A Comparative Follow-Up Study of Patients with Papillary Thyroid Carcinoma Associated or Not with Graves’ Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Data Analyses
2.4. Statistics
3. Results
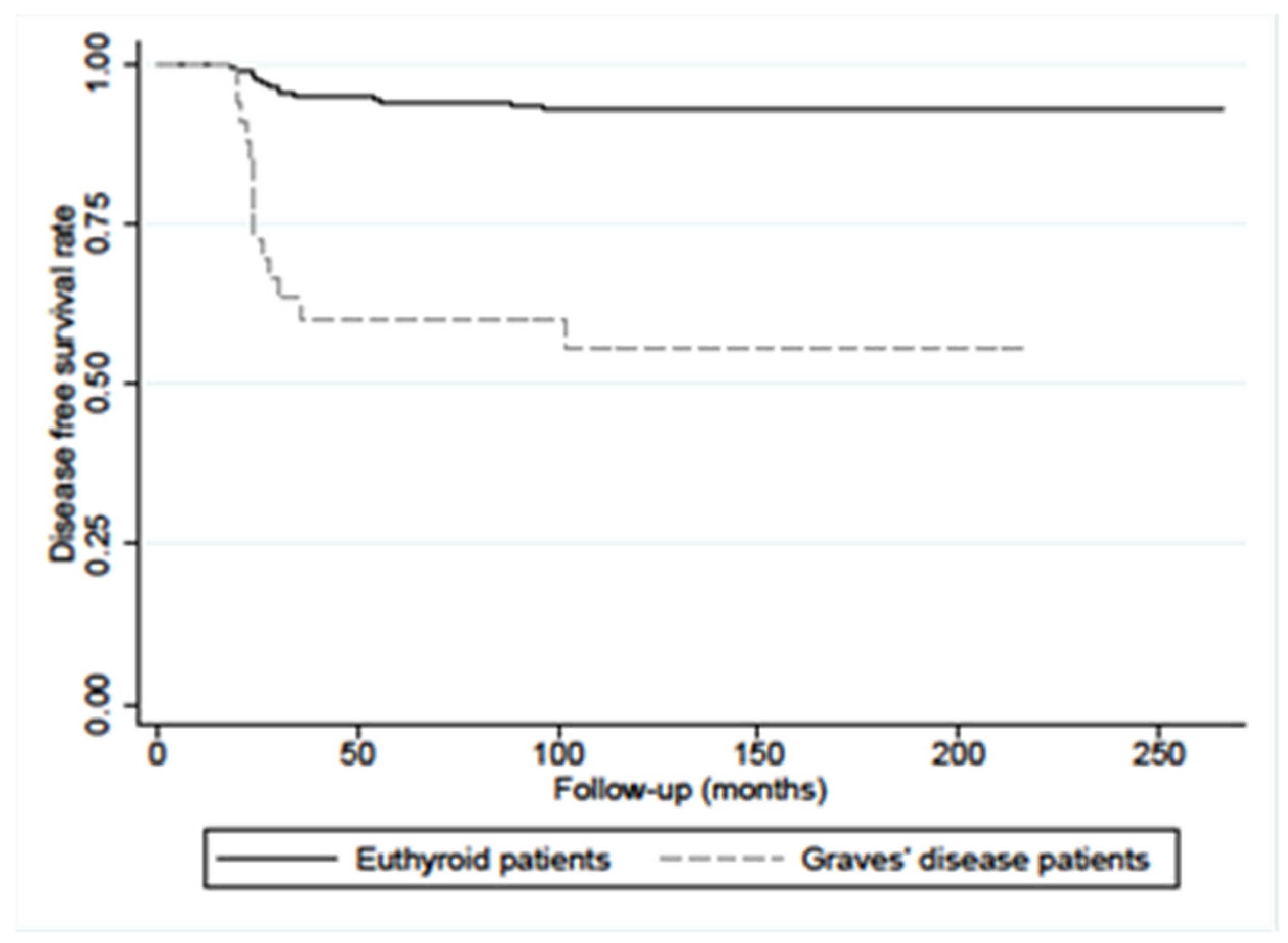
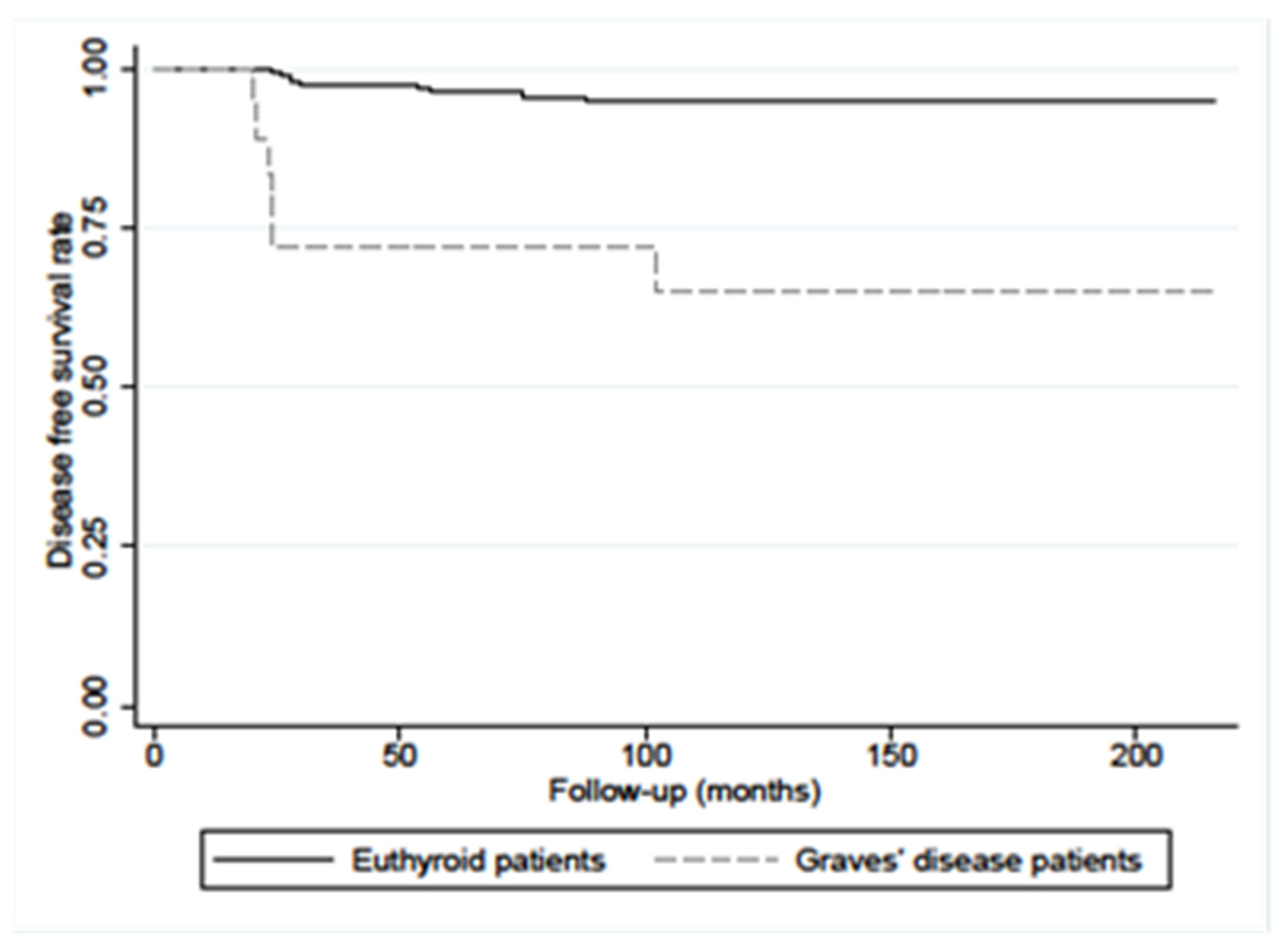
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weber, K.J.; Solorzano, C.C.; Lee, J.K.; Gaffud, M.J.; Prinz, R.A. Thyroidectomy remains an effective treatment option for Graves’ disease. Am. J. Surg. 2006, 191, 400–405. [Google Scholar] [CrossRef]
- Shu, X.; Ji, J.; Li, X.; Sundquist, J.; Sundquist, K.; Hemminki, K. Cancer risk in patients hospitalized for Graves’ disease: A population-based cohort study in Sweden. Br. J. Cancer 2010, 102, 1397–1399. [Google Scholar] [PubMed]
- Chen, Y.K.; Lin, C.L.; Chang, Y.J.; Cheng, F.T.; Peng, C.L.; Sung, F.C.; Cheng, Y.H.; Kao, C.H. Cancer risk in patients with Graves’ disease: A nationwide cohort study. Thyroid 2013, 23, 879–884. [Google Scholar] [PubMed]
- Keskin, C.; Sahin, M.; Hasanov, R.; Aydogan, B.I.; Demir, O.; Emral, R.; Gullu, S.; Erdogan, M.F.; Gedik, V.; Uysal, A.R.; et al. Frequency of thyroid nodules and thyroid cancer in thyroidectomized patients with Graves’ disease. Arch. Med. Sci. 2020, 16, 302–307. [Google Scholar] [PubMed]
- Filetti, S.; Belfiore, A.; Amir, S.M.; Daniels, G.H.; Ippolito, O.; Vigneri, R.; Ingbar, S.H. The role of thyroid-stimulating antibodies of Graves’ disease in differentiated thyroid cancer. N. Engl. J. Med. 1988, 318, 753–759. [Google Scholar]
- Ergin, A.B.; Saralaya, S.; Olansky, L. Incidental papillary thyroid carcinoma: Clinical characteristics and prognostic factors among patients with Graves’ disease and euthyroid goiter, Cleveland Clinic experience. Am. J. Otolaryngol. 2014, 35, 784–790. [Google Scholar]
- Schmidt, J.A.; Allen, N.E.; Almquist, M.; Franceschi, S.; Rinaldi, S.; Tipper, S.J.; Tsilidis, K.K.; Weiderpass, E.; Overvad, K.; Tjønneland, A.; et al. Insulin-like growth factor-i and risk of differentiated thyroid carcinoma in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 976–985. [Google Scholar] [CrossRef]
- Tseng, F.-Y.; Chen, Y.-T.; Chi, Y.-C.; Chen, P.-L.; Yang, W.-S. Serum levels of insulin-like growth factor 1 are negatively associated with log transformation of thyroid-stimulating hormone in Graves’ disease patients with hyperthyroidism or subjects with euthyroidism: A prospective observational study. Medicine 2019, 98, 11. [Google Scholar]
- Poulaki, V.; Mitsiades, C.S.; McMullan, C.; Sykoutri, D.; Fanouraki, G.; Kotoula, V.; Tseleni-Balafouta, S.; Koutras, D.A.; Mitsiades, N. Regulation of vascular endothelial growth factor expression by insulin-like growth factor I in thyroid carcinomas. J. Clin. Endocrinol. Metab. 2003, 88, 5392–5398. [Google Scholar]
- Boutzios, G.; Vasileiadis, I.; Zapanti, E.; Charitoudis, G.; Karakostas, E.; Ieromonachou, P.; Karatzas, T. Higher incidence of tall cell variant of papillary thyroid carcinoma in Graves’ disease. Thyroid 2014, 24, 347–354. [Google Scholar]
- Ozaki, O.; Ito, K.; Kobayashi, K.; Toshima, K.; Iwasaki, H.; Yashiro, T. Thyroid carcinoma in Graves’ disease. World J. Surg. 1990, 14, 437–440. [Google Scholar] [PubMed]
- Cappelli, C.; Braga, M.; De Martino, E.; Castellano, M.; Gandossi, E.; Agosti, B.; Cumetti, D.; Pirola, I.; Mattanza, C.; Cherubini, L.; et al. Outcome of patients surgically treated for various forms of hyperthyroidism with differentiated thyroid cancer: Experience at an endocrine center in Italy. Surg. Today 2006, 36, 125–130. [Google Scholar] [PubMed]
- You, E.; Mascarella, M.A.; Al Jassim, A.; Forest, V.I.; Hier, M.P.; Tamilia, M.; Pusztaszeri, M.; Payne, R.J. Prevalence and aggressiveness of papillary thyroid carcinoma in surgically-treated Graves’ disease patients: A retrospective matched cohort study. J. Otolaryngol. Head Neck Surg. 2019, 48, 40. [Google Scholar]
- Mekraksakit, P.; Rattanawong, P.; Karnchanasorn, R.; Kanitsoraphan, C.; Leelaviwat, N.; Poonsombudlert, K.; Kewcharoen, J.; Dejhansathit, S.; Samoa, R. Prognosis of differentiated thyroid carcinoma in patients with Graves disease: A systematic review and meta-analysis. Endocr. Pract. 2019, 25, 1323–1337. [Google Scholar] [PubMed]
- Belfiore, A.; Garofalo, M.R.; Giuffrida, D.; Runello, F.; Filetti, S.; Fiumara, A.; Ippolito, O.; Vigneri, R. Increased Aggressiveness of Thyroid Cancer in Patients with Graves’ Disease. J. Clin. Endocrinol. Metab. 1990, 70, 830–835. [Google Scholar]
- Pellegriti, G.; Belfiore, A.; Giufrida, D.; Lupo, L.; Vigneri, R. Outcome of differentiated thyroid cancer in Graves patients. J. Clin. Endocrinol. Metab. 1998, 83, 2805–2809. [Google Scholar]
- Chao, T.C.; Lin, J.D.; Jeng, L.; Chen, M.F. Thyroid cancer with concurrent hyperthyroidism. Arch. Surg. 1999, 134, 130–134. [Google Scholar] [PubMed]
- Chao, T.C.; Lin, J.D.; Chen, M.F. Surgical treatment of thyroid cancers with concurrent Graves disease. Ann. Surg. Oncol. 2004, 11, 407–412. [Google Scholar] [CrossRef]
- Pazaitou-Panayiotou, K.; Perros, P.; Boudina, M.; Drimonitis, A.; Siardos, G.; Patakiouta, F.; Vainas, I. Mortality from thyroid cancer in patients with hyperthyroidism: The Theagenion Cancer Hospital experience. Eur. J. Endocrinol. 2008, 159, 799–803. [Google Scholar]
- Lee, J.; Nam, K.H.; Chung, W.Y.; Soh, E.Y.; Park, C.S. Clinicopathologic features and treatment outcomes in differentiated thyroid carcinoma patients with concurrent Graves’ disease. J. Korean Med. Sci. 2008, 23, 796–801. [Google Scholar]
- Pazaitou-Panayiotou, K.; Michalakis, K.; Paschke, R. Thyroid Cancer in Patients with Hyperthyroidism. Horm. Metab. Res. 2012, 44, 255–262. [Google Scholar] [PubMed]
- Ren, M.; Wu, M.C.; Shang, C.Z.; Wang, X.Y.; Zhang, J.L.; Cheng, H.; Xu, M.T.; Yan, L. Predictive factors of thyroid cancer in patients with Graves’ disease. World J. Surg. 2014, 38, 80–87. [Google Scholar]
- Menon, R.; Nair, C.G.; Babu, M.; Jacob, P.; Krishna, G.P. The outcome of papillary thyroid cancer associated with Graves’ disease: A case control study. J. Thyroid Res. 2018, 2018, 8253094. [Google Scholar] [PubMed]
- Premoli, P.; Tanda, M.L.; Piantanida, E.; Veronesi, G.; Gallo, D.; Masiello, E.; Rosetti, S.; Cusini, C.; Boi, F.; Bulla, J.; et al. Features and outcome of differentiated thyroid carcinoma associated with Graves’ disease: Results of a large, retrospective, multicenter study. J. Endocrinol. Investig. 2020, 43, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; Mannarin, C.; Russo, M.; Terranova, R.; Marturano, I.; Vigneri, R.; Belfiore, A. Increased Mortality in Patients With Differentiated Thyroid Cancer Associated With Graves’ Disease. J. Clin. Endocrinol. Metab. 2013, 98, 1014–1021. [Google Scholar]
- Hales, I.B.; McElduf, P.; Crummer, P.; Clifton-Bligh, P.; Delbridge, L.; Hoschl, R.; Poole, A.; Reeve, T.S.; Wilmshurst, E.; Wiseman, J. Does Graves’ disease or thyrotoxicosis affect the prognosis of thyroid cancer? J. Clin. Endocrinol. Metab. 1992, 75, 886–889. [Google Scholar]
- Kasuga, Y.; Sugenoya, A.; Kobayashi, S.; Masuda, H.; Iida, F. The outcome of patients with thyroid carcinoma and Graves’ disease. Surg. Today 1993, 23, 9–12. [Google Scholar]
- Edmonds, C.J.; Tellez, M. Hyperthyroidism and thyroid cancer. Clin. Endocrinol. 1998, 28, 253–259. [Google Scholar]
- Kikuchi, S.; Noguchi, S.; Yamashita, H.; Uchino, S.; Kawamoto, H. Prognosis of small thyroid cancer in patients with Graves’ disease. Br. J. Surg. 2006, 93, 434–439. [Google Scholar]
- Yano, Y.; Shibuya, H.; Kitagawa, W.; Nagahama, M.; Sugino, K.; Ito, K.; Ito, K. Recent outcome of Graves’ disease patients with papillary thyroid cancer. Eur. J. Endocrinol. 2007, 157, 325–329. [Google Scholar]
- Kwon, H.; Moon, B.I. Prognosis of papillary thyroid cancer in patients with Graves’ disease: A propensity score-matched analysis. World J. Surg. Oncol. 2020, 18, 266. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Jin, M.; Kim, M.; Hong, A.R.; Kim, H.K.; Kim, B.H.; Kim, W.B.; Shong, Y.K.; Jeon, M.J.; Kang, H.C. Clinical Characteristics and Prognosis of Coexisting Thyroid Cancer in Patients with Graves’ Disease: A Retrospective Multicenter Study. Endocrinol. Metab. 2021, 36, 1268–1276. [Google Scholar]
- MacFarland, S.P.; Bauer, A.J.; Adzick, N.S.; Surrey, L.F.; Noyes, J.; Kazahaya, K.; Mostoufi-Moab, S. Disease Burden and Outcome in Children and Young Adults with Concurrent Graves Disease and Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 2918–2925. [Google Scholar] [PubMed]
- Lubin, E.; Mechlis-Frish, S.; Zatz, S.; Shimoni, A.; Segal, K.; Avraham, A.; Levy, R.; Feinmesser, R. Serum thyroglobulin and iodine-131 whole-body scan in the diagnosis and assessment of treatment for metastatic differentiated thyroid carcinoma. J. Nucl. Med. 1994, 35, 257–262. [Google Scholar] [PubMed]
- Franceschi, M.; Kusi’c, Z.; Franceschi, D.; Lukinac, L.; Roncevi’c, S. Thyroglobulin determination, neck ultrasonography and iodine-131 whole-body scintigraphy in differentiated thyroid carcinoma. J. Nucl. Med. 1996, 37, 446–451. [Google Scholar] [PubMed]
- Filesi, M.; Signore, A.; Ventroni, G.; Melacrinis, F.F.; Ronga, G. Role of initial iodine-131 whole-body scan and serum thyroglobulin in differentiated thyroid carcinoma metastases. J. Nucl. Med. 1998, 39, 1542–1546. [Google Scholar] [PubMed]
- Chen, L.; Luo, Q.; Shen, Y.; Yu, Y.; Yuan, Z.; Lu, H.; Zhu, R. Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J. Nucl. Med. 2008, 49, 1952–1957. [Google Scholar]
- Aide, N.; Heutte, N.; Rame, J.P.; Rousseau, E.; Loiseau, C.; Henry-Amar, M.; Bardet, S. Clinical relevance of single-photon emission computed tomography/computed tomography of the neck and thorax in post-ablation (131) I scintigraphy for thyroid cancer. J. Clin. Endocrinol. Metab. 2009, 94, 2075–2084. [Google Scholar]
- Avram, A.M.; Fig, L.M.; Frey, K.A.; Gross, M.D.; Wong, K.K. Preablation 131-I Scans With SPECT/CT in Postoperative Thyroid Cancer Patients: What Is the Impact on Staging? J. Clin. Endocrinol. Metab. 2013, 98, 1163–1171. [Google Scholar]
- Avram, A.M.; Esfandiari, N.H.; Wong, K.K. Preablation 131-I Scans with SPECT/CT Contribute to Thyroid Cancer Risk Stratification and 131-I Therapy Planning. J. Clin. Endocrinol. Metab. 2015, 100, 1895–1902. [Google Scholar]
- Tharp, K.; Israel, O.; Hausmann, J.; Bettman, L.; Martin, W.H.; Daitzchman, M.; Sandler, M.P.; Delbeke, D. Impact of 131ISPECT/CT images obtained with an integrated system in the follow-up of patients with thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, H.L.; Li, J.N.; Zou, R.J.; Gu, Z.H.; Wu, J.C. The role of single-photon emission computed tomography/computed tomography for precise localization of metastases in patients with differentiated thyroid cancer. Clin. Imaging 2009, 33, 49–54. [Google Scholar] [PubMed]
- Kohlfuerst, S.; Igerc, I.; Lobnig, M.; Gallowitsch, H.J.; Gomez-Segovia, I.; Matschnig, S.; Mayr, J.; Mikosch, P.; Beheshti, M.; Lind, P. Posttherapeutic 131I SPECT-CT offers high diagnostic accuracy when the findings on conventional planar imaging are inconclusive and allows a tailored patient treatment regimen. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Linke, R.; Uder, M.; Kuwert, T. Five months’ follow-up of patients with and without iodine-positive lymph node metastases of thyroid carcinoma as disclosed by 131I-SPECT/CT at the first radioablation. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 699–705. [Google Scholar] [CrossRef]
- Mustafa, M.; Kuwert, T.; Weber, K.; Knesewitsch, P.; Negele, T.; Haug, A.; Linke, R.; Bartenstein, P.; Schmidt, D. Regional lymphnode involvement in T1 papillary thyroid carcinoma: A bicentric prospective SPECT/CT study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1462–1466. [Google Scholar]
- Ciappuccini, R.; Heutte, N.; Trzepla, G.; Rame, J.-P.; Vaur, D.; Aide, N.; Bardet, S. Postablation 131I scintigraphy with neck and thorax SPECT–CT and stimulated serum thyroglobulin level predict the outcome of patients with differentiated thyroid cancer. Eur. J. Endocrinol. 2011, 164, 961–969. [Google Scholar] [CrossRef]
- Spanu, A.; Nuvoli, S.; Marongiu, A.; Gelo, I.; Mele, L.; De Vito, A.; Rondini, M.; Madeddu, G. The Diagnostic Usefulness of 131I-SPECT/CT at Both Radioiodine Ablation and during Long-Term Follow-Up in Patients Thyroidectomized for Differentiated Thyroid Carcinoma: Analysis of Tissue Risk Factors Ascertained at Surgery and Correlated with Metastasis Appearance. Diagnostics 2021, 11, 1504. [Google Scholar] [CrossRef]
- Spanu, A.; Solinas, M.E.; Chessa, F.; Sanna, D.; Nuvoli, S.; Madeddu, G. 131I SPECT/CT in the Follow-up of Differentiated Thyroid Carcinoma: Incremental Value versus Planar Imaging. J. Nucl. Med. 2009, 50, 184–190. [Google Scholar] [CrossRef]
- Xue, Y.-L.; Qiu, Z.-L.; Song, H.-J.; Luo, Q.-Y. Value of 131I SPECT/CT for the evaluation of differentiated thyroid cancer: A systematic review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 768–778. [Google Scholar]
- Spanu, A.; Nuvoli, S.; Gelo, I.; Mele, L.; Piras, B.; Madeddu, G. Role of Diagnostic 131I SPECT/CT in Long-Term Follow-up of Patients with Papillary Thyroid Microcarcinoma. J. Nucl. Med. 2018, 59, 1510–1515. [Google Scholar]
- Spanu, A.; Nuvoli, S.; Marongiu, A.; Gelo, I.; Mele, L.; Piras, B.; Madeddu, G. Neck lymph node metastasis detection in patients with differentiated thyroid carcinoma (DTC) in long-term follow-up: A 131I-SPECT/CT study. BMC Cancer 2020, 20, 239. [Google Scholar]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.A.; Wiersinga, W. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [PubMed]
- Duh, Q.Y. Thyroid cancer in Graves disease: Incidental cancer versus clinical cancer. Ann. Surg. Oncol. 2004, 11, 356–357. [Google Scholar] [PubMed]
- Ippolito, S.; Piantanida, E.; Tanda, M.L.; Caturegli, P. Graves’ disease insights from a review of the Johns Hopkins surgical pathology archive. J. Endocrinol. Investig. 2020, 43, 1519–1522. [Google Scholar] [CrossRef]
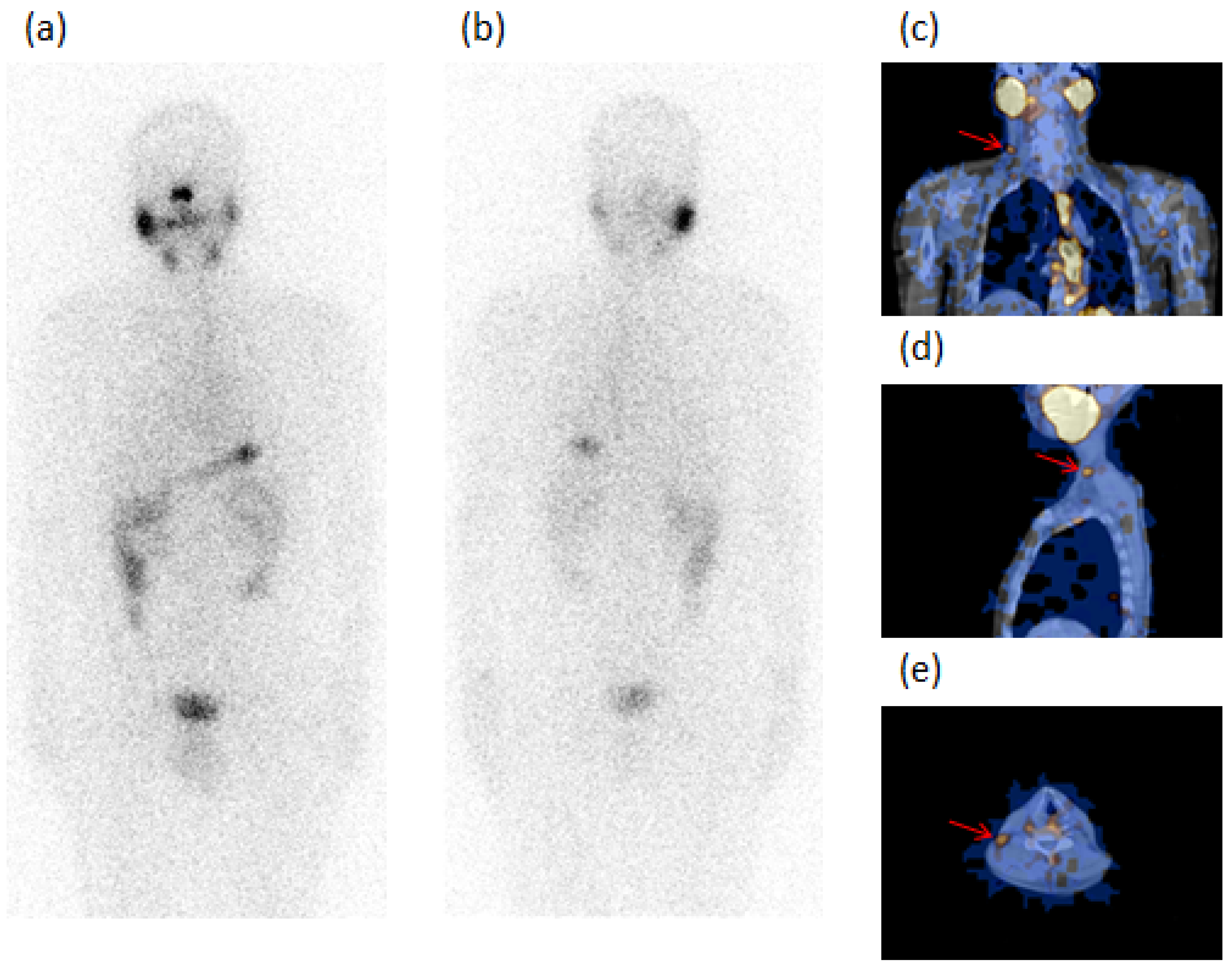
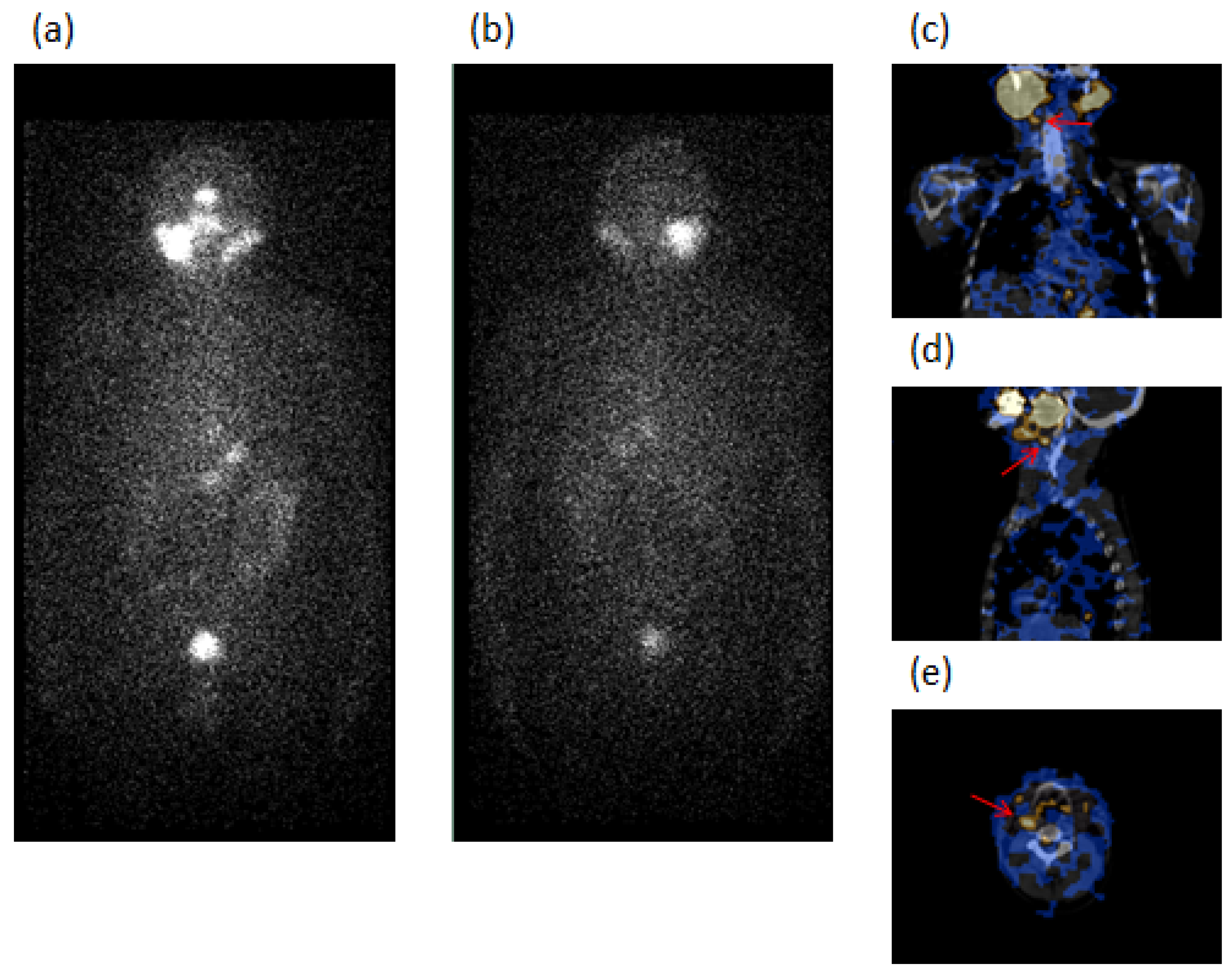
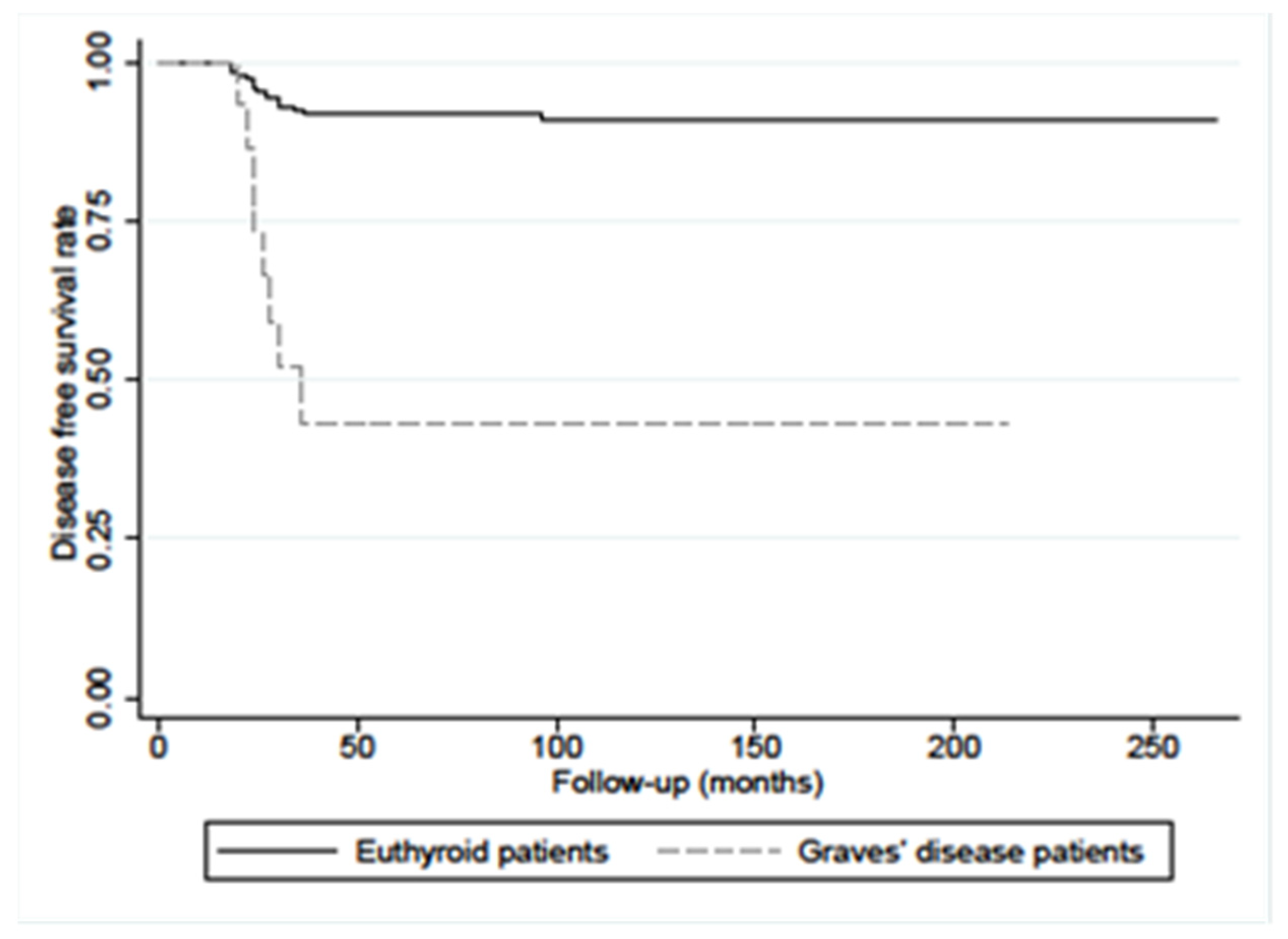
| PC Patients with GD (33 Cases) | PC Patients without GD as Controls (312 Cases) | p-Value | |
|---|---|---|---|
| Age (years) | 26 (78.8%) < 55; 7 (21.2%) ≥ 55 | 217 (69.5) < 55; 95 (30.5%) ≥ 55 | 0.269 |
| Age (years), mean ± SD | 45.1 ± 11.3 (median 45) | 48.3 ± 9.1 (median 48) | 0.0674 |
| Sex (F/M) | 18 (54.5%)/15 (45.5%) | 216 (69.2%)/96 (30.8%) | 0.09 |
| Histology | 28 (84.8%) PC classic variant 5 (15.2%) PC follicular variant | 264 (84.6%) PC classic variant 48 (15.4%) PC follicular variant | 0.972 |
| Tumor size (mm) | 18 (54.5%) ≤ 10/15 (45.5%) > 10 | 164 (52.6%) ≤ 10/148 (47.4%) > 10 | 0.828 |
| Tumor size (mm) mean ± SD | 10.85 ± 7.59 | 11.23 ± 8.90 | 0.813 |
| PC as incidental findings | 15 (45.5%) | 31 (10%) | <0.001 |
| Multifocality | 6 (18.2%) | Absent | <0.001 |
| Minimal extrathyroid extension (mETE) | 3 (9%) | Absent | 0.001 |
| Neck lymph node (LN) metastasis | 0.009 | ||
| N0 | 31 (94%) | 312 (100%) | |
| N1a | 0 | 0 | |
| N1b | 2 (6%) | 0 (0%) | |
| Distant metastasis | Absent | Absent | 1 |
| Exophthalmos | 6 (18.2%) | Absent | <0.001 |
| TRAbs | available in 15 (45.4%) cases, all positive | Not assayed | NA |
| Risk stratification | 0.003 | ||
| High risk (H) | 2 (6%) | 0 | |
| Low risk (L) | 19 (57.6%) | 148 (47.4%) | |
| Very low risk (VL) | 12 (36.4%) | 164 (52.6%) |
| At Surgery | At Follow-Up | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients n. | Sex | Age | Clinical Diagnosis | Histology | Size (mm) | Focality | ETE | Neck LN and Distant Metastasis | Risk Stratification | TNM | Exophthalmos (E) | TRAbs | Planar WBS (n. foci) | SPECT/CT (n. foci) | Tg (ng/mL) | AbTg (IU/mL) |
| GROUP 1 | ||||||||||||||||
| 1 | F | 27 | DTH-ni | PC | 7 | unifocal | VL | T1aN0M0 | Ar | 0 | 1 LTC (oc) | und | absent | |||
| 2 | F | 40 | MNG-i | PC | 5 | unifocal | VL | T1aN0M0 | Ar | 0 | 1 SM (oc) | 0.8 | absent | |||
| 3 | M | 45 | DTH-i | PC | 8 | unifocal | VL | T1aN0M0 | E | Pos | 0 | 1 LTC (oc) + 1 SM (oc) | 2.1 | absent | ||
| 4 | F | 46 | DTH-i | PC | 6 | unifocal | VL | T1aN0M0 | Ar | 0 | 1 LTC (oc) | und | absent | |||
| 5 | M | 48 | MNG-i | PC | 4 | unifocal | VL | T1aN0M0 | Ar | 0 | 1 LTC (oc) | und | absent | |||
| 6 | M | 45 | DTH-ni | PC | 37 | unifocal | L | T2N0M0 | Ar | 2 residues + 1 residue (W) | 2 residues + 1 PT (W) | und | absent | |||
| 7 | F | 27 | MNG-ni | PC | 18 | unifocal | L | T1bN0M0 | Pos | 0 | 2 LTC (oc) | und | absent | |||
| 8 | M | 59 | MNG-ni | PC | 11 | unifocal | L | T1bN0M0 | Pos | 2 unclear | 2 LTC | 1.9 | absent | |||
| 9 | M | 64 | DTH-ni | PC | 11 | unifocal | L | T1bN0M0 | Ar | 0 | 1 SM (oc) | und | 143 | |||
| 10 | M | 67 | DTH-ni | PC/FV | 11 | unifocal | L | T1bN0M0 | Pos | 0 | 1 SM (oc) | und | absent | |||
| 11 | M | 40 | DTH-ni | PC | 20 | unifocal | Neck LN | H | T1bN1bM0 | E | Pos | 0 | 1 LTC (oc) | und | absent | |
| 12 | M | 51 | MNG-ni | PC/FV | 20 | unifocal | Neck LN | H | T1bN1bM0 | Ar | 5 lung | 5 lung | 33 | absent | ||
| 13 | F | 45 | MNG-i | PC | 7 | unifocal | mETE | L | T1aN0M0 | E | Pos | 0 | 1 SM (oc) | 0.8 | absent | |
| 14 | M | 37 | MNG-ni | PC/FV | 20/10 | multifocal | mETE | L | T1bN0M0 | Ar | 0 | 1 LTC (oc) | 1.2 | absent | ||
| GROUP 2 | ||||||||||||||||
| 15 | F | 42 | MNG-i | PC | 2 | unifocal | VL | T1aN0M0 | Ar | 0 | 0 | und | absent | |||
| 16 | F | 38 | DTH-i | PC | 3 | unifocal | VL | T1aN0M0 | Ar | 0 | 0 | und | absent | |||
| 17 | M | 43 | DTH-i | PC | 4 | unifocal | VL | T1aN0M0 | Pos | 0 | 0 | und | absent | |||
| 18 | M | 65 | DTH-i | PC/FV | 8 | unifocal | VL | T1aN0M0 | E | Pos | 0 | 0 | und | absent | ||
| 19 | F | 44 | DTH-i | PC | 4 | unifocal | VL | T1aN0M0 | Pos | 0 | 0 | und | absent | |||
| 20 | F | 54 | DTH-i | PC | 6 | unifocal | VL | T1aN0M0 | Ar | 0 | 0 | und | absent | |||
| 21 | M | 59 | MNG-i | PC | 7 | unifocal | VL | T1aN0M0 | E | Pos | 0 | 0 | und | absent | ||
| 22 | M | 38 | DTH-ni | PC | 20 | unifocal | L | T1bN0M0 | Ar | 0 | 0 | und | absent | |||
| 23 | F | 50 | DTH-ni | PC | 25 | unifocal | L | T1bN0M0 | Pos | 0 | 0 | und | absent | |||
| 24 | F | 21 | DTH-ni | PC/FV | 15 | unifocal | L | T1bN0M0 | Ar | 0 | 0 | und | absent | |||
| 25 | F | 48 | MNG-ni | PC | 12 | unifocal | L | T1bN0M0 | Ar | 0 | 1 residue | und | absent | |||
| 26 | F | 38 | MNG-ni | PC | 11 | unifocal | L | T1bN0M0 | Ar | 0 | 0 | und | absent | |||
| 27 | F | 58 | DTH-ni | PC | 11 | unifocal | L | T1bN0M0 | Ar | 0 | 1 residue | und | absent | |||
| 28 | F | 43 | DTH-ni | PC | 12 | unifocal | mETE | L | T1bN0M0 | Ar | 0 | 0 | und | absent | ||
| 29 | M | 22 | MNG-ni | PC | 10/8 | multicentric | L | T1aN0M0 | Pos | 0 | 2 residues | und | absent | |||
| 30 | F | 46 | MNG-i | PC | 5/2.5 | multicentric | L | T1aN0M0 | E | Pos | 0 | 0 | und | absent | ||
| 31 | F | 45 | MNG-i | PC | 2/2 | multicentric | L | T1aN0M0 | Pos | 0 | 0 | und | absent | |||
| 32 | M | 55 | MNG-i | PC | 8/2 | multicentric | L | T1aN0M0 | Pos | 0 | 2 residues | und | absent | |||
| 33 | F | 40 | MNG-ni | PC | 8/2/2 | multicentric | L | T1aN0M0 | Ar | 0 | 0 | und | absent | |||
| At Surgery | At Follow-Up | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients n. | Sex | Age | Clinical Diagnosis | Histology | Size (mm) | Focality | ETE | Neck LN and Distant Metastasis | Risk Stratification | TNM | Planar WBS (n. foci) | SPECT/CT (n. foci) | Tg (ng/mL) | AbTg (IU/mL) |
| 1 | F | 44 | STN-ni | PC/FV | 17 | unifocal | absent | absent | L | T1bN0M0 | 1 unclear | 1 PT | 2.6 | absent |
| 2 | F | 34 | STN-ni | PC | 30 | unifocal | absent | absent | L | T2N0M0 | 0 | 1 LTC (oc) | und | absent |
| 3 | F | 47 | MNG-ni | PC/FV | 12 | unifocal | absent | absent | L | T1bN0M0 | 0 | 1 LTC (oc) | und | absent |
| 4 | F | 34 | MNG-ni | PC | 15 | unifocal | absent | absent | L | T1bN0M0 | 1 unclear | 1 LTC | 6 | absent |
| 5 | F | 71 | MNG-ni | PC | 11 | unifocal | absent | absent | L | T1bN0M0 | 0 | 1 PT (oc) | 0.9 | absent |
| 6 | F | 40 | STN-ni | PC | 20 | unifocal | absent | absent | L | T1bN0M0 | 0 | 1 PT (oc) | und | absent |
| 7 | M | 54 | STN-ni | PC | 11 | unifocal | absent | absent | L | T1bN0M0 | 0 | 1 PT (oc) | 1.1 | absent |
| 8 | F | 53 | MNG-i | PC | 5 | unifocal | absent | absent | VL | T1aN0M0 | 1 unclear | 1 PT | 13 | absent |
| 9 | F | 28 | MNG-i | PC | 5 | unifocal | absent | absent | VL | T1aN0M0 | 0 | 1 SM (oc) | 2.3 | absent |
| 10 | F | 44 | MNG-i | PC | 5 | unifocal | absent | absent | VL | T1aN0M0 | 0 | 1 SM (oc) | 3.2 | absent |
| 11 | F | 28 | MNG-i | PC | 4 | unifocal | absent | absent | VL | T1aN0M0 | 1 unclear | 1 SM | 2.6 | absent |
| 12 | F | 34 | STN-ni | PC | 12 | unifocal | absent | absent | L | T1bN0M0 | 0 | 1 LTC (oc) | 3 | absent |
| 13 | F | 42 | MNG-ni | PC/FV | 12 | unifocal | absent | absent | L | T1bN0M0 | 1 unclear | 1 LTC | und | absent |
| 14 | F | 38 | STN-ni | PC/FV | 15 | unifocal | absent | absent | L | T1bN0M0 | 1 unclear | 1 LTC | und | absent |
| 15 | F | 46 | MNG-i | PC | 5 | unifocal | absent | absent | VL | T1aN0M0 | 0 | 2 LTC (oc) + 1 SC (oc) | und | absent |
| 16 | F | 64 | MNG-i | PC | 2 | unifocal | absent | absent | VL | T1aN0M0 | 2 residues | 2 residues + 1 SM (oc) | und | absent |
| 17 | F | 52 | MNG-i | PC/FV | 2 | unifocal | absent | absent | VL | T1aN0M0 | 1 residue + 1unclear | 1 residue + 1 LTC | 6 | absent |
| 18 | M | 21 | STN-ni | PC | 20 | unifocal | absent | absent | L | T1bN0M0 | 2 unclear | 2 M | 9.6 | absent |
| 19 | F | 60 | STN-ni | PC/FV | 15 | unifocal | absent | absent | L | T1bN0M0 | 2 unclear | 1 LTC + 1 PV | 4.2 | absent |
| 20 | F | 61 | MNG-ni | PC | 11 | unifocal | absent | absent | L | T1bN0M0 | 1 unclear | 1 SC + 1 SM (oc) | und | absent |
| 21 | F | 62 | MNG-ni | PC | 5 | unifocal | absent | absent | VL | T1aN0M0 | 1 unclear | 1 LTC + 1 PT (oc) | 6.5 | absent |
| Variables | OR (95% CI) | p-Value | aOR (95% CI) | p-Value |
|---|---|---|---|---|
| Age (years) | 0.97 (0.94–1.01) | 0.122 | ||
| Age < 55 years | 1.47 (0.64–3.35) | 0.361 | ||
| Male gender | 1.60 (0.75–3.43) | 0.226 | ||
| PC follicular variant | 2.09 (0.92–4.76) | 0.078 | 2.31 (0.94–5.67) | 0.067 |
| Tumor size (mm) | 1.01 (0.97–1.05) | 0.547 | ||
| Microcarcinoma (≤10 mm) | 0.56 (0.28–1.15) | 0.114 | ||
| Multifocality | 1.79 (0.20–15.80) | 0.599 | ||
| Minimal extrathyroid extension (mETE) | 18.72 (1.65–212.1) | 0.018 | 2.62 (0.20–33.53) | 0.459 |
| Neck lymph node (LN) metastasis | 1 | |||
| Graves’ disease | 10.2 (4.50–23.19) | <0.001 | 9.79 (4.10–23.35) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marongiu, A.; Nuvoli, S.; De Vito, A.; Rondini, M.; Spanu, A.; Madeddu, G. A Comparative Follow-Up Study of Patients with Papillary Thyroid Carcinoma Associated or Not with Graves’ Disease. Diagnostics 2022, 12, 2801. https://doi.org/10.3390/diagnostics12112801
Marongiu A, Nuvoli S, De Vito A, Rondini M, Spanu A, Madeddu G. A Comparative Follow-Up Study of Patients with Papillary Thyroid Carcinoma Associated or Not with Graves’ Disease. Diagnostics. 2022; 12(11):2801. https://doi.org/10.3390/diagnostics12112801
Chicago/Turabian StyleMarongiu, Andrea, Susanna Nuvoli, Andrea De Vito, Maria Rondini, Angela Spanu, and Giuseppe Madeddu. 2022. "A Comparative Follow-Up Study of Patients with Papillary Thyroid Carcinoma Associated or Not with Graves’ Disease" Diagnostics 12, no. 11: 2801. https://doi.org/10.3390/diagnostics12112801
APA StyleMarongiu, A., Nuvoli, S., De Vito, A., Rondini, M., Spanu, A., & Madeddu, G. (2022). A Comparative Follow-Up Study of Patients with Papillary Thyroid Carcinoma Associated or Not with Graves’ Disease. Diagnostics, 12(11), 2801. https://doi.org/10.3390/diagnostics12112801







