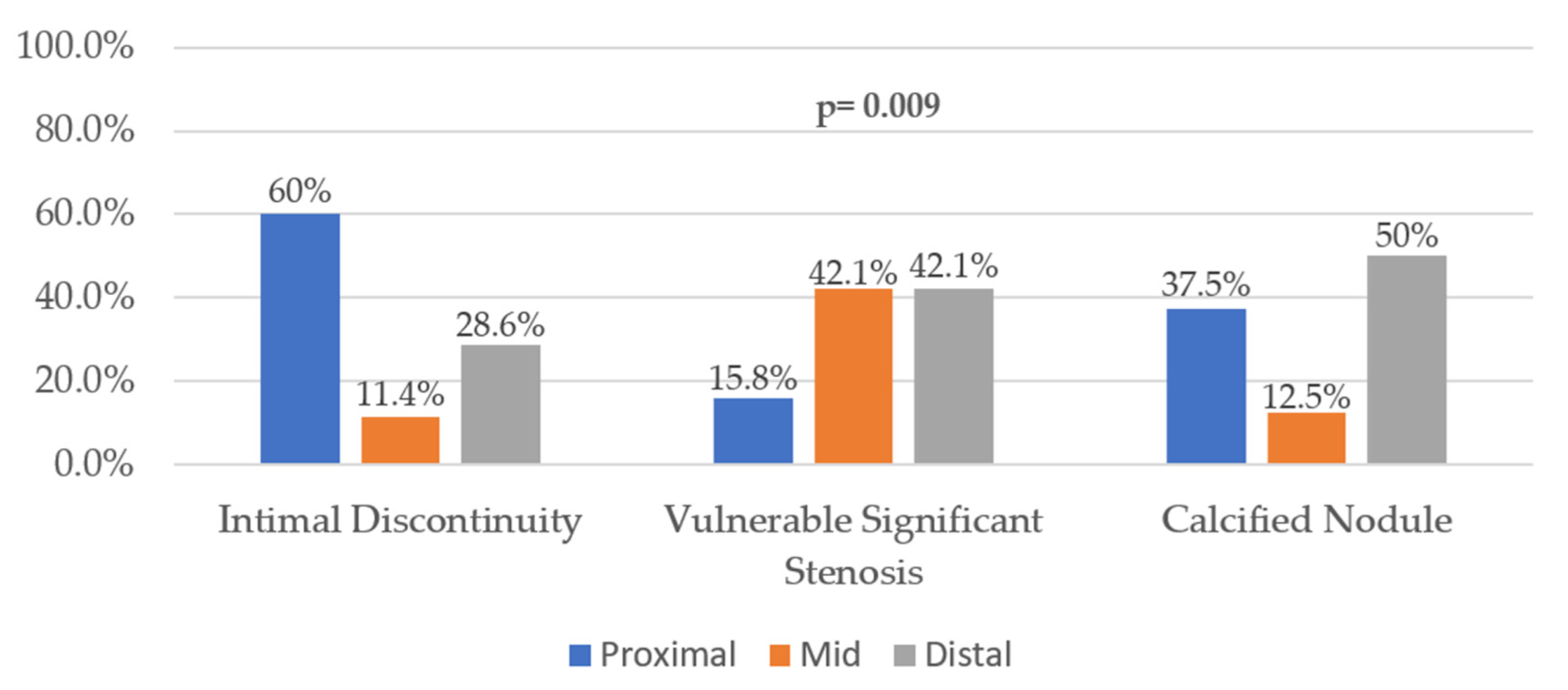
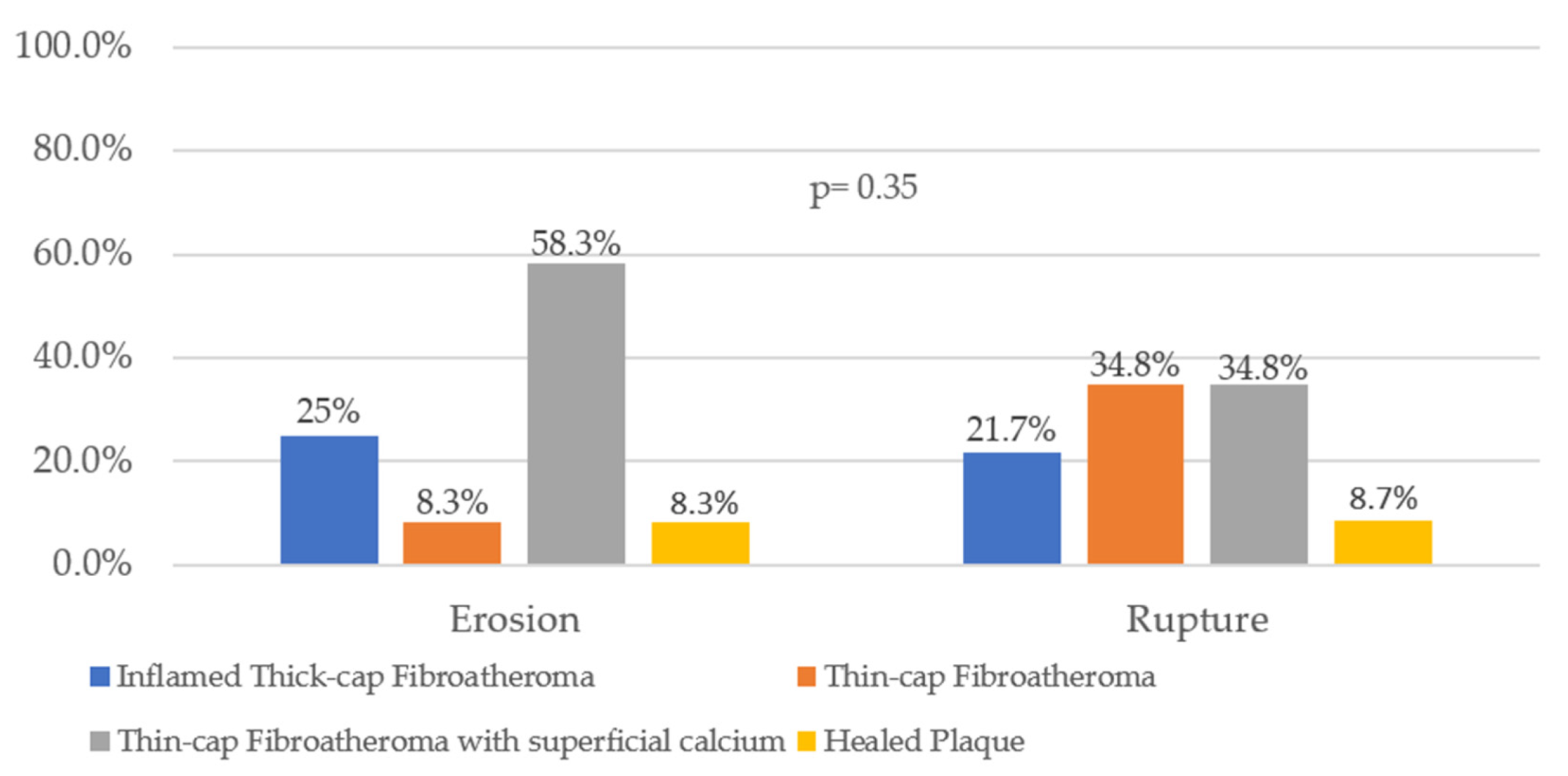
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puymirat, E.; Simon, T.; Cayla, G.; Cottin, Y.; Elbaz, M.; Coste, P.; Lemesle, G.; Motreff, P.; Popovic, B.; Khalife, K.; et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017, 136, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 8), C13–C18. [Google Scholar] [CrossRef] [PubMed]
- Spînu, M.; Onea, L.H.; Homorodean, C.; Olinic, M.; Ober, M.C.; Olinic, D.M. Optical Coherence Tomography-OCT for Characterization of Non-Atherosclerotic Coronary Lesions in Acute Coronary Syndromes. J. Clin. Med. 2022, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Galougahi, K.K.; Maehara, A.; Shlofmitz, R.A.; Ben-Yehuda, O.; Mintz, G.S.; Stone, G.W. Intracoronary Optical Coherence Tomography 2018: Current Status and Future Directions. JACC Cardiovasc. Interv. 2017, 10, 2473–2487. [Google Scholar] [CrossRef]
- Liu, H.; Wingert, A.; Wang, J.; Zhang, J.; Wang, X.; Sun, J.; Chen, F.; Khalid, S.G.; Jiang, J.; Zheng, D. Extraction of Coronary Atherosclerotic Plaques from Computed Tomography Imaging: A Review of Recent Methods. Front. Cardiovasc. Med. 2021, 8, 597568. [Google Scholar] [CrossRef]
- Liu, H.; Wingert, A.; Wang, X.; Zhang, J.; Sun, J.; Chen, F.; Khalid, S.G.; Gong, Y.; Xia, L.; Jiang, J.; et al. Consistency in Geometry Among Coronary Atherosclerotic Plaques Extracted from Computed Tomography Angiography. Front. Physiol. 2021, 12, 715265. [Google Scholar] [CrossRef]
- Halon, D.A.; Lavi, I.; Barnett-Griness, O.; Rubinshtein, R.; Zafrir, B.; Azencot, M.; Lewis, B.S. Plaque Morphology as Predictor of Late Plaque Events in Patients with Asymptomatic Type 2 Diabetes: A Long-Term Observational Study. JACC Cardiovasc. Imaging 2019, 12 Pt 2, 1353–1363. [Google Scholar] [CrossRef]
- Spinu, M.; Olinic, D.M.; Olinic, M.; Homorodean, C. In vivo imaging of complicated atherosclerotic plaque-role of optical coherence tomography (OCT) Rom. J. Morphol. Embryol. 2018, 59, 469–478. [Google Scholar]
- Olinic, D.M.; Spinu, M.; Homorodean, C.; Olinic, M. Vasa vasorum induced LAD dissection and haematoma in an anterior STEMI patient with nearly normal angiography: The role of OCT. Kardiol. Pol. 2017, 75, 504. [Google Scholar] [CrossRef]
- Homorodean, C.; Spinu, M.; Ober, M.C.; Olinic, M.; Olinic, D.M. Spontaneous coronary dissection: Optical coherence tomography insights before and after stenting. Cardiol. J. 2017, 24, 217–218. [Google Scholar] [CrossRef]
- Spinu, M.; Homorodean, C.; Ober, M.C.; Olinic, M.; Mircea, P.A.; Olinic, D.M. Optical Coherence Tomography for In Vivo Identification, Characterization and Optimal Treatment of Spontaneous Recanalization of Coronary Thrombus. In Vivo 2020, 34, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Homorodean, C.; Leucuta, D.; Ober, M.; Homorodean, R.; Spinu, M.; Olinic, M.; Tataru, D.; Olinic, D. Intravascular ultrasound insights into the unstable features of the coronary atherosclerotic plaques: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2022, 52, e13671. [Google Scholar] [CrossRef] [PubMed]
- Ober, M.C.; Homorodean, C.; Bindea, D.; Spinu, M.; Olinic, D.M. Unstable Angina Caused by Honeycomb-Like Coronary Lesion Identified with Use of Optical Coherence Tomography. Tex. Heart Inst. J. 2020, 47, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Homorodean, C.; Iancu, A.C.; Dregoesc, I.M.; Spînu, M.; Ober, M.C.; Tãtaru, D.; Leucuţa, D.; Olinic, M.; Olinic, D.M. Renal Failure Impact on the Outcomes of ST-Segment Elevation Myocardial Infarction Patients Due to a Left Main Coronary Culprit Lesion Treated Using a Primary Percutaneous Coronary Inter-vention. J. Clin. Med. 2019, 8, 565. [Google Scholar] [CrossRef]
- Olinic, D.M.; Spinu, M.; Homorodean, C.; Ober, M.C.; Olinic, M. Real-Life Benefit of OCT Imaging for Optimizing PCI Indications, Strategy, and Results. J. Clin. Med. 2019, 8, 437. [Google Scholar] [CrossRef]
- Homorodean, C.; Iancu, A.C.; Leucuţa, D.; Bãlãnescu, Ş.; Dregoesc, I.M.; Spînu, M.; Ober, M.; Tãtaru, D.; Olinic, M.; Bindea, D.; et al. New Predictors of Early and Late Outcomes after Primary Percutaneous Coronary Intervention in Patients with ST-Segment Elevation Myocardial Infarction and Unprotected Left Main Coronary Artery Culprit Lesion. J. Interv. Cardiol. 2019, 2019, 8238972. [Google Scholar] [CrossRef]
- Marc, M.C.; Iancu, A.C.; Ober, C.D.; Homorodean, C.; Bãlãnescu, Ş.; Sitar, A.V.; Bolboacã, S.; Dregoesc, I.M. Pre-revascularization coronary wedge pressure as marker of adverse long-term left ventricular remodelling in patients with acute ST-segment elevation myocardial infarction. Sci. Rep. 2018, 8, 1897. [Google Scholar] [CrossRef]
- Homorodean, C.; Ober, M.; Iancu, A.; Olinic, M.; Tataru, D.; Spinu, M.; Olinic, D.; Burzotta, F.; Trani, C.; Erglis, A. How should I treat this mini-crush stenting complication? EuroIntervention 2017, 13, 1248–1252. [Google Scholar] [CrossRef]
- Onea, H.L.; Lazar, F.L.; Olinic, D.M.; Homorodean, C.; Cortese, B. The role of optical coherence tomography in guiding percutaneous coronary interventions- is left main the final challenge? Minerva Cardiol. Angiol. 2022. [Google Scholar] [CrossRef]
- Cortese, B.; Piraino, D.; Gentile, D.; Onea, H.L.; Lazar, L. Intravascular imaging for left main stem assessment: An update on the most recent clinical data. Catheter. Cardiovasc. Interv. 2022. [Google Scholar] [CrossRef]
- Kubo, T.; Imanishi, T.; Takarada, S.; Kuroi, A.; Ueno, S.; Yamano, T.; Tanimoto, T.; Matsuo, Y.; Masho, T.; Kitabata, H.; et al. Assessment of culprit lesion morphology in acute myocardial infarction: Ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 2007, 50, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Kume, T.; Akasaka, T.; Kawamoto, T.; Ogasawara, Y.; Watanabe, N.; Toyota, E.; Neishi, Y.; Sukmawan, R.; Sadahira, Y.; Yoshida, K. Assessment of coronary arterial thrombus by optical coherence tomography. Am. J. Cardiol. 2006, 97, 1713–1717. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367, Erratum in Eur. Heart J. 2021, 42, 1908; Erratum in Eur. Heart J. 2021, 42, 1925; Erratum in Eur. Heart J. 2021, 42, 2298. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.-M.; Chowdhary, S.; et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072, Erratum in J. Am. Coll. Cardiol. 2012, 59, 1662. [Google Scholar] [CrossRef]
- Otsuka, F.; Joner, M.; Prati, F.; Virmani, R.; Narula, J. Clinical classification of plaque morphology in coronary disease. Nat. Rev. Cardiol. 2014, 11, 379–389. [Google Scholar] [CrossRef]
- Subban, V.; Raffel, O.C. Optical coherence tomography: Fundamentals and clinical utility. Cardiovasc. Diagn. Ther. 2020, 10, 1389–1414. [Google Scholar] [CrossRef]
- Simera, I.; Moher, D.; Hoey, J.; Schulz, K.F.; Altman, D.G. A catalogue of reporting guidelines for health research. Eur. J. Clin. Investig. 2010, 40, 35–53. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-K.; Mintz, G.S.; Lee, C.W.; Kim, Y.-H.; Lee, S.-W.; Song, J.-M.; Han, K.-H.; Kang, D.-H.; Song, J.-K.; Kim, J.-J.; et al. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: A three-vessel intravascular ultrasound study in 235 patients. Circulation 2004, 110, 928–933. [Google Scholar] [CrossRef]
- Ino, Y.; Kubo, T.; Tanaka, A.; Kuroi, A.; Tsujioka, H.; Ikejima, H.; Okouchi, K.; Kashiwagi, M.; Takarada, S.; Kitabata, H.; et al. Difference of culprit lesion morphologies between ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome: An optical coherence tomography study. JACC Cardiovasc. Interv. 2011, 4, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Gili, S.; Iannaccone, M.; Colombo, F.; Montefusco, A.; Amabile, N.; Calcagno, S.; Capodanno, D.; Scalone, G.; Rognoni, A.; Omedè, P.; et al. Effects of statins on plaque rupture assessed by optical coherence tomography in patients presenting with acute coronary syndromes: Insights from the optical coherence tomography (OCT)-FORMIDABLE registry. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Barros, H.; Maciel, M.J.; Lopes, C. Tobacco smoking and acute myocardial infarction in young adults: A population-based case-control study. Prev. Med. 2007, 44, 311–316. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Hiro, T.; Fujii, T.; Hashimoto, G.; Fujimura, T.; Yamada, J.; Okamura, T.; Matsuzaki, M. Localized elevation of shear stress is related to coronary plaque rupture: A 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J. Am. Coll. Cardiol. 2008, 51, 645–650. [Google Scholar] [CrossRef]
- Thondapu, V.; Mamon, C.; Poon, E.K.W.; Kurihara, O.; Kim, H.O.; Russo, M.; Araki, M.; Shinohara, H.; Yamamoto, E.; Dijkstra, J.; et al. High spatial endothelial shear stress gradient independently predicts site of acute coronary plaque rupture and erosion. Cardiovasc. Res. 2021, 117, 1974–1985. [Google Scholar] [CrossRef]
- Dai, J.; Fang, C.; Zhang, S.; Hou, J.; Xing, L.; Li, L.; Wang, Y.; Wang, J.; Wang, Y.; Tu, Y.; et al. Not All Plaque Erosions Are Equal: Novel Insights From 1,660 Patients with STEMI: A Clinical, Angiographic, and Intravascular OCT Study. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 516–518. [Google Scholar] [CrossRef]
- Yahagi, K.; Kolodgie, F.D.; Otsuka, F.; Finn, A.V.; Davis, H.R.; Joner, M.; Virmani, R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 79–98. [Google Scholar] [CrossRef]
- Shi, X.; Gao, J.; Lv, Q.; Cai, H.; Wang, F.; Ye, R.; Liu, X. Calcification in Atherosclerotic Plaque Vulnerability: Friend or Foe? Front. Physiol. 2020, 11, 56. [Google Scholar] [CrossRef]
- Costopoulos, C.; Huang, Y.; Brown, A.J.; Calvert, P.A.; Hoole, S.P.; West, N.E.; Gillard, J.H.; Teng, Z.; Bennett, M.R. Plaque Rupture in Coronary Atherosclerosis Is Associated with Increased Plaque Structural Stress. JACC Cardiovasc. Imaging 2017, 10, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gong, Y.; Leng, X.; Xia, L.; Wong, K.S.; Ou, S.; Leung, T.W.; Wang, D.; Shi, L. Estimating current and long-term risks of coronary artery in silico by fractional flow reserve, wall shear stress and low-density lipoprotein filtration rate. Biomed. Phys. Eng. Express 2018, 4, 025006. [Google Scholar] [CrossRef]
- Manoharan, G.; Ntalianis, A.; Muller, O.; Hamilos, M.; Sarno, G.; Melikian, N.; Vanderheyden, M.; Heyndrickx, G.R.; Wyffels, E.; Wijns, W.; et al. Severity of coronary arterial stenoses responsible for acute coronary syndromes. Am. J. Cardiol. 2009, 103, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.H.; Mintz, G.S.; Farhat, N.; Marso, S.P.; Taglieri, N.; Verheye, S.; Foster, M.C.; Margolis, M.P.; Templin, B.; Xu, K.; et al. Relation between angiographic lesion severity, vulnerable plaque morphology and future adverse cardiac events (from the Providing Regional Observations to Study Predictors of Events in the Coronary Tree study). Am. J. Cardiol. 2012, 110, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Higuma, T.; Soeda, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Vergallo, R.; Minami, Y.; Ong, D.S.; Lee, H.; et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients With ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2015, 8, 1166–1176, Erratum in JACC Cardiovasc. Interv. 2016, 9, 516. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.L.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Yamamoto, E.; Fracassi, F.; Lee, H.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; et al. Calcified Plaques in Patients with Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2019, 12, 531–540. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Cerrato, E.; Appleton, D.; Moretti, C.; Calcagno, A.; Abouzaki, N.; Vetrovec, G.; Lhermusier, T.; Carrie, D.; Das Neves, B.; et al. Prognostic indicators for recurrent thrombotic events in HIV-infected patients with acute coronary syndromes: Use of registry data from 12 sites in Europe, South Africa and the United States. Thromb. Res. 2014, 134, 558–564. [Google Scholar] [CrossRef]

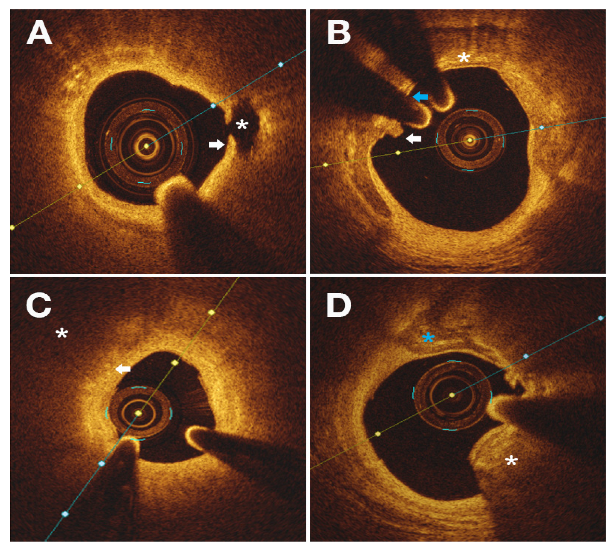
| Histology | OCT Features |
|---|---|
| LRP (thick-cap FA) | High-attenuating and low signal regions with poorly delineated borders surrounded by a signal-rich layer, the fibrous cap |
| Fibrocalcific Plaque | Low-attenuating and low signal regions with sharply defined borders |
| TCFA | Large lipid/necrotic core encapsulated by a thin fibrous cap (<65 µm); superficial calcified plates were also included here |
| Macrophage Infiltration | High-attenuating and high signal dotted or linear structures located at the fibrous cap–necrotic core interface |
| Plaque Rupture | Fibrous cap discontinuity associated with a clearly defined intraplaque cavity |
| Plaque Erosion | Presence of thrombus or luminal irregularity without a visible rupture site |
| Calcified Nodule | High-attenuating and strong signal entity consisting solely of calcium that protrudes into the lumen, leading to thrombosis; a necrotic core is seldom present |
| Healed Plaque | Single or multiple layer aspect with different optical densities enveloping a large necrotic core; calcifications can be present |
| Thrombus | Intraluminal mass that is ± attached to the luminal surface |
| Red thrombus—high attenuation and intense signal | |
| White thrombus—low attenuation and homogenous signal |
| Variables | Overall (n = 70) | ID (n = 35) | SS (n = 19) | CN (n = 16) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 59.7 ± 11.5 | 58.1 ± 11.8 | 58.8 ± 9.4 | 64 ± 12.5 | 0.08 |
| Male sex | 46 (65.7) | 21 (60) | 12 (63.1) | 13 (81.2) | 0.03 |
| CV risk factors | |||||
| Hypertension | 55 (78.6) | 27 (77.1) | 15 (78.9) | 13 (81.2) | 0.66 |
| DM | 18 (25.7) | 11 (31.4) | 4 (21) | 3 (18.8) | 0.05 |
| Dyslipidemia | 46 (65.7) | 23 (65.7) | 14 (73.7) | 9 (56.2) | 0.13 |
| Smoking habit | 14 (20) | 8 (22.9) | 3 (15.8) | 3 (18.8) | 0.34 |
| Overweight | 9 (12.9) | 3 (8.6) | 4 (21) | 2 (12.5) | 0.06 |
| Clinical presentation | |||||
| STEMI | 17 (24.3) | 12 (34.3) | 2 (10.5) | 3 (18.8) | 0.04 |
| NSTEMI | 19 (27.1) | 8 (22.9) | 3 (15.8) | 8 (50) | 0.03 |
| UAP | 34 (48.6) | 15 (42.9) | 14 (73.7) | 5 (31.2) | 0.03 |
| Prior medication | |||||
| Aspirin | 28 (40) | 12 (34.3) | 9 (47.4) | 7 (43.6) | 0.6 |
| Statin | 36 (51.4) | 15 (42.9) | 13 (68.4) | 8 (50) | 0.1 |
| Management | 0.03 | ||||
| PCI | 52 (74.3) | 24 (68.6) | 19 (94.7) | 9 (56.3) | |
| CABG | 4 (5.7) | 2 (5.7) | 1 (5.3) | 2 (12.5) | |
| Conservative | 14 (20) | 9 (25.7) | 0 (0) | 5 (31.2) | |
| Variables | Overall (n = 70) | ID (n = 35) | SS (n = 19) | CN (n = 16) | p-Value |
|---|---|---|---|---|---|
| Culprit vessel | 0.64 | ||||
| LM | 9 (12.9) | 6 (17.1) | 0 (0) | 3 (18.8) | 0.43 |
| LAD | 55 (78.6) | 25 (71.4) | 17 (89.5) | 13 (81.3) | 0.71 |
| LCx | 2 (2.9) | 1 (2.9) | 1 (5.3) | 0 (0) | 0.78 |
| RCA | 4 (5.7) | 3 (8.6) | 1 (5.3) | 0 (0) | 0.66 |
| Lesion severity (%) | <0.001 | ||||
| 50–70 | 20 (28.6) | 18 (51.4) | 0 (0) | 2 (12.5) | |
| 70–90 | 19 (27.1) | 7 (20) | 2 (10.5) | 10 (62.5) | |
| >90 | 31 (44.3) | 10 (28.6) | 17 (89.5) | 4 (25) | |
| Lesion length (mm) | 16.5 ± 9.9 | 16.8 ± 9.3 | 12.4 ± 6.7 | 20.8 ± 12.6 | 0.04 |
| Multivessel disease * | 39 (55.7) | 19 (54.3) | 8 (42.1) | 12 (75) | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onea, H.-L.; Spinu, M.; Homorodean, C.; Olinic, M.; Lazar, F.-L.; Ober, M.C.; Stoian, D.; Itu, L.M.; Olinic, D.M. Distinctive Morphological Patterns of Complicated Coronary Plaques in Acute Coronary Syndromes: Insights from an Optical Coherence Tomography Study. Diagnostics 2022, 12, 2837. https://doi.org/10.3390/diagnostics12112837
Onea H-L, Spinu M, Homorodean C, Olinic M, Lazar F-L, Ober MC, Stoian D, Itu LM, Olinic DM. Distinctive Morphological Patterns of Complicated Coronary Plaques in Acute Coronary Syndromes: Insights from an Optical Coherence Tomography Study. Diagnostics. 2022; 12(11):2837. https://doi.org/10.3390/diagnostics12112837
Chicago/Turabian StyleOnea, Horea-Laurentiu, Mihail Spinu, Calin Homorodean, Maria Olinic, Florin-Leontin Lazar, Mihai Claudiu Ober, Diana Stoian, Lucian Mihai Itu, and Dan Mircea Olinic. 2022. "Distinctive Morphological Patterns of Complicated Coronary Plaques in Acute Coronary Syndromes: Insights from an Optical Coherence Tomography Study" Diagnostics 12, no. 11: 2837. https://doi.org/10.3390/diagnostics12112837
APA StyleOnea, H.-L., Spinu, M., Homorodean, C., Olinic, M., Lazar, F.-L., Ober, M. C., Stoian, D., Itu, L. M., & Olinic, D. M. (2022). Distinctive Morphological Patterns of Complicated Coronary Plaques in Acute Coronary Syndromes: Insights from an Optical Coherence Tomography Study. Diagnostics, 12(11), 2837. https://doi.org/10.3390/diagnostics12112837








