The Diagnostic Efficiency of Quantitative Diffusion Weighted Imaging in Differentiating Medulloblastoma from Posterior Fossa Tumors: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
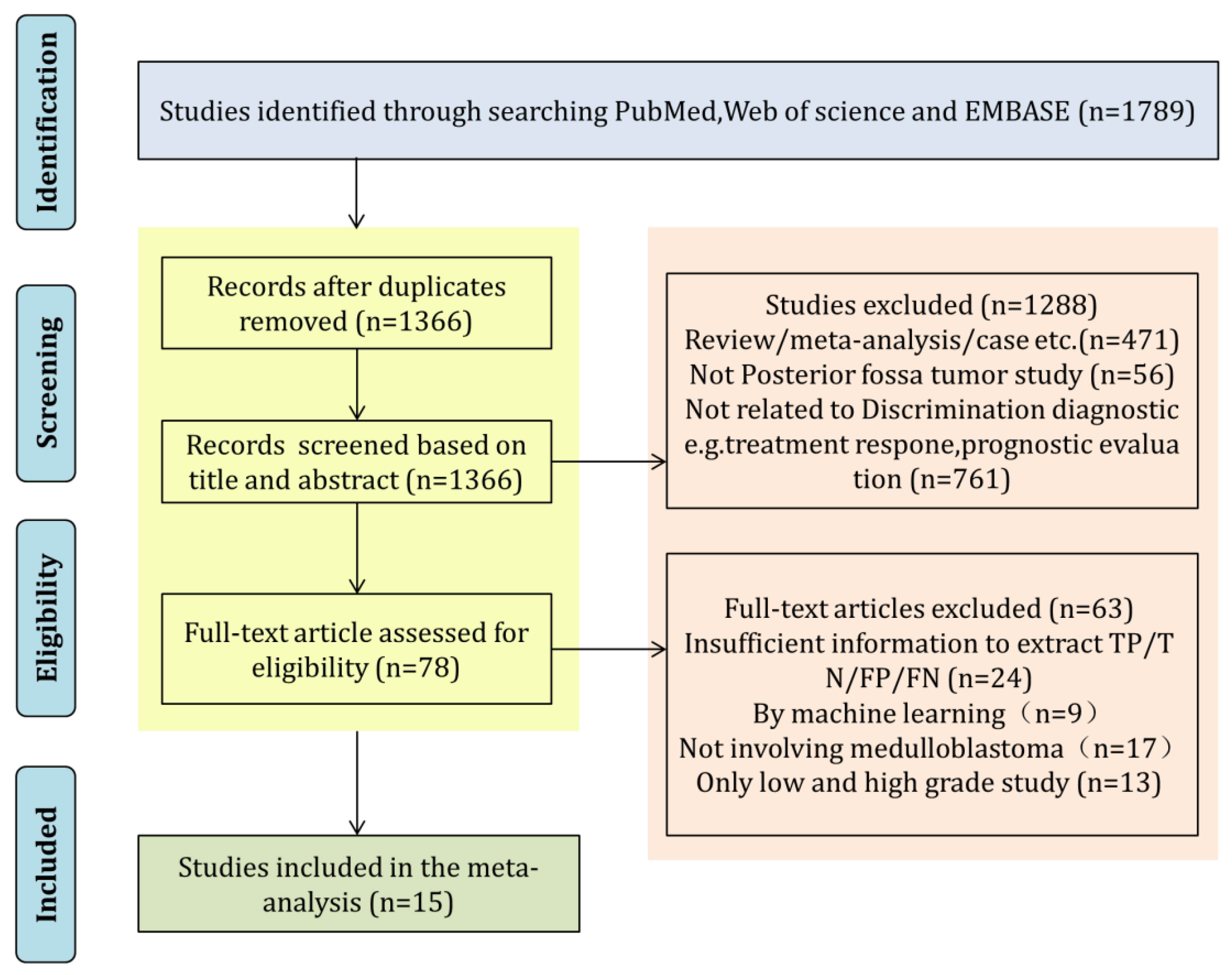
3.1. Literature Search and Article Selection
3.2. Basic Characteristics of the Included Studies
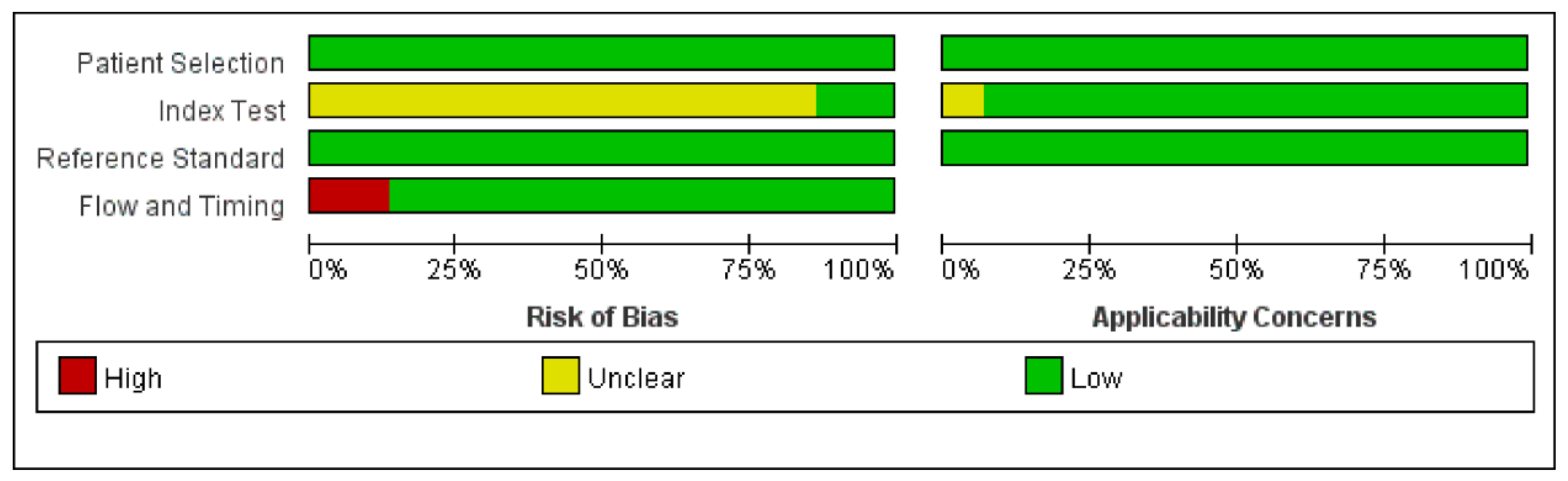
3.3. Quality Assessment
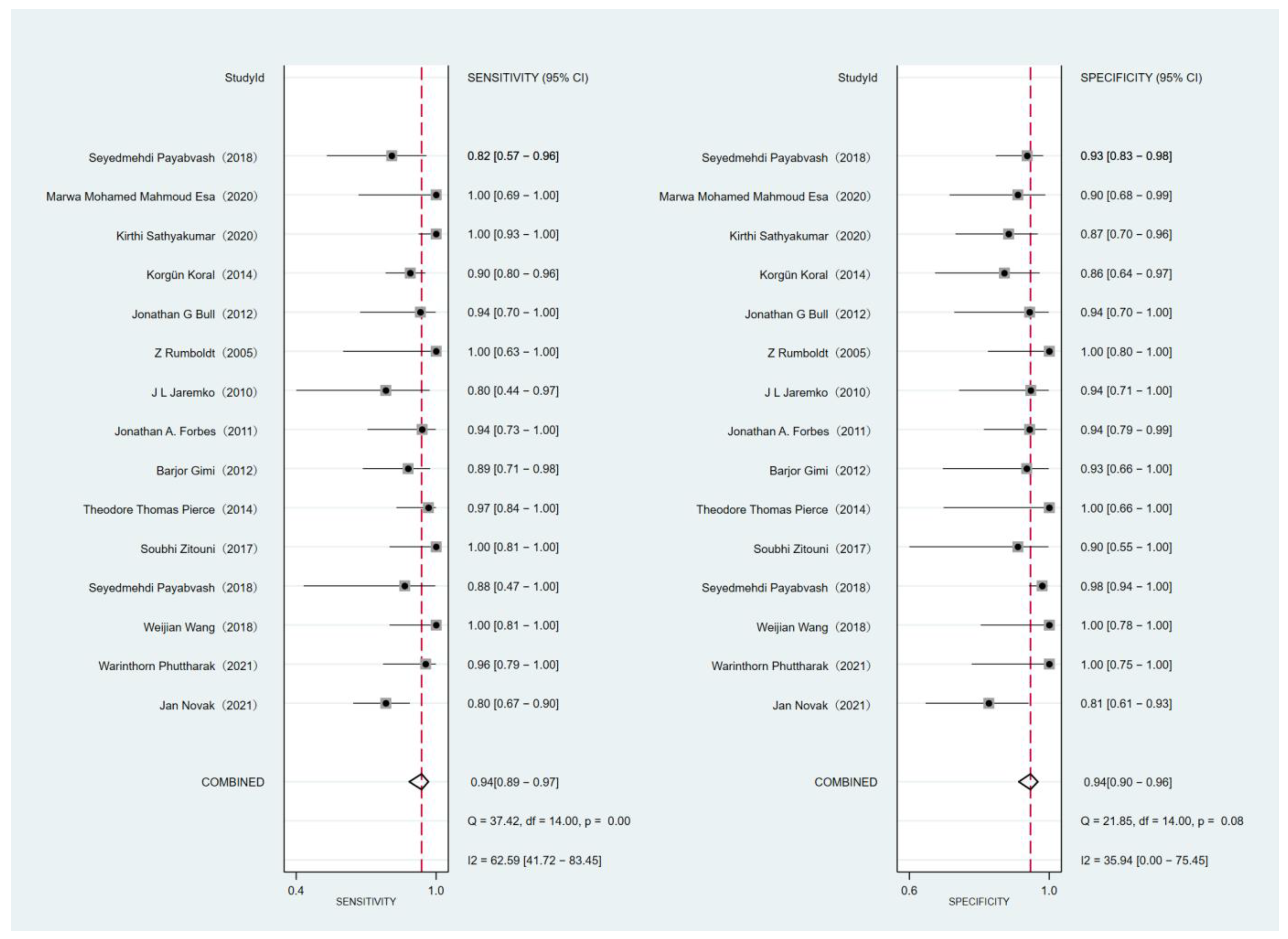
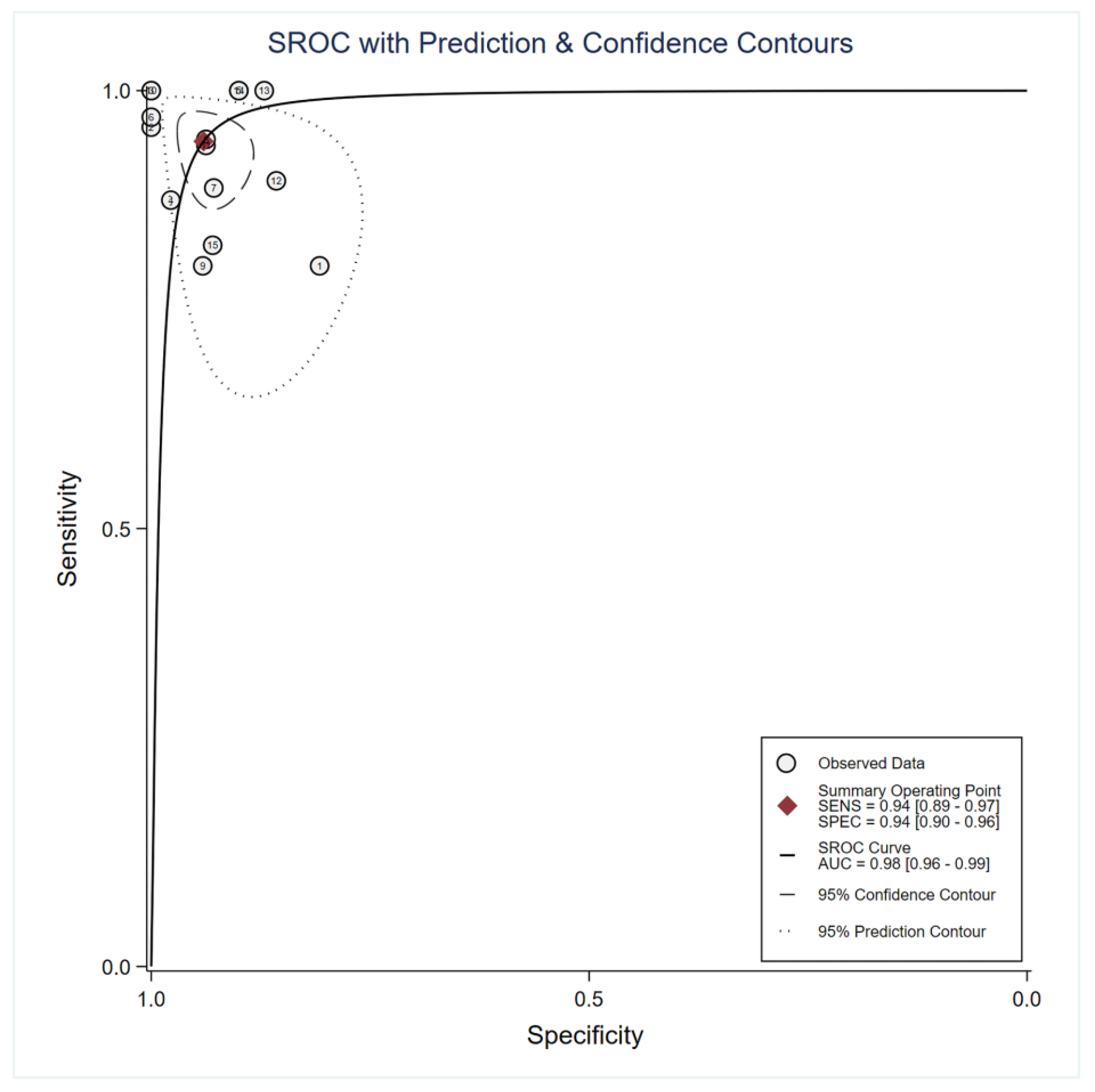
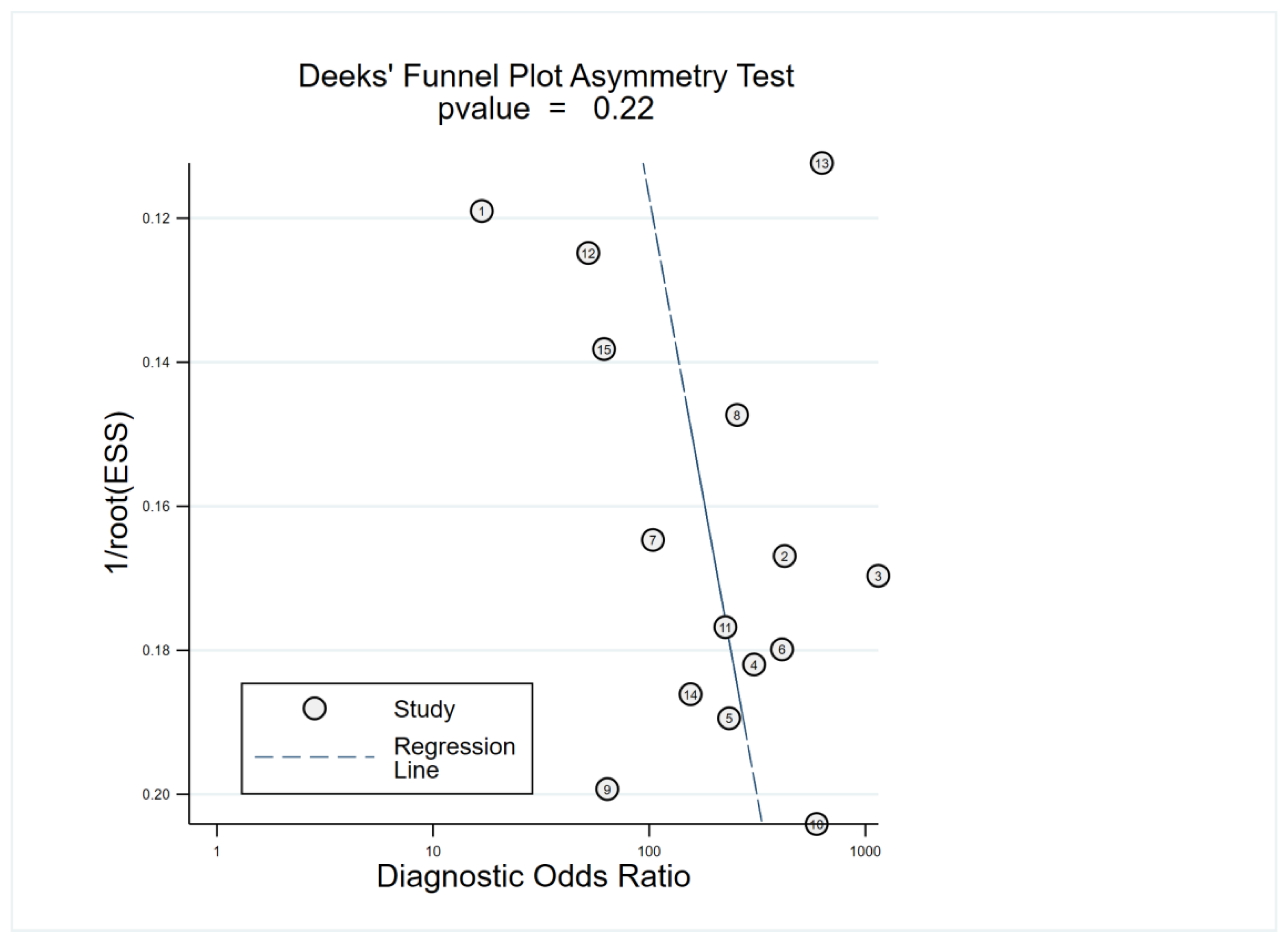
3.4. Main Statistical Analysis Results
3.5. Subgroup Analysis and Meta-Regression
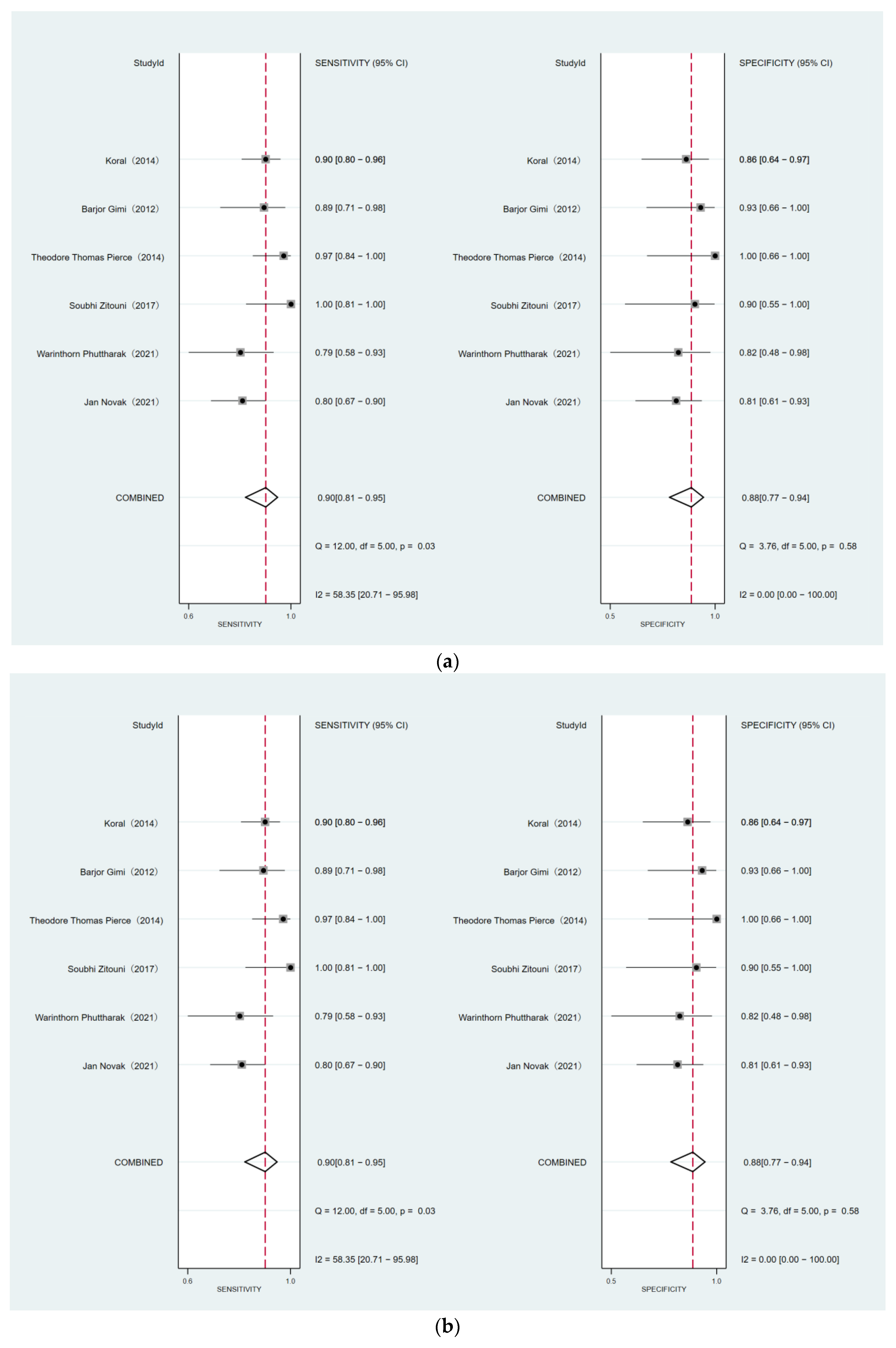
3.6. Comparison of DWI for Differentiating MB from EP and PA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Brien, D.F.; Brien, D.F.; Caird, J.; Kennedy, M.; Roberts, G.A.; Marks, J.C.; Allcutt, D.A. Posterior fossa tumours in childhood: Evaluation of presenting clinical features. Ir. Med. J. 2001, 94, 52–53. [Google Scholar] [PubMed]
- Gajjar, A.; Bowers, D.C.; Karajannis, M.A.; Leary, S.; Witt, H.; Gottardo, N.G. Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape. J. Clin. Oncol. 2015, 33, 2986–2998. [Google Scholar] [CrossRef]
- Rumboldt, Z.; Camacho, D.L.; Lake, D.; Welsh, C.T.; Castillo, M. Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR Am. J. Neuroradiol. 2006, 27, 1362–1369. [Google Scholar] [PubMed]
- Poretti, A.; Meoded, A.; Huisman, T.A. Neuroimaging of pediatric posterior fossa tumors including review of the literature. J. Magn. Reson. Imaging 2012, 35, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Taylor, M.D. Medulloblastoma: From Myth to Molecular. J. Clin. Oncol. 2017, 35, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Guerrini-Rousseau, L.; Abbas, R.; Huybrechts, S.; Kieffer-Renaux, V.; Puget, S.; Andreiuolo, F.; Beccaria, K.; Blauwblomme, T.; Bolle, S.; Dhermain, F.; et al. Role of neoadjuvant chemotherapy in metastatic medulloblastoma: A comparative study in 92 children. Neuro Oncol. 2020, 22, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, A.; Blüml, S. Neuroimaging of pediatric brain tumors: From basic to advanced magnetic resonance imaging (MRI). J. Child Neurol. 2009, 24, 1343–1365. [Google Scholar] [CrossRef]
- Dong, J.; Li, L.; Liang, S.; Zhao, S.; Zhang, B.; Meng, Y.; Zhang, Y.; Li, S. Differentiation Between Ependymoma and Medulloblastoma in Children with Radiomics Approach. Acad. Radiol. 2021, 28, 318–327. [Google Scholar] [CrossRef]
- Humphries, P.D.; Sebire, N.J.; Siegel, M.J.; Olsen, Ø.E. Tumors in pediatric patients at diffusion-weighted MR imaging: Apparent diffusion coefficient and tumor cellularity. Radiology 2007, 245, 848–854. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kumabe, T.; Higano, S.; Watanabe, M.; Tominaga, T. Minimum apparent diffusion coefficient is significantly correlated with cellularity in medulloblastomas. Neurol Res. 2009, 31, 940–946. [Google Scholar] [CrossRef]
- Wang, Y.; Miller, F.H.; Chen, Z.E.; Merrick, L.; Mortele, K.J.; Hoff, F.L.; Hammond, N.A.; Yaghmai, V.; Nikolaidis, P. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics 2011, 31, E47–E64. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Lin, G.; Zhong, W. A meta-analysis of the added value of diffusion weighted imaging in combination with contrast-enhanced magnetic resonance imaging for the diagnosis of small hepatocellular carcinoma lesser or equal to 2 cm. Oncol. Lett. 2020, 20, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Hung, K.C.; Shih, Y.J.; Lim, S.W.; Yang, C.C.; Kuo, Y.T.; Chen, J.H.; Ko, C.C. Preoperative Apparent Diffusion Coefficient Values for Differentiation between Low and High Grade Meningiomas: An Updated Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 630. [Google Scholar] [CrossRef]
- Lin, G.; Zong, X.; Li, Y.; Tan, W.; Sun, W.; Zhang, S.; Gan, Y.; Zeng, H. Whole-Body MRI Is an Effective Imaging Modality for Hematological Malignancy Treatment Response Assessment: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 827777. [Google Scholar] [CrossRef]
- Kan, P.; Liu, J.K.; Hedlund, G.; Brockmeyer, D.L.; Walker, M.L.; Kestle, J.R. The role of diffusion-weighted magnetic resonance imaging in pediatric brain tumors. Childs Nerv. Syst. 2006, 22, 1435–1439. [Google Scholar] [CrossRef]
- Schneider, J.F.; Confort-Gouny, S.; Viola, A.; Le Fur, Y.; Viout, P.; Bennathan, M.; Chapon, F.; Figarella-Branger, D.; Cozzone, P.; Girard, N. Multiparametric differentiation of posterior fossa tumors in children using diffusion-weighted imaging and short echo-time 1H-MR spectroscopy. J. Magn. Reason. Imaging. 2007, 26, 1390–1398. [Google Scholar] [CrossRef]
- Jaremko, J.L.; Jans, L.B.; Coleman, L.T.; Ditchfield, M.R. Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. AJNR Am. J. Neuroradiol. 2010, 31, 1613–1616. [Google Scholar] [CrossRef]
- Quadery, F.A.; Okamoto, K. Diffusion-weighted MRI of haemangioblastomas and other cerebellar tumours. Neuroradiology 2003, 45, 212–219. [Google Scholar] [CrossRef]
- She, D.J.; Lu, Y.P.; Xiong, J.; Cao, D.R.; Geng, D.Y.; Yin, B. Comparison of conventional, diffusion, and perfusion MRI between infratentorial ganglioglioma and pilocytic astrocytoma. Acta Radiol. 2019, 60, 1687–1694. [Google Scholar] [CrossRef]
- He, Y.X.; Qu, C.X.; He, Y.Y.; Shao, J.; Gao, Q. Conventional MR and DW imaging findings of cerebellar primary CNS lymphoma: Comparison with high-grade glioma. Sci. Rep. 2020, 10, 10007. [Google Scholar] [CrossRef] [PubMed]
- Koral, K.; Zhang, S.; Gargan, L.; Moore, W.; Garvey, B.; Fiesta, M.; Seymour, M.; Yang, L.; Scott, D.; Choudhury, N. Diffusion MRI improves the accuracy of preoperative diagnosis of common pediatric cerebellar tumors among reviewers with different experience levels. AJNR Am. J. Neuroradiol. 2013, 34, 2360–2365. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.C.; Graves, M.J.; Gilbert, F.J.; Patterson, A.J. A Meta-analysis of the Diagnostic Performance of Diffusion MRI for Breast Lesion Characterization. Radiology 2019, 291, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Phuttharak, W.; Wannasarnmetha, M.; Wara-Asawapati, S.; Yuthawong, S. Diffusion MRI in Evaluation of Pediatric Posterior Fossa Tumors. Asian Pac. J. Cancer Prev. 2021, 22, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Zarinabad, N.; Rose, H.; Arvanitis, T.; MacPherson, L.; Pinkey, B.; Oates, A.; Hales, P.; Grundy, R.; Auer, D.; et al. Classification of paediatric brain tumours by diffusion weighted imaging and machine learning. Sci. Rep. 2021, 11, 2987. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, J.; Zhang, Y.; Wang, C. Use of Apparent Diffusion Coefficient Histogram in Differentiating Between Medulloblastoma and Pilocytic Astrocytoma in Children. Med. Sci. Monit. 2018, 24, 6107–6112. [Google Scholar] [CrossRef]
- Payabvash, S.; Tihan, T.; Cha, S. Differentiation of Cerebellar Hemisphere Tumors: Combining Apparent Diffusion Coefficient Histogram Analysis and Structural MRI Features. J. Neuroimaging 2018, 28, 656–665. [Google Scholar] [CrossRef]
- Zitouni, S.; Koc, G.; Doganay, S.; Saracoglu, S.; Gumus, K.Z.; Ciraci, S.; Coskun, A.; Unal, E.; Per, H.; Kurtsoy, A.; et al. Apparent diffusion coefficient in differentiation of pediatric posterior fossa tumors. Jpn. J. Radiol. 2017, 35, 448–453. [Google Scholar] [CrossRef]
- Pierce, T.T.; Provenzale, J.M. Evaluation of apparent diffusion coefficient thresholds for diagnosis of medulloblastoma using diffusion-weighted imaging. Neuroradiol. J. 2014, 27, 63–74. [Google Scholar] [CrossRef]
- Gimi, B.; Cederberg, K.; Derinkuyu, B.; Gargan, L.; Koral, K.M.; Bowers, D.C.; Koral, K. Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad. Radiol. 2012, 19, 794–800. [Google Scholar] [CrossRef]
- Forbes, J.A.; Reig, A.S.; Smith, J.G.; Jermakowicz, W.; Tomycz, L.; Shay, S.D.; Sun, D.A.; Wushensky, C.A.; Pearson, M.M. Findings on preoperative brain MRI predict histopathology in children with cerebellar neoplasms. Pediatr. Neurosurg. 2011, 47, 51–59. [Google Scholar] [CrossRef]
- Bull, J.G.; Saunders, D.E.; Clark, C.A. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur. Radiol. 2012, 22, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Koral, K.; Alford, R.; Choudhury, N.; Mossa-Basha, M.; Gargan, L.; Gimi, B.; Gao, A.; Zhang, S.; Bowers, D.C.; Koral, K.M.; et al. Applicability of apparent diffusion coefficient ratios in preoperative diagnosis of common pediatric cerebellar tumors across two institutions. Neuroradiology 2014, 56, 781–788. [Google Scholar] [CrossRef]
- Sathyakumar, K.; Mani, S.; Pathak, G.H.; Prabhu, K.; Chacko, A.G.; Chacko, G. Neuroimaging of pediatric infratentorial tumors and the value of diffusion-weighted imaging (DWI) in determining tumor grade. Acta Radiol. 2021, 62, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Payabvash, S.; Tihan, T.; Cha, S. Volumetric voxelwise apparent diffusion coefficient histogram analysis for differentiation of the fourth ventricular tumors. Neuroradiol. J. 2018, 31, 554–564. [Google Scholar] [CrossRef]
- Esa, M.; Mashaly, E.M.; El-Sawaf, Y.F.; Dawoud, M.M. Diagnostic accuracy of apparent diffusion coefficient ratio in distinguishing common pediatric CNS posterior fossa tumors. Egypt. J. Radiol. Nucl. Med. 2020, 51, 1–11. [Google Scholar] [CrossRef]
- Wang, Q.P.; Lei, D.Q.; Yuan, Y.; Xiong, N.X. Accuracy of ADC derived from DWI for differentiating high-grade from low-grade gliomas: Systematic review and meta-analysis. Medicine 2020, 99, e19254. [Google Scholar] [CrossRef]
- Dury, R.J.; Lourdusamy, A.; Macarthur, D.C.; Peet, A.C.; Auer, D.P.; Grundy, R.G.; Dineen, R.A. Meta-Analysis of Apparent Diffusion Coefficient in Pediatric Medulloblastoma, Ependymoma, and Pilocytic Astrocytoma. J. Magn. Reason. Imaging 2021, 56, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.Y.; Yao, Q.Y.; Wu, L.M.; Xu, J.R. Breast Lesions: Diagnosis Using Diffusion Weighted Imaging at 1.5T and 3.0T-Systematic Review and Meta-analysis. Clin. Breast Cancer 2018, 18, e305–e320. [Google Scholar] [CrossRef]
- Van Rijswijk, C.S.; Kunz, P.; Hogendoorn, P.C.; Taminiau, A.H.; Doornbos, J.; Bloem, J.L. Diffusion-weighted MRI in the characterization of soft-tissue tumors. J. Magn. Reason. Imaging 2002, 15, 302–307. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Wang, Q.; Wu, C.J.; Wang, X.N.; Zhang, J.; Liu, H.; Liu, X.S.; Shi, H.B. The histogram analysis of diffusion-weighted intravoxel incoherent motion (IVIM) imaging for differentiating the gleason grade of prostate cancer. Eur. Radiol. 2015, 25, 994–1004. [Google Scholar] [CrossRef]
- Lu, S.S.; Kim, S.J.; Kim, N.; Kim, H.S.; Choi, C.G.; Lim, Y.M. Histogram analysis of apparent diffusion coefficient maps for differentiating primary CNS lymphomas from tumefactive demyelinating lesions. AJR Am. J. Roentgenol. 2015, 204, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Hu, H.; Su, G.Y.; Zhang, L.; Liu, H.; Hong, X.N.; Shi, H.B.; Wu, F.Y. Orbital Indeterminate Lesions in Adults: Combined Magnetic Resonance Morphometry and Histogram Analysis of Apparent Diffusion Coefficient Maps for Predicting Malignancy. Acad. Radiol. 2016, 23, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Robba, T.; Chianca, V.; Albano, D.; Clementi, V.; Piana, R.; Linari, A.; Comandone, A.; Regis, G.; Stratta, M.; Faletti, C.; et al. Diffusion-weighted imaging for the cellularity assessment and matrix characterization of soft tissue tumour. Radiol. Med. 2017, 122, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, A.C.; Dale, B.M.; Boll, D.T.; Merkle, E.M. Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology 2009, 250, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, C.; Yan, J.; Sun, J.; Zhao, X.; Zhang, L.; Yin, L. Accuracy of quantitative diffusion-weighted imaging for differentiating benign and malignant pancreatic lesions: A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 7746–7759. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Bayne, A.M.; Chakrabarty, A.; Kenny, T.; Chumas, P. Incidence of childhood CNS tumours in Britain and variation in rates by definition of malignant behaviour: Population-based study. BMC Cancer 2019, 19, 139. [Google Scholar] [CrossRef]
- Benesch, M.; Mynarek, M.; Witt, H.; Warmuth-Metz, M.; Pietsch, T.; Bison, B.; Pfister, S.M.; Pajtler, K.W.; Kool, M.; Schüller, U.; et al. Newly Diagnosed Metastatic Intracranial Ependymoma in Children: Frequency, Molecular Characteristics, Treatment, and Outcome in the Prospective HIT Series. Oncologist 2019, 24, e921–e929. [Google Scholar] [CrossRef]
- Von Bueren, A.O.; Kortmann, R.D.; von Hoff, K.; Friedrich, C.; Mynarek, M.; Müller, K.; Goschzik, T.; Zur Mühlen, A.; Gerber, N.; Warmuth-Metz, M.; et al. Treatment of Children and Adolescents With Metastatic Medulloblastoma and Prognostic Relevance of Clinical and Biologic Parameters. J. Clin. Oncol. 2016, 34, 4151–4160. [Google Scholar] [CrossRef]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood brain tumors: Current management, biological insights, and future directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef]
- Murphy, E.S.; Parsai, S.; Kano, H.; Sheehan, J.P.; Martinez-Alvarez, R.; Martinez-Moreno, N.; Kondziolka, D.; Simonova, G.; Liscak, R.; Mathieu, D.; et al. Outcomes of stereotactic radiosurgery for pilocytic astrocytoma: An international multiinstitutional study. J. Neurosurg. 2019, 134, 162–170. [Google Scholar] [CrossRef]
- Higano, S.; Yun, X.; Kumabe, T.; Watanabe, M.; Mugikura, S.; Umetsu, A.; Sato, A.; Yamada, T.; Takahashi, S. Malignant astrocytic tumors: Clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 2006, 241, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, Y.; Hirai, T.; Morishita, S.; Kitajima, M.; Murakami, R.; Korogi, Y.; Makino, K.; Nakamura, H.; Ikushima, I.; Yamura, M.; et al. Diffusion-weighted imaging of metastatic brain tumors: Comparison with histologic type and tumor cellularity. AJNR Am. J. Neuroradiol. 2006, 27, 1419–1425. [Google Scholar] [PubMed]
- Gonçalves, F.G.; Zandifar, A.; Ub Kim, J.D.; Tierradentro-García, L.O.; Ghosh, A.; Khrichenko, D.; Andronikou, S.; Vossough, A. Application of Apparent Diffusion Coefficient Histogram Metrics for Differentiation of Pediatric Posterior Fossa Tumors: A Large Retrospective Study and Brief Review of Literature. Clin. Neuroradiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shlobin, N.A.; Lam, S.K.; DeCuypere, M. Artificial Intelligence Applications in Pediatric Brain Tumor Imaging: A Systematic Review. World Neurosurg. 2022, 157, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Di Noia, C.; Grist, J.T.; Riemer, F.; Lyasheva, M.; Fabozzi, M.; Castelli, M.; Lodi, R.; Tonon, C.; Rundo, L.; Zaccagna, F. Predicting Survival in Patients with Brain Tumors: Current State-of-the-Art of AI Methods Applied to MRI. Diagnostics 2022, 12, 2125. [Google Scholar] [CrossRef] [PubMed]
| First Author/Year | Country | No. of Patients(n) | Age of Patients(y) | MB(n) | Other PFT(n) | Study Design | MRI Field Strength (T) | b Values (sec/mm2) | Selection of ROI | Diagnostic Parameters |
|---|---|---|---|---|---|---|---|---|---|---|
| Warinthorn Phuttharak (2021) [23] | Thailand | 37 | 7.94 | 24 | 13 | retrospective | 1.5T/3.0T | 0, 1000 | single layer | ADC ratio |
| Jan Novak (2021) [24] | UK | 81 | 5.8 | 55 | 26 | retrospective | 1.5T/3.0T | 0, 1000 | whole tumors | ADC histogram |
| Weijian Wang (2018) [25] | China | 33 | 3–15 | 18 | 15 | retrospective | 3.0T | 0, 1000 | single layer | ADC histogram |
| Seyedmehdi Paybvash (2018) [26] | USA | 142 | 47 | 8 | 134 | retrospective | 1.5T/3.0T | 0, 1000 | whole tumors | ADC histogram |
| Soubhi Zitouni (2017) [27] | Turkey | 28 | 7.76 | 18 | 10 | retrospective | 1.5T | 0, 1000 | multiple layers | ADC ratio |
| Theodore Thomas Pierce (2014) [28] | USA | 42 | 6.5 | 33 | 9 | retrospective | NA | NA | multiple layers | ADC min |
| Barjor Gimi (2012) [29] | USA | 41 | 5.9 | 27 | 14 | retrospective | 1.5T/3.0T | 0, 1000 | multiple layers | ADC ratio |
| Jonathan A. Forbes (2011) [30] | USA | 50 | <18 | 18 | 32 | retrospective | 1.5T | NA | single layer | rDWI |
| J L Jaremko (2010) [17] | Australia | 27 | 5.8 | 10 | 17 | retrospective | 1.5T/3.0T | 0, 1000 | single layer | ADC min |
| Z Rumboldt (2005) [3] | USA | 25 | 9 | 8 | 17 | retrospective | 1.5T | 0, 1000 | multiple layers | ADC mean |
| Jonathan G Bull (2012) [31] | UK | 32 | 6.1 | 16 | 16 | retrospective | 1.5T | 0, 1000 | whole tumors | ADC histogram |
| Korgün Koral (2014) [32] | USA | 79 | 6.8 | 58 | 21 | retrospective | 1.5T/3.0T | 0, 1000 | multiple layers | ADC ratio |
| Kirthi Sathyakumar (2020) [33] | India | 82 | 8.24 | 51 | 31 | retrospective | 1.5T | 0, 1000 | multiple layers | ADC mean |
| Seyedmehdi Payabvash (2018) [34] | USA | 74 | 25.4 | 17 | 57 | retrospective | 1.5T/3.0T | 0, 1000 | whole tumors | ADC histogram |
| Marwa Mohamed Mahmoud Esa (2020) [35] | Egypt | 30 | 8.7 | 10 | 20 | prospective | 1.5T | 0, 1000 | multiple layers | ADC ratio |
| Covariates | Subgroups | No. of Study | Sensitivity (95% CI) | p | Specificity (95% CI) | p |
|---|---|---|---|---|---|---|
| Age | <18 | 13 | 0.95 (0.91–0.98) | 0.25 | 0.94 (0.90–0.97) | 0.63 |
| >18 | 2 | 0.91 (0.79–1.00) | 0.96 (0.90–1.00) | |||
| Region | Asia | 4 | 0.99 (0.98–1.00) | 0.02 | 0.94 (0.87–1.00) | 0.16 |
| Non-Asia | 11 | 0.90 (0.86–0.95) | 0.94 (0.91–0.97) | |||
| Magnetic field strength | 1.5 T | 6 | 0.89 (0.84–0.94) | <0.001 | 0.93 (0.87–0.98) | 0.03 |
| 3.0 T or mixed | 8 | 0.98 (0.96–1.00) | 0.94 (0.91–0.98) | |||
| DWI parameters | only lesions | 9 | 0.94 (0.89–0.99) | 0.17 | 0.92 (0.87–0.98) | <0.001 |
| with normal tissue | 6 | 0.95 (0.90–0.99) | 0.95 (0.91–0.98) | |||
| ADC histogram | ADC histogram | 5 | 0.93 (0.85–1.00) | 0.26 | 0.97 (0.94–0.99) | 0.18 |
| Not-histogram | 10 | 0.95 (0.91–0.99) | 0.91 (0.87–0.96) | |||
| ROI | single layer | 4 | 0.93 (0.89–0.98) | 0.13 | 0.93 (0.89–0.96) | 0.01 |
| multiple layers | 11 | 0.96 (0.90–1.00) | 0.97 (0.93–1.00) |
| No.of Studies | No.of Patients | Sensitivity | Heterogeneity | p | Specificity | Heterogeneity | p | ||
|---|---|---|---|---|---|---|---|---|---|
| p Value | I2(%) | p Value | I2(%) | ||||||
| Medulloblastoma vs. ependymoma | |||||||||
| 6 | 316 | 0.90 (0.81, 0.95) | 0.03 | 58.35 | 0.74 | 0.88 (0.77, 0.94) | 0.58 | 0.00 | 0.01 |
| Medulloblastoma vs. pilocytic astrocytoma | |||||||||
| 4 | 122 | 0.96 (0.77, 1.00) | 0.03 | 67.38 | 0.99 (0.65, 1.00) | 0.14 | 45.18 | ||
| First Author/Year | MRI Field Strength (T) | Selection of ROI | Diagnostic Parameters | MB | EP | The Optimal Threshold Value |
|---|---|---|---|---|---|---|
| Warinthorn Phuttharak (2021) | 1.5T/3.0T | single layer | ADC ratio | 0.91 ± 0.17 | 1.3 ± 0.35 | 1.00 |
| Jan Novak (2021) | 1.5T/3.0T | whole tumors | ADC histogram (mean) | (0.87 ± 0.15) × 10−3 mm2 s−1 | (1.13 ± 0.16) × 10−3 mm2 s−1 | 0.98 × 10−3 mm2 s−1 |
| Soubhi Zitouni (2017) | 1.5T | multiple layers | ADC ratio | 1.02 ± 0.30 | 1.50 ± 0.20 | 1.18 |
| Theodore Thomas Pierce (2014) | NA | multiple layers | ADC min | (0.54 ± 0.09) × 10−3 mm2 s−1 | (0.88 ± 0.13) × 10−3 mm2 s−1 | 0.68 × 10−3 mm2 s−1 |
| Barjor Gimi (2012) | 1.5T/3.0T | multiple layers | ADC ratio | 0.97–0.99 | 1.54–1.58 | 1.20 |
| Korgün Koral (2014) | 1.5T/3.0T | multiple layers | ADC ratio | —— | —— | 1.20 |
| Warinthorn Phuttharak (2021) | 1.5T/3.0T | single layer | ADC ratio | 0.91 ± 0.17 | 2.11 ± 0.51 | 1.17 |
| Weijian Wang (2018) | 3.0T | single layer | ADC histogram (mean) | 106.5 ± 15.8 | 200.9 ± 31.4 | 137.7 |
| J L Jaremko (2010) | 1.5T/3.0T | single layer | ADC min | —— | —— | 0.8 × 10−3 mm2 s−1 |
| Z Rumboldt (2005) | 1.5T | multiple layer | ADC mean | (1.65 ± 0.27) × 10−3 mm2 s−1 | (0.66 ± 0.15) × 10−3 mm2 s−1 | —— |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Zhang, S.; Tan, W.; Lin, G.; Zhuang, Y.; Zeng, H. The Diagnostic Efficiency of Quantitative Diffusion Weighted Imaging in Differentiating Medulloblastoma from Posterior Fossa Tumors: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2796. https://doi.org/10.3390/diagnostics12112796
Luo Y, Zhang S, Tan W, Lin G, Zhuang Y, Zeng H. The Diagnostic Efficiency of Quantitative Diffusion Weighted Imaging in Differentiating Medulloblastoma from Posterior Fossa Tumors: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(11):2796. https://doi.org/10.3390/diagnostics12112796
Chicago/Turabian StyleLuo, Yi, Siqi Zhang, Weiting Tan, Guisen Lin, Yijiang Zhuang, and Hongwu Zeng. 2022. "The Diagnostic Efficiency of Quantitative Diffusion Weighted Imaging in Differentiating Medulloblastoma from Posterior Fossa Tumors: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 11: 2796. https://doi.org/10.3390/diagnostics12112796
APA StyleLuo, Y., Zhang, S., Tan, W., Lin, G., Zhuang, Y., & Zeng, H. (2022). The Diagnostic Efficiency of Quantitative Diffusion Weighted Imaging in Differentiating Medulloblastoma from Posterior Fossa Tumors: A Systematic Review and Meta-Analysis. Diagnostics, 12(11), 2796. https://doi.org/10.3390/diagnostics12112796








