Diagnosis of Cervical Cancer and Pre-Cancerous Lesions by Artificial Intelligence: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Search Strategy and Information Sources
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction and Synthesis
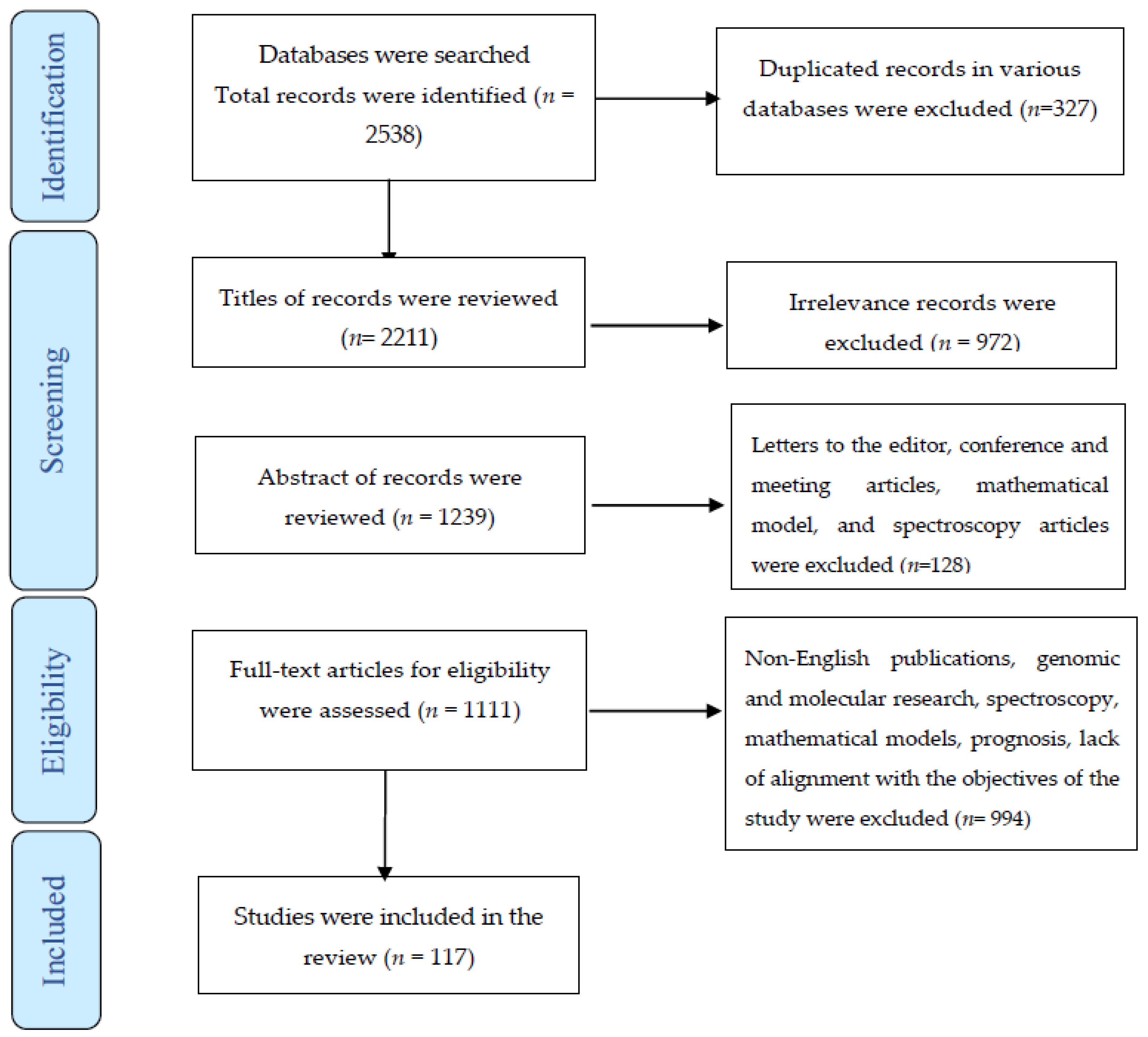
3. Results
3.1. Search Results
3.1.1. Application of AI for Cervical Cancer and Its Cost-Effectiveness
3.1.2. Application of AI in Predicting Cervical Cancer
3.1.3. Application of AI in Cervical Cancer Screening
3.1.4. Application of AI in Cytology for the Detection of Cervical Cancer
3.1.5. Application of AI in Colposcopy for the Detection of Cervical Cancer
4. Discussion
Limitations and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Volume 3. [Google Scholar]
- Takiar, R.; Nadayil, D.; Nandakumar, A. Projections of number of cancer cases in India (2010-2020) by cancer groups. Asian Pac. J. Cancer Prev. 2010, 11, 1045–1049. [Google Scholar]
- Salehiniya, H.; Momenimovahed, Z.; Allahqoli, L.; Momenimovahed, S.; Alkatout, I. Factors related to cervical cancer screening among Asian women. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6109–6122. [Google Scholar]
- Bogani, G.; Sopracordevole, F.; Di Donato, V.; Ciavattini, A.; Ghelardi, A.; Lopez, S.; Simoncini, T.; Plotti, F.; Casarin, J.; Serati, M.; et al. High-risk HPV-positive and -negative high-grade cervical dysplasia: Analysis of 5-year outcomes. Gynecol. Oncol. 2021, 161, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Canfell, K. Towards the global elimination of cervical cancer. Papillomavirus Res. 2019, 8, 100170. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Zarchi, M.; Allahqoli, L.; Nehmati, A.; Kashi, A.M.; Taghipour-Zahir, S.; Alkatout, I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health 2020, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Werner, C.L.; Darragh, T.M.; Guido, R.S.; Mathews, C.; Moscicki, A.-B.; Mitchell, M.M.; Schiffman, M.; Wentzensen, N.; Massad, L.S.; et al. ASCCP Colposcopy Standards: Role of Colposcopy, Benefits, Potential Harms, and Terminology for Colposcopic Practice. J. Low. Genit. Tract Dis. 2017, 21, 223–229. [Google Scholar] [CrossRef]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Brawley, O.W.; Wender, R.C. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA A Cancer J. Clin. 2017, 67, 100–121. [Google Scholar] [CrossRef]
- Valenti, G.; Vitale, S.G.; Tropea, A.; Biondi, A.; Laganà, A.S. Tumor markers of uterine cervical cancer: A new scenario to guide surgical practice? Updates Surg. 2017, 69, 441–449. [Google Scholar] [CrossRef]
- van den Helder, R.; Steenbergen, R.D.; van Splunter, A.P.; Mom, C.H.; Tjiong, M.Y.; Martin, I.; Rosier-van Dunné, F.M.; van der Avoort, I.A.; Bleeker, M.C.; van Trommel, N.E. HPV and DNA Methylation Testing in Urine for Cervical Intraepithelial Neoplasia and Cervical Cancer Detection. Clin. Cancer Res. 2022, 28, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- John, J.H.; Halder, A.; Purwar, S.; Pushpalatha, K.; Gupta, P.; Dubey, P. Study to determine efficacy of Urinary HPV 16 &18 detection in predicting premalignant and malignant lesions of Uterine Cervix. Int. J. Gynecol. Obstet. 2022. [Google Scholar] [CrossRef]
- Sravani, A.B.; Ghate, V.; Lewis, S. Human papillomavirus infection, cervical cancer and the less explored role of trace elements. Biol. Trace Element Res. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- WHO; IARC. Prevention of Cervical Cancer through Screening Using Visual Inspection with Acetic Acid (VIA) and Treatment with Cryotherapy. A Demonstration Project in Six African Countries: Malawi, Madagascar, Nigeria, Uganda, the United Republic of Tanzania, and Zambia. 2012; 1–40. [Google Scholar]
- Hou, X.; Shen, G.; Zhou, L.; Li, Y.; Wang, T.; Ma, X. Artificial Intelligence in Cervical Cancer Screening and Diagnosis. Front. Oncol. 2022, 12, 851367. [Google Scholar] [CrossRef] [PubMed]
- Catarino, R.; Schäfer, S.; Vassilakos, P.; Petignat, P.; Arbyn, M. Accuracy of combinations of visual inspection using acetic acid or lugol iodine to detect cervical precancer: A meta-analysis. BJOG: Int. J. Obstet. Gynaecol. 2017, 125, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, N.F.; Karimi-Zarchi, M.; Allahqoli, L.; Sekhavat, L.; Gitas, G.; Rahmani, A.; Fallahi, A.; Hassanlouei, B.; Alkatout, I. Accuracy of the Triple Test Versus Colposcopy for the Diagnosis of Premalignant and Malignant Cervical Lesions. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 3501–3507. [Google Scholar] [CrossRef]
- Nazari, Z.; Torabizadeh, G.; Khalilian, A.; Ghadami, S.; Karimi-Zarchi, M.; Allahqoli, L.; Gharacheh, M.; Salehiniya, H.; Alkatout, I. Is cryotherapy effective in all women with low-grade cervical intraepithelial neoplasia? Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4211–4218. [Google Scholar]
- D’Oria, O.; Corrado, G.; Laganà, A.S.; Chiantera, V.; Vizza, E.; Giannini, A. New Advances in Cervical Cancer: From Bench to Bedside. Int. J. Environ. Res. Public Health 2022, 19, 7094. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, L.; Wang, L.; Zhang, M.; Zhao, Z.; Fang, L.; Cong, S.; Zhou, M.; Wang, L. Significant variations in the cervical cancer screening rate in China by individual-level and geographical measures of socioeconomic status: A multilevel model analysis of a nationally representative survey dataset. Cancer Med. 2018, 7, 2089–2100. [Google Scholar] [CrossRef]
- Bedell, S.L.; Goldstein, L.S.; Goldstein, A.R.; Goldstein, A.T. Cervical Cancer Screening: Past, Present, and Future. Sex. Med. Rev. 2020, 8, 28–37. [Google Scholar] [CrossRef]
- Pollack, A.E.; Tsu, V.D. Preventing cervical cancer in low-resource settings: Building a case for the possible. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2005, 89 (Suppl. S2), S1–S3. [Google Scholar] [CrossRef] [PubMed]
- 24. Wang, A.C.; Wang, L.Q.; Li, J.; Li, M.X.; Tu, L.L.; Zhang, Y.X.; Liu, A.J. Artificial intelligence aided measurement of cervical squamous epithelial thickness and its correlation with cervical precancerous lesions. Zhonghua Bing Li Xue Za Zhi Chin. J. Pathol. 2021, 50, 339-43. [Google Scholar]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA A Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunović, H. Artificial intelligence in retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, M.; Hashimoto, K. Artificial intelligence in gynecologic cancers: Current status and future challenges – A systematic review. Artif. Intell. Med. 2021, 120, 102164. [Google Scholar] [CrossRef] [PubMed]
- Shanthi, P.; Hareesha, K.; Kudva, R. Automated detection and classification of cervical cancer using pap smear microscopic images: A comprehensive review and future perspectives. Eng. Sci. 2022, 19, 20–41. [Google Scholar]
- Bao, H.; Sun, X.; Zhang, Y.; Pang, B.; Li, H.; Zhou, L.; Wu, F.; Cao, D.; Wang, J.; Turic, B.; et al. The artificial intelligence-assisted cytology diagnostic system in large-scale cervical cancer screening: A population-based cohort study of 0.7 million women. Cancer Med. 2020, 9, 6896–6906. [Google Scholar] [CrossRef]
- Holmström, O.; Linder, N.; Kaingu, H.; Mbuuko, N.; Mbete, J.; Kinyua, F.; Törnquist, S.; Muinde, M.; Krogerus, L.; Lundin, M.; et al. Point-of-Care Digital Cytology With Artificial Intelligence for Cervical Cancer Screening in a Resource-Limited Setting. JAMA Netw. Open 2021, 4, e211740. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Jenny, J.; Isenegger, I.; Boon, M.E.; Husain, O.N. Consistency of a Double PAPNET Scan of Cervical Smears. Acta Cytol. 1997, 41, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Michelow, P.M.; Hlongwane, F.; Leiman, G. Simulation of Primary Cervical Cancer Screening by the PAPNET System in an Unscreened, High-Risk Community. Acta Cytol. 1997, 41, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Mango, L.J.; Valente, P.T. Neural Network–Assisted Analysis and Microscopic Rescreening in Presumed Negative Cervical Cytologic Smears. Acta Cytol. 1998, 42, 227–232. [Google Scholar] [CrossRef]
- Sherman, M.E.; Schiffman, M.; Herrero, R.; Kelly, D.; Bratti, C.; Mango, L.J.; Alfaro, M.; Hutchinson, M.L.; Mena, F.; Hildesheim, A.; et al. Performance of a semiautomated Papanicolaou smear screening system: Results of a population-based study conducted in Guanacaste, Costa Rica. Cancer 1998, 84, 273–280. [Google Scholar] [CrossRef]
- Nieminen, P.; Hakama, M.; Viikki, M.; Tarkkanen, J.; Anttila, A. Prospective and randomised public-health trial on neural network-assisted screening for cervical cancer in Finland: Results of the first year. Int. J. Cancer 2002, 103, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Suri, J.; Ali, M.; Sharma, V. Novel benchmark database of digitized and calibrated cervical cells for artificial intelligence based screening of cervical cancer. J. Ambient Intell. Humaniz. Comput. 2016, 7, 593–606. [Google Scholar] [CrossRef]
- Kudva, V.; Prasad, K.; Guruvare, S. Andriod Device-Based Cervical Cancer Screening for Resource-Poor Settings. J. Digit. Imaging 2018, 31, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Bell, D.; Antani, S.; Xue, Z.; Yu, K.; Horning, M.P.; Gachuhi, N.; Wilson, B.; Jaiswal, M.S.; Befano, B.; et al. An Observational Study of Deep Learning and Automated Evaluation of Cervical Images for Cancer Screening. JNCI J. Natl. Cancer Inst. 2019, 111, 923–932. [Google Scholar] [CrossRef]
- Sompawong, N.; Mopan, J.; Pooprasert, P.; Himakhun, W.; Suwannarurk, K.; Ngamvirojcharoen, J.; Vachiramon, T.; Tantibundhit, C. Automated Pap Smear Cervical Cancer Screening Using Deep Learning. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 7044–7048. [Google Scholar] [CrossRef]
- Hu, L.; Horning, M.P.; Banik, D.; Ajenifuja, O.K.; Adepiti, C.A.; Yeates, K.; Mtema, Z.; Wilson, B.; Mehanian, C. Deep learning-based image evaluation for cervical precancer screening with a smartphone targeting low resource settings—Engineering approach. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual International Conference, Montreal, QC, Canada, 20–24 July 2020; pp. 1944–1999. [Google Scholar]
- Sahoo, G.R.; Dey, R.; Das, N.; Ghosh, N.; Pradhan, A. Two dimensional multifractal detrended fluctuation analysis of low coherence images for diagnosis of cervical pre-cancer. Biomed. Phys. Eng. Express 2020, 6, 025011. [Google Scholar] [CrossRef]
- Saini, S.K.; Bansal, V.; Kaur, R.; Juneja, M. ColpoNet for automated cervical cancer screening using colposcopy images. Mach. Vis. Appl. 2020, 31, 1–15. [Google Scholar] [CrossRef]
- Sanyal, P.; Ganguli, P.; Barui, S. Performance characteristics of an artificial intelligence based on convolutional neural network for screening conventional Papanicolaou-stained cervical smears. Med. J. Armed Forces India 2019, 76, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Win, K.P.; Kitjaidure, Y.; Hamamoto, K.; Aung, T.M. Computer-Assisted Screening for Cervical Cancer Using Digital Image Processing of Pap Smear Images. Appl. Sci. 2020, 10, 1800. [Google Scholar] [CrossRef]
- Xiang, Y.; Sun, W.; Pan, C.; Yan, M.; Yin, Z.; Liang, Y. A novel automation-assisted cervical cancer reading method based on convolutional neural network. Biocybern. Biomed. Eng. 2020, 40, 611–623. [Google Scholar] [CrossRef]
- Xue, Z.; Novetsky, A.P.; Einstein, M.H.; Marcus, J.Z.; Befano, B.; Guo, P.; Demarco, M.; Wentzensen, N.; Long, L.R.; Schiffman, M.; et al. A demonstration of automated visual evaluation of cervical images taken with a smartphone camera. Int. J. Cancer 2020, 147, 2416–2423. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, S.; Yu, J.; Rao, G.; Xiao, Y.; Han, W.; Zhu, W.; Lv, X.; Li, N.; Cai, J.; et al. Robust whole slide image analysis for cervical cancer screening using deep learning. Nat. Commun. 2021, 12, 5639. [Google Scholar] [CrossRef]
- Tan, X.; Li, K.; Zhang, J.; Wang, W.; Wu, B.; Wu, J.; Li, X.; Huang, X. Automatic model for cervical cancer screening based on convolutional neural network: A retrospective, multicohort, multicenter study. Cancer Cell Int. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Cai, D.; Kong, Y.; Ye, H.; Ma, Z.; Lv, H.; Tuo, L.; Pan, Q.; Liu, Z.; Han, X. Cervical cytology screening facilitated by an artificial intelligence microscope: A preliminary study. Cancer Cytopathol. 2021, 129, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Liou, Y.A.; Lin, Y.J.; Chang, C.C.; Chu, P.H.; Lee, Y.C.; Wang, C.H.; Chao, T.K. Artificial intelligence-assisted fast screening cervical high grade squamous intraepithelial lesion and squamous cell carcinoma diagnosis and treatment planning. Sci. Rep. 2021, 11, 16244. [Google Scholar] [CrossRef]
- Wentzensen, N.; Lahrmann, B.; Clarke, M.A.; Kinney, W.; Tokugawa, D.; Poitras, N.; Locke, A.; Bartels, L.; Krauthoff, A.; Walker, J.; et al. Accuracy and Efficiency of Deep-Learning–Based Automation of Dual Stain Cytology in Cervical Cancer Screening. JNCI J. Natl. Cancer Inst. 2020, 113, 72–79. [Google Scholar] [CrossRef]
- Fu, L.; Xia, W.; Shi, W.; Cao, G.-X.; Ruan, Y.-T.; Zhao, X.-Y.; Liu, M.; Niu, S.-M.; Li, F.; Gao, X. Deep learning based cervical screening by the cross-modal integration of colposcopy, cytology, and HPV test. Int. J. Med. Inform. 2022, 159, 104675. [Google Scholar] [CrossRef]
- Kahng, J.; Kim, E.-H.; Kim, H.-G.; Lee, W. Development of a cervical cancer progress prediction tool for human papillomavirus-positive Koreans: A support vector machine-based approach. J. Int. Med. Res. 2015, 43, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Al-Wesabi, Y.M.S.; Choudhury, A.; Won, D. Classification of cervical cancer dataset. arXiv 2018, arXiv:1812.10383. [Google Scholar]
- Ahmed, M.; Kabir, M.M.J.; Kabir, M.; Hasan, M. Identification of the Risk Factors of Cervical Cancer Applying Feature Selection Approaches. In Proceedings of the 2019 3rd International Conference on Electrical, Computer & Telecommunication Engineering (ICECTE), Rajshahi, Bangladesh, 26–28 December 2019; pp. 201–204. [Google Scholar] [CrossRef]
- Alam, T.M.; Milhan, M.; Atif, M.; Wahab, A.; Mushtaq, M. Cervical Cancer Prediction through Different Screening Methods using Data Mining. Int. J. Adv. Comput. Sci. Appl. 2019, 10, 388–396. [Google Scholar] [CrossRef]
- Chen, H.; Yang, L.; Li, L.; Li, M.; Chen, Z. An efficient cervical disease diagnosis approach using segmented images and cytology reporting. Cogn. Syst. Res. 2019, 58, 265–277. [Google Scholar] [CrossRef]
- Geetha, R.; Sivasubramanian, S.; Kaliappan, M.; Vimal, S.; Annamalai, S. Cervical Cancer Identification with Synthetic Minority Oversampling Technique and PCA Analysis using Random Forest Classifier. J. Med. Syst. 2019, 43, 1–19. [Google Scholar] [CrossRef]
- Nithya, B.; Ilango, V. Evaluation of machine learning based optimized feature selection approaches and classification methods for cervical cancer prediction. SN Appl. Sci. 2019, 1, 641. [Google Scholar] [CrossRef]
- Alsmariy, R.; Healy, G.; Abdelhafez, H. Predicting cervical cancer using machine learning methods. Int. J. Adv. Comput. Sci. Appl. 2020, 11, 173–184. [Google Scholar] [CrossRef]
- Asadi, F.; Salehnasab, C.; Ajori, L. Supervised Algorithms of Machine Learning for the Prediction of Cervical Cancer. J. Biomed. Phys. Eng. 2020, 10, 513–522. [Google Scholar] [CrossRef]
- Doornewaard, H.; van de Seijp, H.; Woudt, J.M.; van der Graaf, Y.; Tweel, J.G.V.D. Negative Cervical Smears Before CIN 3/Carcinoma. Acta Cytol. 1997, 41, 74–78. [Google Scholar] [CrossRef]
- Giovagnoli, M.R.; Cenci, M.; Olla, S.V.; Vecchione, A. Cervical False Negative Cases Detected by Neural Network–Based Technology. Critical review of cytologic errors. Acta Cytol. 2002, 46, 1105–1109. [Google Scholar] [CrossRef]
- Bao, H.; Bi, H.; Zhang, X.; Zhao, Y.; Dong, Y.; Luo, X.; Zhou, D.; You, Z.; Wu, Y.; Liu, Z.; et al. Artificial intelligence-assisted cytology for detection of cervical intraepithelial neoplasia or invasive cancer: A multicenter, clinical-based, observational study. Gynecol. Oncol. 2020, 159, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, H.; Wang, X.; Wang, Q.; Wang, L.; Heng, P.-A. Dual-path network with synergistic grouping loss and evidence driven risk stratification for whole slide cervical image analysis. Med. Image Anal. 2021, 69, 101955. [Google Scholar] [CrossRef]
- Sheela Shiney, T.S.; Rose, R.J. Deep Auto Encoder Based Extreme Learning System for Automatic Segmentation of Cervical Cells. IETE J. Res. 2021. [Google Scholar] [CrossRef]
- Bai, B.; Du, Y.; Liu, P.; Sun, P.; Li, P.; Lv, Y. Detection of cervical lesion region from colposcopic images based on feature reselection. Biomed. Signal Process. Control 2020, 57, 101785. [Google Scholar] [CrossRef]
- Miyagi, Y.; Takehara, K.; Nagayasu, Y.; Miyake, T. Application of deep learning to the classification of uterine cervical squamous epithelial lesion from colposcopy images combined with HPV types. Oncol. Lett. 2019, 19, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Nikookar, E.; Naderi, E.; Rahnavard, A. Cervical Cancer Prediction by Merging Features of Different Colposcopic Images and Using Ensemble Classifier. J. Med. Signals Sens. 2021, 11, 67–78. [Google Scholar] [PubMed]
- Peng, G.; Dong, H.; Liang, T.; Li, L.; Liu, J. Diagnosis of cervical precancerous lesions based on multimodal feature changes. Comput. Biol. Med. 2021, 130, 104209. [Google Scholar] [CrossRef]
- Viñals, R.; Vassilakos, P.; Rad, M.; Undurraga, M.; Petignat, P.; Thiran, J.-P. Using Dynamic Features for Automatic Cervical Precancer Detection. Diagnostics 2021, 11, 716. [Google Scholar] [CrossRef]
- Meng, Z.; Zhao, Z.; Li, B.; Su, F.; Guo, L. A Cervical Histopathology Dataset for Computer Aided Diagnosis of Precancerous Lesions. IEEE Trans. Med. Imaging 2021, 40, 1531–1541. [Google Scholar] [CrossRef]
- Diniz, D.N.; Vitor, R.F.; Bianchi, A.G.C.; Delabrida, S.; Carneiro, C.M.; Ushizima, D.M.; de Medeiros, F.N.S.; Souza, M.J.F. An ensemble method for nuclei detection of overlapping cervical cells. Expert Syst. Appl. 2021, 185, 115642. [Google Scholar] [CrossRef]
- Kruczkowski, M.; Drabik-Kruczkowska, A.; Marciniak, A.; Tarczewska, M.; Kosowska, M.; Szczerska, M. Predictions of cervical cancer identification by photonic method combined with machine learning. Sci. Rep. 2022, 12, 3762. [Google Scholar] [CrossRef] [PubMed]
- Elakkiya, R.; Teja, K.S.S.; Deborah, L.J.; Bisogni, C.; Medaglia, C. Imaging based cervical cancer diagnostics using small object detection - generative adversarial networks. Multimed. Tools Appl. 2021, 81, 191–207. [Google Scholar] [CrossRef]
- Dong, Y.; Bai, J.; Zhang, Y.; Shang, G.; Zhao, Y.; Li, S.; Yan, N.; Hao, S.; Zhang, W. Automated Quantitative Cytology Imaging Analysis System in Cervical Cancer Screening in Shanxi Province, China. Cancer Clin. Oncol. 2017, 6, p51. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Lee, H.; Lee, S.; Song, J.-Y.; Lee, J.-K.; Lee, N.-W. Role of Artificial Intelligence Interpretation of Colposcopic Images in Cervical Cancer Screening. Healthcare 2022, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ahmed, K.; Bui, F.M.; Paul, B.K.; Ibrahim, S.M.; Quinn, J.M.; Moni, M.A. Machine learning-based statistical analysis for early stage detection of cervical cancer. Comput. Biol. Med. 2021, 139, 104985. [Google Scholar] [CrossRef]
- Luo, W. Predicting Cervical Cancer Outcomes: Statistics, Images, and Machine Learning. Front. Artif. Intell. 2021, 4. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.; Gordon, T. A general cardiovascular risk profile: The Framingham study. Am. J. Cardiol. 1976, 38, 46–51. [Google Scholar] [CrossRef]
- Dillak, R.Y.; Manulangga, G.C.; Lalandos, J.L. Early warning system for cervical cancer diagnosis using ridge polynomial neural network and chaos optimization algorithm. J. Theor. Appl. Inf. Technol. 2018, 96, 1989–1998. [Google Scholar]
- Garg, S.K.; Kapil, M. A Cervical Cancer Prediction Model Using REPTree Classifier. J. Comput. Theor. Nanosci. 2019, 16, 4438–4442. [Google Scholar] [CrossRef]
- Kar, S.; Majumder, D.D. A Novel Approach of Mathematical Theory of Shape and Neuro-Fuzzy Based Diagnostic Analysis of Cervical Cancer. Pathol. Oncol. Res. 2019, 25, 777–790. [Google Scholar] [CrossRef]
- Tian, R.; Cui, Z.; He, D.; Tian, X.; Gao, Q.; Ma, X.; Yang, J.; Wu, J.; Das, B.C.; Severinov, K.; et al. Risk stratification of cervical lesions using capture sequencing and machine learning method based on HPV and human integrated genomic profiles. Carcinogenesis 2019, 40, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Hussain, E.; Mahanta, L.B.; Das, C.R.; Talukdar, R.K. A comprehensive study on the multi-class cervical cancer diagnostic prediction on pap smear images using a fusion-based decision from ensemble deep convolutional neural network. Tissue Cell 2020, 65, 101347. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.F.; Attique, M.; Son, Y. Data-Driven Cervical Cancer Prediction Model with Outlier Detection and Over-Sampling Methods. Sensors 2020, 20, 2809. [Google Scholar] [CrossRef]
- Weegar, R.; Sundström, K. Using machine learning for predicting cervical cancer from Swedish electronic health records by mining hierarchical representations. PLoS ONE 2020, 15, e0237911. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, S.; Ahmed, R.; Rehana, H.; Chakraborty, S.; Islam, S.; Bhuiyan, T. Machine learning to reveal an astute risk predictive framework for Gynecologic Cancer and its impact on women psychology: Bangladeshi perspective. BMC Bioinform. 2021, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, Q.M.; Ahmad, M. An Enhanced Ensemble Diagnosis of Cervical Cancer: A Pursuit of Machine Intelligence Towards Sustainable Health. IEEE Access 2021, 9, 12374–12388. [Google Scholar] [CrossRef]
- Jahan, S.; Islam, M.D.S.; Islam, L.; Rashme, T.Y.; Prova, A.A.; Paul, B.K.; Mosharof, M.K. Automated invasive cervical cancer disease detection at early stage through suitable machine learning model. SN Appl. Sci. 2021, 3, 1–17. [Google Scholar] [CrossRef]
- Khan, I.U.; Aslam, N.; Alshehri, R.; Alzahrani, S.; Alghamdi, M.; Almalki, A.; Balabeed, M. Cervical Cancer Diagnosis Model Using Extreme Gradient Boosting and Bioinspired Firefly Optimization. Sci. Program. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Mehmood, M.; Rizwan, M.; Ml, M.G.; Abbas, S. Machine Learning Assisted Cervical Cancer Detection. Front. Public Health 2021, 9, 2024. [Google Scholar] [CrossRef]
- Mudawi, N.A.; Alazeb, A. A Model for Predicting Cervical Cancer Using Machine Learning Algorithms. Sensors 2022, 22, 4132. [Google Scholar] [CrossRef]
- Suman, S.K.; Hooda, N. Predicting risk of Cervical Cancer: A case study of machine learning. J. Stat. Manag. Syst. 2019, 22, 689–696. [Google Scholar] [CrossRef]
- Maxim, L.D.; Niebo, R.; Utell, M.J. Screening tests: A review with examples. Inhal. Toxicol. 2014, 26, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Obuchowski, N.A.; Graham, R.J.; Baker, M.E.; Powell, K.A. Ten criteria for effective screening: Their application to multislice CT screening for pulmonary and colorectal cancers. Am. J. Roentgenol. (1976) 2001, 176, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Sharma, D.; Mishra, S. (Eds.) Computer based automatic segmentation of pap smear cells for cervical cancer detection. In Proceedings of the 2018 5th International Conference on Signal Processing and Integrated Networks (SPIN), Delhi, India, 22–23 February 2018. [Google Scholar]
- Yang, L.; Tuzel, O.; Chen, W.; Meer, P.; Salaru, G.; Goodell, L.A.; Foran, D.J. PathMiner: A Web-based tool for computer-assisted diagnostics in pathology. IEEE Trans. Inf. Technol. Biomed. A Publ. IEEE Eng. Med. Biol. Soc. 2009, 13, 291–299. [Google Scholar] [CrossRef]
- Sherman, M.E.; Mango, L.J.; Kelly, D.; Paull, G.; Ludin, V.; Copeland, C.; Solomon, D.; Schiffman, M.H. PAPNET analysis of reportedly negative smears preceding the diagnosis of a high-grade squamous intraepithelial lesion or carcinoma. Mod. Pathol. 1994, 7, 578–581. [Google Scholar]
- Kok, M.R.; Boon, M.E. Consequences of neural network technology for cervical screening: Increase in diagnostic consistency and positive scores. Cancer 1996, 78, 112–117. [Google Scholar] [CrossRef]
- Cenci, M.; Nagar, C.; Giovagnoli, M.R.; Vecchione, A. The PAPNET system for quality control of cervical smears: Validation and limits. Anticancer Res. 1998, 17, 4731–4734. [Google Scholar]
- Kemp, R.A.; MacAulay, C.; Garner, D.; Palcic, B. Detection of Malignancy Associated Changes in Cervical Cell Nuclei Using Feed-Forward Neural Networks. Anal. Cell. Pathol. 1997, 14, 31–40. [Google Scholar] [CrossRef]
- Koss, L.G.; Sherman, M.E.; Cohen, M.B.; Anes, A.R.; Darragh, T.M.; Lemos, L.B.; McClellan, B.J.; Rosenthal, D.L.; Keyhani-Rofagha, S.; Schreiber, K.; et al. Significant reduction in the rate of false-negative cervical smears with neural network-based technology (PAPNET testing system). Hum. Pathol. 1997, 28, 1196–1203. [Google Scholar] [CrossRef]
- Keenan, S.; Diamond, J.; McCluggage, W.G.; Bharucha, H.; Thompson, D.; Bartels, P.H.; Hamilton, P.W. An automated machine vision system for the histological grading of cervical intraepithelial neoplasia (CIN). J. Pathol. 2000, 192, 351–362. [Google Scholar] [CrossRef]
- Dickman, E.D.; Doll, T.J.; Chiu, C.K.; Ferris, D.G. Identification of cervical neoplasia using a simulation of human vision. J. Low. Genit. Tract Dis. 2001, 5, 144–152. [Google Scholar] [PubMed]
- Parker, M.F.; Mooradian, G.C.; Okimoto, G.S.; O’Connor, D.M.; Miyazawa, K.; Saggese, S.J. Initial neural net construction for the detection of cervical intraepithelial neoplasia by fluorescence imaging. Am. J. Obstet. Gynecol. 2002, 187, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Boon, M.E.; Ouwerkerk-Noordam, E.; Suurmeijer, A.; Kok, L.P. Diagnostic Parameters in Liquid-Based Cervical Cytology Using a Coagulant Suspension Fixative. Acta Cytol. 2005, 49, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Dounias, G.; Bjerregaard, B.; Jantzen, J.; Tsakonas, A.; Ampazis, N.; Panagi, G.; Panourgias, E. Automated identification of cancerous smears using various competitive intelligent techniques. Oncol. Rep. 2006, 15, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Mat-Isa, N.A.; Mashor, M.Y.; Othman, N.H. An automated cervical pre-cancerous diagnostic system. Artif. Intell. Med. 2008, 42, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Crookes, D.; Eldin, O.S.; Wang, S.; Hamilton, P.; Diamond, J. Assisted Diagnosis of Cervical Intraepithelial Neoplasia (CIN). IEEE J. Sel. Top. Signal Process. 2009, 3, 112–121. [Google Scholar] [CrossRef][Green Version]
- Al-Batah, M.S.; Isa, N.A.M.; Klaib, M.F.; Al-Betar, M.A. Multiple Adaptive Neuro-Fuzzy Inference System with Automatic Features Extraction Algorithm for Cervical Cancer Recognition. Comput. Math. Methods Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Sokouti, B.; Haghipour, S.; Tabrizi, A.D. A framework for diagnosing cervical cancer disease based on feedforward MLP neural network and ThinPrep histopathological cell image features. Neural Comput. Appl. 2012, 24, 221–232. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, H.Y.; Kim, D.W. A study on development of automation diagnosis of liquid based cytology. Sains Malays. 2015, 44, 1729–1738. [Google Scholar]
- Kyrgiou, M.; Pouliakis, A.; Panayiotides, J.G.; Margari, N.; Bountris, P.; Valasoulis, G.; Paraskevaidi, M.; Bilirakis, E.; Nasioutziki, M.; Loufopoulos, A.; et al. Personalised management of women with cervical abnormalities using a clinical decision support scoring system. Gynecol. Oncol. 2016, 141, 29–35. [Google Scholar] [CrossRef]
- Hyeon, J.; Choi, H.-J.; Lee, B.D.; Lee, K.N. Diagnosing cervical cell images using pre-trained convolutional neural network as feature extractor. In Proceedings of the 2017 IEEE International Conference on Big Data and Smart Computing (BigComp) 2017, Jeju Island, Korea, 13–16 February 2017; pp. 390–393. [Google Scholar] [CrossRef]
- Abdoh, S.F.; Rizka, M.A.; Maghraby, F.A. Cervical Cancer Diagnosis Using Random Forest Classifier With SMOTE and Feature Reduction Techniques. IEEE Access 2018, 6, 59475–59485. [Google Scholar] [CrossRef]
- Arya, M.; Mittal, N.; Singh, G. Texture-based feature extraction of smear images for the detection of cervical cancer. IET Comput. Vis. 2018, 12, 1049–1059. [Google Scholar] [CrossRef]
- Aljakouch, K.; Hilal, Z.; Daho, I.; Schuler, M.; Krauß, S.D.; Yosef, H.K.; Dierks, J.; Mosig, A.; Gerwert, K.; El-Mashtoly, S.F. Fast and Noninvasive Diagnosis of Cervical Cancer by Coherent Anti-Stokes Raman Scattering. Anal. Chem. 2019, 91, 13900–13906. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwari, K.V.; Poornima, B. Cervical cancer cell identification & detection using fuzzy C mean and K nearest neighbor techniques. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 1080–1084. [Google Scholar]
- Lasyk, L.; Barbasz, J.; Żuk, P.; Prusaczyk, A.; Włodarczyk, T.; Prokurat, E.; Olszewski, W.; Bidziński, M.; Baszuk, P.; Gronwald, J. An evaluation of the construction of the device along with the software for digital archiving, sending the data, and supporting the diagnosis of cervical cancer. Contemp. Oncol./Współczesna Onkol. 2019, 23, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, T.; Huang, X.; Wang, X.; Li, C.; Jerwick, J.; Ning, Y.; Zeng, X.; Wang, B.; Wang, Y.; et al. Computer-Aided Diagnosis of Label-Free 3-D Optical Coherence Microscopy Images of Human Cervical Tissue. IEEE Trans. Biomed. Eng. 2019, 66, 2447–2456. [Google Scholar] [CrossRef]
- Moscon, L.M.; Macedo, N.D.; Nunes, C.S.M.; Boasquevisque, P.C.R.; de Andrade, T.U.; Endringer, D.C.; Lenz, D. Automated detection of anomalies in cervix cells using image analysis and machine learning. Comp. Clin. Pathol. 2018, 28, 177–182. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, C.; Bao, K.; Xu, C. Recognition and Clinical Diagnosis of Cervical Cancer Cells Based on our Improved Lightweight Deep Network for Pathological Image. J. Med. Syst. 2019, 43, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; He, J.; Chen, T.; Liu, Z.; Chen, D. Abnormal region detection in cervical smear images based on fully convolutional network. IET Image Process. 2019, 13, 583–590. [Google Scholar] [CrossRef]
- Konstandinou, C.; Kostopoulos, S.; Glotsos, D.; Pappa, D.; Ravazoula, P.; Michail, G.; Kalatzis, I.; Asvestas, P.; Lavdas, E.; Cavouras, D.; et al. GPU-enabled design of an adaptable pattern recognition system for discriminating squamous intraepithelial lesions of the cervix. Biomed. Eng./Biomed. Tech. 2019, 65, 315–325. [Google Scholar] [CrossRef]
- Ma, D.; Liu, J.; Li, J.; Zhou, Y. Cervical cancer detection in cervical smear images using deep pyramid inference with refinement and spatial-aware booster. IET Image Process. 2020, 14, 4717–4725. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, G.; Mu, C.; Guan, B.; Wang, M. Cervical Cancer Cell Detection Based on Deep Convolutional Neural Network. In Proceedings of the 2020 39th Chinese Control Conference (CCC), Shenyang, China, 27–29 July 2020. [Google Scholar] [CrossRef]
- Cao, L.; Yang, J.; Rong, Z.; Li, L.; Xia, B.; You, C.; Lou, G.; Jiang, L.; Du, C.; Meng, H.; et al. A novel attention-guided convolutional network for the detection of abnormal cervical cells in cervical cancer screening. Med. Image Anal. 2021, 73, 102197. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Zhou, J.; Zhang, C. Detection of cervical cells based on improved SSD network. Multimed. Tools Appl. 2021, 81, 13371–13387. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Shen, X.; Zhou, Y.; Xiao, B.; Li, T.-Q. Detection of Cervical Cancer Cells in Whole Slide Images Using Deformable and Global Context Aware Faster RCNN-FPN. Curr. Oncol. 2021, 28, 3585–3601. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Pan, C.; Sun, W.; Liu, Q.; Du, Y. Global context-aware cervical cell detection with soft scale anchor matching. Comput. Methods Programs Biomed. 2021, 204, 106061. [Google Scholar] [CrossRef]
- Liang, Y.; Tang, Z.; Yan, M.; Chen, J.; Liu, Q.; Xiang, Y. Comparison detector for cervical cell/clumps detection in the limited data scenario. Neurocomputing 2021, 437, 195–205. [Google Scholar] [CrossRef]
- Pal, A.; Xue, Z.; Desai, K.; Banjo, A.A.; Adepiti, C.A.; Long, L.R.; Schiffman, M.; Antani, S. Deep multiple-instance learning for abnormal cell detection in cervical histopathology images. Comput. Biol. Med. 2021, 138, 104890. [Google Scholar] [CrossRef]
- Jia, D.; He, Z.; Zhang, C.; Yin, W.; Wu, N.; Li, Z. Detection of cervical cancer cells in complex situation based on improved YOLOv3 network. Multimedia Tools Appl. 2022, 81, 8939–8961. [Google Scholar] [CrossRef]
- Asiedu, M.N.; Simhal, A.; Chaudhary, U.; Mueller, J.L.; Lam, C.T.; Schmitt, J.W.; Venegas, G.; Sapiro, G.; Ramanujam, N. Development of Algorithms for Automated Detection of Cervical Pre-Cancers With a Low-Cost, Point-of-Care, Pocket Colposcope. IEEE Trans. Biomed. Eng. 2018, 66, 2306–2318. [Google Scholar] [CrossRef]
- Zimmer-Stelmach, A.; Zak, J.; Pawlosek, A.; Rosner-Tenerowicz, A.; Budny-Winska, J.; Pomorski, M.; Fuchs, T.; Zimmer, M. The Application of Artificial Intelligence-Assisted Colposcopy in a Tertiary Care Hospital within a Cervical Pathology Diagnostic Unit. Diagnostics 2022, 12, 106. [Google Scholar] [CrossRef]
- Park, S.Y.; Follen, M.; Milbourne, A.; Rhodes, H.; Malpica, A.; MacKinnon, N.B.; MacAulay, C.; Markey, M.K.; Richards-Kortum, R.R. Automated image analysis of digital colposcopy for the detection of cervical neoplasia. J. Biomed. Opt. 2008, 13, 014029. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Venkataraman, S.; Gustafsson, U.; Oyama, J.C.; Ferris, D.G.; Lieberman, R. Using acetowhite opacity index for detecting cervical intraepithelial neoplasia. J. Biomed. Opt. 2009, 14, 014020. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Sargent, D.; Lieberman, R.; Gustafsson, U. Domain-Specific Image Analysis for Cervical Neoplasia Detection Based on Conditional Random Fields. IEEE Trans. Med. Imaging 2011, 30, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Liu, P.-Z.; Du, Y.-Z.; Luo, Y.-M. Automatic segmentation of cervical region in colposcopic images using K-means. Australas. Phys. Eng. Sci. Med. 2018, 41, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Ramapraba, P.S.; Chitra, M.P.; Prem Kumar, M. Effective lesion detection of colposcopic images using active contour method. Biomed. Res. 2017, 28, S255-S64. [Google Scholar]
- Cho, B.-J.; Choi, Y.J.; Lee, M.-J.; Kim, J.H.; Son, G.-H.; Park, S.-H.; Kim, H.-B.; Joo, Y.-J.; Cho, H.-Y.; Kyung, M.S.; et al. Classification of cervical neoplasms on colposcopic photography using deep learning. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Xue, P.; Tang, C.; Chang, J.; Chu, C.; Ma, K.; Li, Q.; Zheng, Y.; Qiao, Y. Computer-Aided Cervical Cancer Diagnosis Using Time-Lapsed Colposcopic Images. IEEE Trans. Med. Imaging 2020, 39, 3403–3415. [Google Scholar] [CrossRef]
- Luo, Y.-M.; Zhang, T.; Li, P.; Liu, P.-Z.; Sun, P.; Dong, B.; Ruan, G. MDFI: Multi-CNN Decision Feature Integration for Diagnosis of Cervical Precancerous Lesions. IEEE Access 2020, 8, 29616–29626. [Google Scholar] [CrossRef]
- Xue, P.; Tang, C.; Li, Q.; Li, Y.; Shen, Y.; Zhao, Y.; Chen, J.; Wu, J.; Li, L.; Wang, W.; et al. Development and validation of an artificial intelligence system for grading colposcopic impressions and guiding biopsies. BMC Med. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Yuan, C.; Yao, Y.; Cheng, B.; Cheng, Y.; Li, Y.; Li, Y.; Liu, X.; Cheng, X.; Xie, X.; Wu, J.; et al. The application of deep learning based diagnostic system to cervical squamous intraepithelial lesions recognition in colposcopy images. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Yue, Z.; Ding, S.; Zhao, W.; Wang, H.; Ma, J.; Zhang, Y.; Zhang, Y. Automatic CIN Grades Prediction of Sequential Cervigram Image Using LSTM With Multistate CNN Features. IEEE J. Biomed. Health Inform. 2019, 24, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Adweb, K.M.A.; Cavus, N.; Sekeroglu, B. Cervical Cancer Diagnosis Using Very Deep Networks Over Different Activation Functions. IEEE Access 2021, 9, 46612–46625. [Google Scholar] [CrossRef]
- Chandran, V.; Sumithra, M.G.; Karthick, A.; George, T.; Deivakani, M.; Elakkiya, B.; Subramaniam, U.; Manoharan, S. Diagnosis of Cervical Cancer based on Ensemble Deep Learning Network using Colposcopy Images. BioMed Res. Int. 2021, 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.; Fregnani, J.H.T.G.; Brenes, D.; Schwarz, R.A.; Salcedo, M.P.; Possati-Resende, J.C.; Antoniazzi, M.; Fonseca, B.O.; Santana, I.V.V.; Matsushita, G.M.; et al. Cervical lesion assessment using real-time microendoscopy image analysis in Brazil: The CLARA study. Int. J. Cancer 2021, 149, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.-H.; Xue, P.; Chen, J.; Ma, K.; Qian, T.; Zheng, Y.; Qiao, Y.-L. GRAND: A large-scale dataset and benchmark for cervical intraepithelial Neoplasia grading with fine-grained lesion description. Med. Image Anal. 2021, 70, 102006. [Google Scholar] [CrossRef]
- Yan, L.; Li, S.; Guo, Y.; Ren, P.; Song, H.; Yang, J.; Shen, X. Multi-state colposcopy image fusion for cervical precancerous lesion diagnosis using BF-CNN. Biomed. Signal Process. Control 2021, 68, 102700. [Google Scholar] [CrossRef]
- Yue, Z.; Ding, S.; Li, X.; Yang, S.; Zhang, Y. Automatic Acetowhite Lesion Segmentation via Specular Reflection Removal and Deep Attention Network. IEEE J. Biomed. Health Inform. 2021, 25, 3529–3540. [Google Scholar] [CrossRef]
- Ito, Y.; Miyoshi, A.; Ueda, Y.; Tanaka, Y.; Nakae, R.; Morimoto, A.; Shiomi, M.; Enomoto, T.; Sekine, M.; Sasagawa, T.; et al. An artificial intelligence-assisted diagnostic system improves the accuracy of image diagnosis of uterine cervical lesions. Mol. Clin. Oncol. 2021, 16, 1–6. [Google Scholar] [CrossRef]
- Bargahi, N.; Ghasemali, S.; Jahandar-Lashaki, S.; Nazari, A. Recent advances for cancer detection and treatment by microfluidic technology, review and update. Biol. Proced. Online 2022, 24, 1–20. [Google Scholar] [CrossRef]
- Li, Y.; Yi, J.; Chen, H.; Peng, D. Theory and application of artificial intelligence in financial industry. Data Sci. Financ. Econ. 2021, 1, 96–116. [Google Scholar] [CrossRef]
- Ahmad, Z.; Rahim, S.; Zubair, M.; Abdul-Ghafar, J. Artificial intelligence (AI) in medicine, current applications and future role with special emphasis on its potential and promise in pathology: Present and future impact, obstacles including costs and acceptance among pathologists, practical and philosophical considerations. A comprehensive review. Diagn. Pathol. 2021, 16, 1–16. [Google Scholar] [CrossRef]
- Tarawneh, A.S.; Hassanat, A.B.; Celik, C.; Chetverikov, D.; Rahman, M.S.; Verma, C. (Eds.) Deep face image retrieval: A comparative study with dictionary learning. In Proceedings of the 2019 10th International Conference on Information and Communication Systems (ICICS), Irbid, Jordan, 11–13 June 2019. [Google Scholar]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Casarin, J.; Bogani, G.; Papadia, A.; Ditto, A.; Pinelli, C.; Garzon, S.; Donadello, N.; Laganà, A.S.; Cromi, A.; Mueller, M.; et al. Preoperative Conization and Risk of Recurrence in Patients Undergoing Laparoscopic Radical Hysterectomy for Early Stage Cervical Cancer: A Multicenter Study. J. Minim. Invasive Gynecol. 2020, 28, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Di Donna, M.C.; Giallombardo, V.; Lo Balbo, G.; Cucinella, G.; Sozzi, G.; Capozzi, V.A.; Abbate, A.; Laganà, A.S.; Garzon, S.; Chiantera, V. Conventional Laparoscopy versus Robotic-Assisted Aortic Lymph-Nodal Staging for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3332. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Raffaelli, R.; Ban Frangež, H.; Lukanovič, D.; Franchi, M. Vaginal Stenosis After Cervical Cancer Treatments: Challenges for Reconstructive Surgery. J. Investig. Surg. Off. J. Acad. Surg. Res. 2021, 34, 754–755. [Google Scholar] [CrossRef]
- La Rosa, V.; Laganà, A.; Fanale, D.; Vitale, S. Comment on: Survey of cervical cancer survivors regarding quality of life and sexual function. J. Cancer Res. Ther. 2017, 13, 598–599. [Google Scholar] [CrossRef]
- Schulte-Frohlinde, R.; Georges, D.; Clifford, G.M.; Baussano, I. Predicting Cohort-Specific Cervical Cancer Incidence From Population-Based Surveys of Human Papilloma Virus Prevalence: A Worldwide Study. Am. J. Epidemiol. 2021, 191, 402–412. [Google Scholar] [CrossRef]
- Nsugbe, E. Towards the use of cybernetics for an enhanced cervical cancer care strategy. Intell. Med. 2022. [Google Scholar] [CrossRef]
- Sørbye, S.W.; Suhrke, P.; Revå, B.W.; Berland, J.; Maurseth, R.J.; Al-Shibli, K. Accuracy of cervical cytology: Comparison of diagnoses of 100 Pap smears read by four pathologists at three hospitals in Norway. BMC Clin. Pathol. 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Amorim, J.G.; Cerentini, A.; Macarini, L.A.B.; Matias, A.V.; von Wangenheim, A. Systematic Literature Review of Computer Vision-Aided Cytology; Federal University of Santa Catarina: Florianópolis, Brazil, 2020. [Google Scholar]
- Fan, A.; Wang, C.; Zhang, L.; Yan, Y.; Han, C.; Xue, F. Diagnostic value of the 2011 International Federation for Cervical Pathology and Colposcopy Terminology in predicting cervical lesions. Oncotarget 2018, 9, 9166–9176. [Google Scholar] [CrossRef]
- Mitchell, M.F.; Schottenfeld, D.; Tortolero-Luna, G.; Cantor, S.B.; Richards-Kortum, R. Colposcopy for the diagnosis of squamous intraepithelial lesions: A meta-analysis. Obstet. Gynecol. 1998, 91, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.B.; Sharma, P.; Bhandari, P.; East, J.; Antonelli, G.; Lorenzetti, R.; Vieth, M.; Speranza, I.; Spadaccini, M.; Desai, M.; et al. Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia. Gastroenterology 2022, 163, 295–304.e5. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future - Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Hassanat, A.B.A. Furthest-Pair-Based Decision Trees: Experimental Results on Big Data Classification. Information 2018, 9, 284. [Google Scholar] [CrossRef]
- Chankong, T.; Theera-Umpon, N.; Auephanwiriyakul, S. Automatic cervical cell segmentation and classification in Pap smears. Comput. Methods Programs Biomed. 2014, 113, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Alkatout, I.; Karimi-Zarchi, M.; Allahqoli, L. Gynecological cancers and the global COVID-19 pandemic. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 272–278. [Google Scholar] [CrossRef]
| First Author/Year | Sample Size | Methods | Datasets | Main Results | Drawbacks of Studies |
|---|---|---|---|---|---|
| Kahng et al., 2015 [55] | 731 | SVM | Patient records (PAP smear report, age, and the presence of high-risk HPV genotypes) | Four features (PAP, HPV16, HPV52, and HPV35) were found to be the most effective in predicting cancer. | Not reported |
| Al-Wesabi et al., 2018 [56] | 858 | DT and KNN | 858 samples and 32 features, as well as four classes | When factors including age, first sexual intercourse, pregnancies, smoking, hormonal contraceptives, and genital herpes were taken into account, the accuracy of cancer prediction was 97%. | Not reported |
| Dillak et al., 2018 [83] | 400 | RPNN and COA | Subjects (of which 250 were used for training and 150 were used for testing) | The accuracy of the suggested method was 96%. | Not reported |
| Ahmed et al., 2019 [57] | 858 | RFE and RF | Patient record (age, age at first sexual intercourse, number of sexual partners, pregnancies, Schiller, Hinselmann, cytology) smoking, smoking in years, IUD, IUD use in years, STDs, years of STDs, and hormonal contraceptives (years). | With an accuracy of 91.04%, this model successfully identified six risk variables for cervical cancer: Schiller, Hinselmann, cytology, first sexual experience (age), number of pregnancies, and age. | Not reported |
| Alam et al., 2019 [58] | 858 patients | DMT and SMOTE | Patient records (age, pregnancies, smoking patterns, chronological records of STDs, and contraceptive usage) | A very high prediction was noted for Boosted DT, which had an AUROC of 0.978. | Not reported |
| Chen et al., 2019 [59] | 365 patients | Boruta algorithm and RF | The age of the patient, uterine cervix images, ThinPrep Pap test, and HPV test | The proposed multi-modal diagnostic approach provides the final diagnosis with 83.1% accuracy | The main limitations of this study were the small sample size and the unbalanced distribution of the patient population |
| Garg et al., 2019 [84] | - | REPTree | 32 essential clinical characteristics, including age, the use of hormonal contraceptives, the number of sexual partners, pregnancies, smoking, etc., as well as four classifications (Hinselmann, Schiller, cytology, and biopsy) | Age, the use of hormonal contraceptives, age at the first sexual encounter, genital herpes. STDs, number of pregnancies, and smoking are the main predictive factors that improve classification in comparison with other factors. | Not reported |
| Geetha et al., 2019 [60] | 858 cases | RF, SMOTE and PCA | Patient data with 32 risk factors and four objective variables: Hinselmann, Schiller, cytology, and biopsy | When factors including age, first sexual encounter, pregnancy, smoking, hormonal contraceptives, and STDs such as genital herpes were taken into account, the accuracy of cancer prediction was 97%. | SMOTE was only applied to two-dimensional data. SMOTE loses effectiveness as dimensions increase since adjacent nodes are not taken into account, leading to overlapping and inaccurate results. |
| Kar et al., 2019 [85] | 15 samples | NFS | Patients’ records | The application of NFS for early-stage detection of cervical cancer produced satisfactory results with 100% accuracy. | Not reported |
| Kumar Suman and Hooda, 2019 [96] | 858 patients | RF, Neural Network, SVM, AdaBoost, Bayes Net, DT | Patient demographics, habits and medical records | The accuracy and AUC of the Bayes Net algorithm were 96.38% and 0.95, respectively. | Not reported |
| Nithya et al., 2019 [61] | 858 patients | C5.0, RF, Rpart, KNN and SVM | Patient data with 36 attributes (32 input features and 4 target variables: Hinselmann, Schiller, cytology, biopsy) | Overall, C5.0 and RF classifiers identified women presenting clinical signs of cervical cancer fairly accurately and thoroughly. | Not reported |
| Tian et al., 2019 [86] | 34 paired samples | MLA (RF) | Adjacent cervical tissues of 14 CIN2+, 10 HPV+ and 10 CIN1 patients. | The probability of accuracy was 0.814 for CIN2+, and 0.922 for HPV+ and CIN1. | The sample size was small. |
| Alsmariy et al., 2020 [62] | 858 cases | SMOTE | 32 risk factors (demographic, habits, and historical medical records) with four target variables (Hinselmann, Schiller, cytology, and biopsy) | The accuracy, sensitivity, and PPA ratios of all target variables were increased in the SMOTE voting model by 0.93%, 5.13%, 39.26%, and 29%, respectively.Using the PCA technique shortened the time taken to execute computations and also improved the effectiveness of the model. | Not reported |
| Asadi et al., 2020 [63] | 145 patients | SVM, QUEST, C&R tree, MLP and RBF | Patient data with 23 attributes | The percentages of MLP’s accuracy, sensitivity, specificity, and AUC are 90.90, 90.00, 91.67, and 91.50. The level of personal health, marital status, socioeconomic standing, dose of contraceptives used, education level, and the number of caesarean deliveries were all found to be significant predictors in all algorithms. | Not reported |
| Ijaz et al., 2020 [88] | 858 patients | DBSCAN, SMOTET, RF, iForest | Sexual partners, first sexual encounter, pregnancies, smoking, hormonal contraception, IUDs, STDs, CIN, HPV, and four objective variables: Hinselmann, Schiller, cytology, and biopsy. | DBSCAN with SMOTE and DBSCAN with SMOTETomek were outperformed by combinations of iForest with SMOTE and iForest with SMOTETomek. | Algorithm (which was a combination of outlier technique and became balancing with RF) ran more slowly and required more memory. |
| Weegar, 2020 [89] | 1321 patients with cervical cancer | LSTM neural network | Clinical codes, lab findings, and free text notes on patients, taken from electronic health records. | FR achieved the best results with an AUC of 0.70. | Not reported |
| Asaduzzaman et al., 2021 [90] | 161 patients | ML models | Risk factors for cervical cancer included children, age at first sexual encounter, husband’s age, Pap tests, and age. | The best scores were noted for LR (84.8%) and Sklearn (79.3%). | Not reported |
| Ilyas et al., 2021 [91] | 858 subjects | DT, SVM, RF, KNN, NB, MP, J48 Trees, and LR | Three target variables and cervical cancer risk factors: Hinselmann, Schiller, and cytology | The study shows a high prediction accuracy to 94%, which is significantly higher than the prediction accuracies of individual classification methods tested on the same benchmarked datasets. | Not reported |
| Jahan et al., 2021 [92] | 858 patients’ cases for 32 features | MLP, RF, KNN, DT, LR, SVC, GB, and AdaBoost | Demographics, behaviors, and medical records, as well as four target variables: Hinselmann, Schiller, cytology, and biopsy | Classification models claim the highest accuracy for specific top features such as multilayer perceptrons. The highest accuracy was 98.10% for 30 features. | Not reported |
| Khan et al., 2021 [93] | 858 records | XGBoost, AdaBoost, and RF | Data on 32 risk variables for cervical cancer, including age, cancer, CIN, HPV, and characteristics with no missing values and four targets (Hinselmann, Schiller, cytology, and biopsy) | When compared to 30 features, the performance of the Hinselmann test with the chosen feature produced better results and can be used to diagnose cervical cancer. The accuracy, sensitivity, specificity, PPA, and NPA values for the +e model were 98.83, 97.5, 99.2, 99.17, and 97.63, respectively. | The dataset suffers from huge imbalance, and augmented data was generated using SMOTE. |
| Mehmood et al., 2021 [94] | 858 instances | RF and shallow neural network | Demographic data, patient behaviors, and medical history | CervDetect predicted cervical cancer with an accuracy of 93.6%, false-positive and negative rates of 6.4% and 100%, respectively. | Not reported |
| Mudawi et al., 2022 [95] | 585 persons | MLA | Demographics, medical background, and risk factors such as age, IUD use, smoking, STDs, and others. | The RF, DT, adaptive boosting, and gradient boosting algorithms yielded the maximum classification score of 100% for the prediction of cervical cancer. SVM, on the other hand, achieved an accuracy of 99%. | Since the DT method is extremely unstable, even a small change in the data will significantly change the layout of the best decision tree. It is insufficiently reliable SMOTE. |
| First Author/Year | Sample Size | Methods | Datasets | Main Results |
|---|---|---|---|---|
| Jenny et al., 1997 [33] | 516 | PAPNET scan | Women’s cervical smears with abnormal histopathological diagnoses | In conventional screening, the false negative rate fell from 5.7% to 0.8%. |
| Mango et al., 1998 [35] | Over 10,000 | PAPNET vs. conventional microscopic rescreening | Cervical smear | The false negative yield was 6.2% (142/2293) when applying NNA analysis, as opposed to 0.6% (82/13761) when using conventional rescreening. |
| Michelow et al., 1997 [34] | 3106 | PAPNET system vs. manual screening | Consecutive normal and abnormal cervical smears | In low-grade lesions, the PAPNET significantly outperformed traditional screening (89.6% vs. 63.8%, respectively). There was no significant difference between PAPNET and manual detection for more serious abnormalities, such as HSIL or invasive cancer (87.5% vs. 94.6%). |
| Sherman et al., 1998 [36] | 7323 | PAPNET system vs. conventional microscopic screening | ThinPrep slides of women participating in a population-based study | In the hypothetical scenario, 4.3% and 6.5% of women would have been referred for colposcopy by PAPNET-assisted and manual screening, respectively. Smears taken from women with high-grade SIL or carcinoma were correctly identified by PAPNET-assisted cytological screening. |
| Nieminen et al., 2003 [37] | 108,686 | PAPNET system vs. conventional method | Cervical smears | Papnet was able to recognize 92.5% of normal cytologies, while conventional smears had a specificity of 92.9%. |
| Sarwar et al., 2016 [38] | 8091 | Novel hybrid ensemble technique | Cervical smears | Algorithms developed using a digital database demonstrated efficiencies in the range of 93% to 95%, whereas multi-class problem algorithms showed efficiencies in the range of 69% to 78%. The hybrid ensemble approach outperformed all other algorithms and achieved an efficiency of approximately 98% for 2-class problems and approximately 86% for 7-class problems. |
| Kudva et al., 2018 [39] | 102 | SVM and DT | Digitized cervical images from screening | This algorithm had a sensitivity of 99.05%, specificity of 97.16%, and accuracy of 97.94%. |
| Hu et al., 2019 [40] | 9406 | DL-based visual evaluation algorithm | Digitized cervical images from screening | AI identified cumulative precancerous/cancer cases with greater accuracy than conventional cytology ((AUC ¼ 0.91) vs. (AUC ¼ 0.71)). |
| Sompawong et al., 2019 [41] | 1024 | Mask Regional CNN (Mask R-CNN) | Pap smear histological slides | The obtained results had a sensitivity, specificity, and accuracy of 72.5%, 94.3%, and 89.8%, respectively. |
| Bao et al., 2020 [30] | 98,549 | AI-assisted cytology system vs. manual reading | Pap smear histological slides | Overall, 94.7% of manual readings and AI results concurred. The CIN2+ detection rate increased with the severity of cytological abnormalities, based on both manual reading and AI. AI-assisted cytology was 5.8% more sensitive for CIN2+ detection than manual reading and had a slightly lower specificity than the latter. |
| Hussain et al., 2020 [87] | 1670 images | DL | A hospital-based dataset of Pap smear samples | The suggested method is assessed using three datasets: the Herlev, conventional, and liquid-based cytology datasets. The ensemble classifier produced the best results with 0.989 accuracy, 0.978 sensitivity, and 0.979 specificity. |
| Hu et al., 2020 [42] | 7334 | AVE | Cervigram images | By refactoring to a new deep learning-based detection framework, the core AVE algorithm can be operated in approximately 30 s with equivalent accuracy on a basic smartphone. On a low-end smartphone, an image quality algorithm can identify the cervix and evaluate image quality in about one second with an AUC of 0.95 on the ROC curve. |
| Sahoo et al., 2020 [43] | 256 | 2D MFDFA | Low-coherence images | The specificities and sensitivities between normal and CIN1, CIN1 and CINII, and normal and CIN2 were found to be 94%, 88%, and 93%; and 96%, 98%, and 100% respectively. |
| Saini et al., 2020 [44] | 800 | ColpoNet | Colposcopy images | ColpoNet achieved an accuracy of 81.353%. ColpoNet outperformed AlexNet, VGG16, ResNet50, LeNet, and GoogleNet. |
| Sanyal et al., 2020 [45] | 1838 | CNN | Microphotographs from cervical smears | The accuracy, sensitivity, specificity, PPV, and NPV by CNN were 95.46%, 94.28%, 96.01%, 91.66%, and 97.30%, respectively. False positives were reported when the CNN failed to recognize overlapping cells (2.7% microphotographs). |
| Win et al., 2020 [46] | 917 Herlev datasets and 966 SIPaKMeD | RF, LD, SVM, KNN, boosted trees, and bagged trees | Pap smear images | Using the SIPaKMeD dataset, the two-class classification accuracy was 98.27%, while the five-class classification accuracy was 94.09%. |
| Xiang et al., 2020 [47] | 1014 | YOLOv3 | Annotated cervical cell images | On cervical cell image-level screening, the model yielded a sensitivity of 97.5% and a specificity of 67.8%. Produced a cervical cell-level diagnosis with a best mean average precision of 63.4%. |
| Xue et al., 2020 [48] | 3221 women | AVE | 7587 filtered images fromMobileODT | For all ROC curves, the AUC values for discrimination of the most likely precancerous cases from the least likely cases were above 0.90. AVE is able to classify images of the cervix with confidence scores that are strongly related to expert evaluations of severity for the same images. |
| Cheng et al., 2021 [49] | 1170 patient-wise | WSI | Cervical smear slides | Achieved 95.1% sensitivity and 93.5% specificity for classifying slides, which compares favorably with the average performance of three independent cytopathologists. Additionally, it was able to identify the top 10 lesion cells on 447 positive slides with an 88.5% true positive rate. |
| Holmstrom et al., 2021 [31] | 740 | DLS | Smears of HIV-positive women | For the detection of cervical cellular atypia, sensitivities were 95.7% compared with the pathologist’s assessment of digital slides, and 100% compared with the pathologist’s assessment of physical slides. Specificities were 84.7% compared with the pathologist’s assessment of digital slides, and 78.4% compared with the pathologist assessment of physical slides. The corresponding AUCs were 0.94 and 0.96. Accuracy and NPV were both high, especially for the detection of high-grade lesions. Compared to the pathologist’s evaluation of digital slides, there was a significant level of interrater agreement. |
| Tan et al., 2021 [50] | 13,775 | Robust DCNN model | ThinPrep cytology test | With an AUC of 0.67, the proposed cervical cancer screening system had a sensitivity and specificity of 99.4% and 34.8%, respectively. |
| Tang et al., 2021 [51] | 10,601 cases | Manual reading compared with AI assistance | Abnormal cervical epithelial cells | Sensitivity for the detection of LSIL and HSIL increased remarkably from 0.837 to 0.923 and 0.830 to 0.917, respectively. |
| Wang et al., 2021 [52] | 143 images | DL-based cervical lesions diagnosis system | De-identified, digitized whole-slide images of conventional Pap smear samples | A high precision (0.93), recall (0.90), F-measure (0.88), and Jaccard index (0.84) were achieved with the DL-based technique. According to the run-time analysis, the suggested technique processes a WSI in only 210 s, which is 20 times faster than U-Net and 19 times faster than SegNet. |
| Wentzensen et al., 2021 [53] | 4253 patients | Cloud-based whole-slide imaging platform with a deep-learning classifier compared with conventional Pap and manual DS | Cervical images | AI-based DS had a lower positive rate than cytology and manual DS, equal sensitivity, and much higher specificity when compared to both Pap and manual DS. When compared to Pap, AI-based DS reduced referrals to colposcopy by 31% (41.9% vs. 60.1%). |
| Fu et al., 2022 [54] | 2160 women | DL | Cervical images | With an AUC of 0.921, the cross-modal integrated model achieved the best performance. |
| First Author/Year | Sample Size | Methods | Datasets | Main Results |
|---|---|---|---|---|
| Sherman et al., 1994 [101] | 20 | PAPNET system vs. conventional microscopy screening | Cervical smears | Each PAPNET analysis (conducted by pathologists) identified SILs in 10 individuals who were missed in the initial screening and selected smears for rescreening in 19 (95%) of 20 patients. |
| Kok and Boon, 1996 [102] | 25,767 conventional and 65,527 PAPNET smears | PAPNET system vs. conventional screening | Cervical smears | The consistency of screening was much higher for PAPNET than for traditional screening with regard to invasive cancer and high-grade SIL smears. A higher screening sensitivity was demonstrated by the higher positive results for invasive and in situ cancer on histology. |
| Cenci et al., 1997 [103] | 3000 | PAPNET system | Conventional cervical smears | PAPNET detects false negative cytological errors rapidly and accurately. |
| Doornewaard et al., 1997 [64] | 46 cases, 920 control smears | PAPNET system | Histologically confirmed CIN3 or carcinoma | Twenty percent of negative smears were positive. Two were reclassified as high-grade and seven as low-grade squamous intraepithelial lesions. In the 920 smears that constituted the control group, 1 of the 31 initially positive smears was misidentified. Fourteen newly discovered positive cases (1.6%) were found in the control group of 889 negative smears; all of these were low-grade SIL. |
| Kemp et al., 1997 [104] | 344 slides for cell-by-cell classification, 395 slides for slide-by-slide classification | Linear discriminant functions, feed-forward neural networks, Quickprop algorithm | Conventional spatula-collected cervical cell smears | For the test data, a linear discriminant function had an accurate classification rate of 61.6%, whereas neural networks had a cell-by-cell score of up to 72.5%. Neural networks achieved a high rate of 76.2% valid classifications, and the discriminant function achieved a mere 67.6%. |
| Koss et al., 1997 [105] | 487 negative smears | PAPNET testing system | Archival negative smears (index smears) from 228 women with biopsy-proven high-grade precancerous lesions or invasive cervical carcinoma | PAPNET enhanced the detection rate of SILs in control smears by 25% and raised the yield of quality control rescreening by 5.1 times when compared to historical performance data from various participating laboratories. |
| Keenan et al., 2000 [106] | 230 | ML | Smears | 62.3% of the CIN cases had the proper category assigned to them. |
| Dickman et al., 2001 [107] | 8 training images, 8 test images | GTV system | Cervigrams, 35-mm colpophotographs and direct computer-captured colposcopy images | GTV achieved 100% sensitivity and 98% specificity in detecting CIN3 after being trained on one set of photos and tested on another set of images. Following training on one set of digitized cervical colposcopy pictures and testing on another set of images, GTV also achieved a sensitivity of 88% and a specificity of 93% for the detection of cervical cancer. |
| Giovagnoli et al., 2002 [65] | 12 FNs | PAPNET | Cervical smears | When used in cervical screening, Nnbt can assist the diagnosis of misread smears in addition to allowing the detection of FNs due to screening errors. |
| Parker et al., 2002 [108] | 17 women | Neural net | Abnormal Papanicolaou smears | Average correct classification rates for the intrapatient and interpatient nets were 96.5% and 97.5%, respectively. For grade I cervical intraepithelial neoplasia, the sensitivity, specificity, positive predictive value, and negative predictive value were 98.2%, 98.9%, 71.4%, and 99.9%, respectively. |
| Boon et al., 2005 [109] | 1010 | Neural network scanner: PAPNET | Cervical cell samples suspended in the coagulant fixative BoonFix in liquid-based PapSpin slides | A change in the diagnostic parameter was noticed on the PapSpin slide for 151 of 151 exceptional cases, or 85%. In 94% of the cases, it was simpler to determine whether inflammatory cells were adherent to epithelial cells, whereas the adhesion of microbes varied between 43% and 100%. |
| Dounias et al., 2006 [110] | 500 | Hard C-means/fuzzy C-means/Gustafson–Kessel clustering/feature selection/ANFIS neuro-fuzzy classification/nearest neighbor classification/entropy information-based inductive machine learning/genetic programming-derived crisp rule-based system/(LMAM/OLMAM) type second order neural networks | Pap-smear images collected automatically with the aid of software especially designed to recognize, under the electronic microscope, the regions of nucleus-cytoplasm-background. | In the 2-class problem, the vast majority of the techniques performed exceptionally well, frequently achieving a test accuracy of 90%. However, in the 7-class problem, most of the techniques only achieved an average testing accuracy of approximately 75%. Genetic programming demonstrated the best average generalization capabilities in both types of issues considered, achieving 89% and 81% accuracy for the 2- and 7-class problems, respectively. Second-order neural networks scored highest in the 2-class problem, with an accuracy of 99%. |
| Mat-Isa et al., 2008 [111] | 550 | A new artificial neural network architecture known as hierarchical hybrid multilayered perceptron | Pap smears | The proposed network achieved 96.67% sensitivity, 100% specificity, and 97.50% accuracy. False positives and negatives were 1.33% and 3.00%, respectively. |
| Wang et al., 2009 [112] | 31 available digital slides | SVMs | Cytology images | Initial findings point to the system’s potential as a training and diagnostic tool for pathologists. |
| Al-Batah et al., 2014 [113] | 500 | Moving 𝑘-mean, SBRG, ANFIS | Single cell images captured from the slides by using the AutoCapture system | Based on the five-fold analysis method, MANFIS produced a training accuracy rate of 96.3% and a testing accuracy rate of 94.2%. |
| Sokouti et al., 2014 [114] | 100 patients | LMFFNN | Cervical cell images | Using the suggested strategy, cervical cell images were successfully classified at a 100% correct classification rate. Additionally, using the LMFFNN technique, the rates of sensitivity and specificity were 100%. Good concurrence was noted between the values obtained from the ANN model and the expert decision. |
| Kim et al., 2015 [115] | 30 | Image processing by the Hough transform extraction algorithm | Cell images | Using a liquid-based cytology software, the accuracy was 91.5%. The software’s Hough transform extraction algorithm evaluation yielded a success rate of 95%. The Hough transform extraction technique was found to have potential advantages over extraction algorithms for imaging. |
| Kyrgiou, et al., 2016 [116] | 3561 patients | ANN implemented by a MLP | Detailed patient characteristics and the colposcopic impression. | The sensitivity for predicting CIN2 or worse was 93.0%, the specificity was 99.2%, and the positive and negative predictive values were also high (93.3% and 99.2%, respectively). |
| Hyeon et al., 2017 [117] | 71,344 | CNN as feature extractor/classifiers: LR, RF, AadaBoost, SVM | Pap smear microscopic images from Seegene Medical Foundation | SVM performed the best, achieving an F1 score of 78%. |
| Abdoh et al., 2018 [118] | 858 | RF, feature reduction, recursive feature elimination, PCA | Historical medical records, habits, and demographic information | With regard to all features, the SMOTE-RF model had the best accuracy, sensitivity, PPA, and NPA. The SMOTE method is able to increase sensitivity and PPA ratios. For all target variables, sensitivity increased from 86% to 96% and PPA increased from 30% to 98%. |
| Arya et al., 2018 [119] | 330 and 917 | Texture-based feature extraction/classifiers: ANN, SVM | Generated dataset MNITJ (330), DTU/Herlev Pap smear benchmark dataset (917) | With the help of ANN, the suggested texture features technique achieved 99.50% accuracy, 99.90% sensitivity, and 99.90% specificity. For the categorization of single cell images, an accuracy of 99% was achieved using the SVM quadratic classifier, with a sensitivity and specificity of 98.04% and 98.00%, respectively. |
| Aljakouch et al., 2019 [120] | - | DCCN | Pap-smears | The distinction between healthy and malignant Pap smears was made with 100% accuracy by DCNNs based on CARS, SHG/TPF, or Raman images. |
| Bhuvaneshwari and Poornima, 2019 [121] | 20 Pap smear images | Fuzzy c means segmentation, k- k-NN classifier | The single cell microscopic image data were collected from cancer registry hospitals. | On multi-cell and overlapped cells, the approach works quite well. For the KNN classifier this technique achieved a precision of 95%. |
| Lasyk, et al., 2019 [122] | 2058 | U-NET and CNN | Liquid-based cytology samples | Normal and abnormal samples could be distinguished with 100% sensitivity and specificity. |
| Ma et al., 2019 [123] | 92 patients, 141, 467 images | CNN and SVM | Gray-scale cervical tissue images | The classification accuracy for five groups of cervical tissue-normal, ectropion, LSIL and HSIL, and cancer-was 88.3%. The approach yielded an area-under-the-curve value of 0.959 in the binary classification [low-risk (normal, ectropion, and LSIL) against HSIL and cancer] with a sensitivity and specificity of 86.7% and 93.5%, respectively. |
| Moscon et al., 2019 [124] | 15 | Machine-based learning image | Samples of cervix cells | A high sensitivity (99%, 99%) and specificity (98%, 97%) was noted for distinguishing normal cells and HSIL. However, sensitivity (78%) and specificity (79%) were lower for LSIL cells. |
| Wang et al., 2019 [125] | 917 | Deep network model | Cervical cytology images | The experimental results demonstrated that the lightweight deep model performs better than the previous compared models and is able to obtain a model accuracy of 94.1% when applied to a cervical cell dataset with fewer parameters. |
| Zhang et al., 2019 [126] | 62 | R-FCN | Cervical cell images | According to experimental findings, detecting abnormal regions in cervical smear images is accomplished with an average precision of 93.2%. The suggested approach shows promise for the creation of computer-aided cervical cytological screening systems. |
| Bao et al., 2020 [66] | 188,542 | CNN | Digital cytological images from database of routine screening | Compared to manual reading, AI-assisted reading recognized 92.6% of CIN 2 and 96.1% of CIN 3+. AI-assisted reading showed higher specificity (relative specificity 1.36) and equal sensitivity (relative specificity 1.01) compared to expert cytologists, but higher specificity (1.12) and sensitivity (1.12) compared to cytology doctors. |
| Guruvare et al., 2020 [127] | 66 | PNN classifier, the exhaustive search feature selection method, the leave-one-out and the bootstrap validation methods | Microscopy images of H&E-stained biopsy material from two different medical centers | The accuracy of the pattern recognition system was 93% and 88.6% when using the leave-one-out and bootstrap validation methods, respectively. |
| Ma et al., 2020 [128] | 4107 | Cervical cancer detection booster based on FPN and Retinanet | Slide images of cervical smears | The sensitivity of the suggested technique at four false positives per image and the average precision were both increased compared to baseline (Retinanet) by 2.79% and 7.2%, respectively. |
| Xia et al., 2020 [129] | 4036 | SPFNet | Cervical cytology images | The experimental findings demonstrated that the framework outperformed more traditional detection methods by 78.4% AP in cervical cancer cell identification tests. |
| Ali et al., 2021 [80] | - | RF, IBK/feature selection techniques | Kaggle data repository for cervical cancer | The best results were achieved by RF and IBk for Hinselmann (99.16%) and Schiller (98.58%), respectively. |
| Cao et al., 2021 [130] | 325 | CNN, vs. Faster R-CNN | ThinPrep Pap test slide datasets | An independent testing dataset with 3970 cervical cytology images achieved an overall sensitivity, specificity, accuracy, and AUC of 95.83%, 94.81%, 95.08%, and 0.991, respectively, which is comparable to a pathologist with 10 years of expertise. The feature pyramid network model is almost 380 times faster than an average pathologist. |
| Diniz et al., 2021 [75] | 45 training imagesand 900 test images | DT, Nearest Centroid, and k-NN | Cervical cytology images | The suggested ensemble method maintained high precision while achieving the highest results in terms of F1 (0.993) and recall values (0.999). |
| Jia et al., 2021 [131] | 1462 | SSD | Benchmarked cervical cells dataset | The accuracy and mAP of the suggested optimized SSD network were 90.8% and 81.53%, respectively, which is 7.54% and 4.92% higher than YOLO and conventional SSD, respectively. |
| Li et al., 2021 [132] | 800 | Novel framework based on Faster RCNN-FPN | Cervical image dataset | With a mAP of 0.505 and an AUC of 0.670, the proposed model is superior to all other state-of-the-art models. When integrated with traditional computer vision approaches for tagging the negative picture samples, the mAP increased by 6–9%. |
| Liang. et al., 2021 [133] | 12,909 cervical images with 58,995 ground truth boxes corresponding to 10 categories objects | A global context-aware network based on YOLOv3 algorithm | Cervical cell dataset | With the sacrifice of a 2.6% delay in inference time, the suggested approaches ultimately achieve increases a mAP of 5.7% and specificity of 18.5%. |
| Liang et al., 2021 [134] | 7410 and a small-sized dataset of 762 randomly selected images | Faster R-CNN with FPN | Cervical microscopic images | With a mAP of 26.3%, the suggested comparison detector improved on the small dataset. Using the medium-sized dataset for training, the comparison detector improved its mAP by 48.8%. |
| Lin et al., 2021 [67] | 19,303 | CNN with dual-path encoder | Cervical slide images from multiple medical centers | The technique performed effectively, with a high sensitivity of 0.907 and a specificity of 0.80. |
| Meng et al., 2021 [74] | 100 slides from 71 patients | MobileNet-v2, VGG, GoogLeNet, Inception-v3, DenseNet, and ResNet/segmentation networks including FCN, SegNet, DeepLab v3+, U-Net, HookNet | Cervical histopathology image dataset | The dice coefficient approaches 0.7833, showing that the suggested weakly supervised ensemble technique is effective. |
| Pal et al., 2021 [135] | 1331 images | Multiple instance learning | Cervical histopathology images | A framework for multiple instance learning with sparse attention that can provide a classification accuracy of up to 84.55% on the test set. |
| Sheela Shiney et al., 2021 [68] | - | AMBSS algorithm and SVM | Pap images | The achieved accuracy was 85.4%. AMBSS with quasi-Newton-based feedforward neural network classification was employed to increase accuracy, and a classification accuracy of 96.0% was achieved. Additionally, The AMBSS classification using a deep auto encoder-based extreme learning machine achieved an accuracy rate of 99.1%. |
| Jia et al., 2022 [136] | YOLO algorithm, improved algorithm k-means++ is used to replace the clustering algorithm k-means in the original yolov3, NMS algorithm | Experimental verification showed that the network achieved a mAP of 78.87% which is 8.02%, 8.22%, and 4.83% higher than that of SSD, YOLOv3, and ResNet50, respectively. |
| First Author/Year | Sample Size | Methods | Datasets | Main Results |
|---|---|---|---|---|
| Park et al., 2008 [139] | 29 patients | K-means clustering algorithm | Digital images of the cervix | Diagnostic performance: 88% specificity and 79% sensitivity. |
| Li et al., 2009 [140] | 99 human subjects | Automated image analysis | Images captured with a digital colposcope | The proposed opacity index demonstrated 94% and 87% sensitivity and specificity, respectively. |
| Park et al., 2011 [141] | 48 patients | CRFs | Clinical data | The suggested automated diagnostic approach can supplement or even replace conventional colposcopy, permit more objective tissue specimen sampling, and reduce the incidence of cervical cancer in low-income nations by offering an economical screening option. |
| Ramapraba et al., 2017 [143] | 400 images | DWT and KNN | Cervical images | The cervical acetowhite lesion can be found with 94% sensitivity in less than 40 s. |
| Asiedu et al., 2019 [137] | 134 | ML | Pocket colposcope patients | The suggested framework successfully distinguished cervical intraepithelial neoplasia (CIN+) from benign and normal tissue with sensitivity, specificity, and accuracy rates of 81.3%, 78.6%, and 80.0%, respectively. This is better than the average values obtained by three expert doctors on the same dataset (77% sensitivity, 51% specificity, and 63% accuracy) for differentiating normal/benign cases from CIN+. |
| Bai et al., 2020 [69] | 817 | CLDNet | 6536 Colposcopy images from attendees of cancer screening | The average precision of the model extraction lesion region is 92.53%, and the average recall rate is 85.56%. |
| Cho et al., 2020 [144] | 6000 cases | Pre-trained CNN | Photographs, colposcopy-directed biopsy and conization | The AUC of the CIN system for differentiating high-risk lesions from low-risk lesions by Resnet-152 was 0.781 and the AUC of the LAST system was 0.708. |
| Li et al., 2020 [145] | 7668 | GCNs | Colposcopy images | Similar to an in-service colposcopist, the suggested deep learning framework achieves a classification accuracy of 78.33%. |
| Luo et al., 2020 [146] | Positive samples of 494 cases, negative samples of 615 cases | Multi-CNN | Colposcopy images obtained through a lighted magnifying glass and clinical diagnosis reports | Results with two data splits were compared as follows: single-class split: AUC = 0.756; multi-class split: AUC = 0.764. The suggested multi-decision feature fusion technique can produce computer-aided diagnosis outcomes that are more in line with clinical diagnosis requirements. |
| Miyagi et al., 2020 [70] | 330 patients | DL | Colposcopy images | For diagnosing HSI, the AI classifier and oncologists performed with accuracy, sensitivity, specificity rates, and a Youden’s J index of 0.823 and 0.797, 0.800 and 0.831, 0.882 and 0.773, and 0.682 and 0.604, respectively. The 95% confidence interval for the area under the receiver-operating characteristic curve was 0.721–0.928. The best cutoff value was 0.692. |
| Xue et al., 2020 [147] | 19,435 patients | CAIADS | Colposcopy images, clinical information, and pathological results | While the specificities were comparable (low-grade or worse 51.8% vs. 52.0%; high-grade or worse 93.9% vs. 94.9%), CAIADS demonstrated higher sensitivity for detecting pathological HSIL+ than colposcopies interpreted by colposcopists at either biopsy threshold (low-grade or worse 90.5% vs. 83.5%; high-grade or worse 71.9% vs. 60.4%). |
| Yuan et al., 2020 [148] | 5384 | ResNet | Colposcopy images of three subsets: the training set, the test set and the validation set, in a ratio of 8:1:1 | With an AUC of 0.93, the classification model’s sensitivity, specificity, and accuracy in differentiating between negative and positive cases were 85.38%, 82.62%, and 84.10%, respectively. |
| Yue et al., 2020 [149] | 4753 | C-RCNN | Cervigram images | Achieved a test accuracy of 96.13%, specificity of 98.22%, and sensitivity of 95.09%. The AUC was more than 0.94. |
| Adweb et al., 2021 [150] | 4000 pre-cancerous, 800 healthy samples | ReLU-ResNet, PReLU-ResNet and Leaky-ReLU | Cervical images from colposcopy | The accuracy of designed residual networks with leaky and parametric rectified linear unit (Leaky-RELU and PRELU) activation functions (accuracies of 90.2% and 100%, respectively) was similar. |
| Chandran et al., 2021 [151] | 5679 | CYENET and VGG19 (TL) | Colposcopy photographs | For VGG19, the classification accuracy was 73.3%. High sensitivity, specificity, and kappa scores of 92.4%, 96.2%, and 88% were achieved by the proposed CYENET. |
| Hunt et al., 2021 [152] | 1486 | Multi-task CNN | High-resolution microendoscopy images | For the detection of CIN3+, HRME with morphologic image analysis was just as sensitive (95.6% vs. 96.2%) and specific (56.6% vs. 58.7%) as colposcopy. Compared to colposcopy, HRME with morphologic image analysis had a slightly lower sensitivity (91.7% vs. 95.6%) and specificity (59.7% vs. 63.4%, p = 0.02) for the identification of CIN2+. |
| Li et al., 2021 [153] | 8604 | CAD systems | Colposcopy images from grading cervical intraepithelial neoplasia | The grading accuracy of CIN increased by more than 10% with TAE and ABV. |
| Nikookar et al., 2021 [71] | 287 | NavieBayes, AdaBoost, RF, R tree, SVM, Decision tree, Logit boost | Cervigrams from digital colposcopy dataset | Random tree is the best performing classifier on the dataset acquired by applying the Fmvs aggregation function. |
| Peng et al., 2021 [72] | 960 | CNN | Original colposcopy image | In 60 tests, the suggested technique achieved a classification accuracy of 86.3%, sensitivity of 84.1%, and specificity of 89.8%. |
| Viñals et al., 2021 [73] | 21,851 positive pixels and 93,725 negative pixels | ANN | VIA videos | The sensitivity and specificity of the suggested solution were 0.9 and 0.87, respectively. |
| Yan et al., 2021 [154] | 1400 | BF-CNN vs. ResNet18 | Cervicograms | Similar sensitivity (74.6%) and the best accuracy (85.5%), specificity (95.7%), and AUC (0.909) were achieved with F-CNN. |
| Yue et al., 2021 [155] | 609 | CICN | Clinical cervigram | DenseNet-121 achieved the highest accuracy (0.906) and AUC (0.973) |
| Kim et al., 2022 [79] | 234 patients | ML | Cervical images | Compared to each clinician’s colposcopic impressions, AI was associated with greater sensitivity, equivalent specificity, and equivalent positive predictive value. |
| Elakkiya et al., 2022 [77] | 858 | SOD-GAN | Cervical samples and colposcopy images | The suggested method demonstrated good accuracy through all stages, achieving a sensitivity of approximately 97% with a loss of less than 1%. |
| Ito et al., 2022 [156] | 463 | AISD | Colposcopy images | The accuracy of AI was 57.8% for normal, 35.4% for cervical intraepithelial neoplasia (CIN)1, 40.5% for CIN2–3, and 44.2% for invasive cancer. Before learning about the AI image diagnosis, the accuracy of gynecologists’ diagnoses based on cervical pathology images was 54.4% for CIN2–3 and 38.9% for invasive cancer. Their accuracy increased to 58.0% for CIN2–3 and 48.5% for invasive cancer after they learned about the AISD. |
| Zimmer-Stelmach et al., 2022 [138] | 48 | AI colposcopy assessment | Colposcopy examinations | With a significantly lower sensitivity (66.7% vs. 100%) but a higher specificity (46.7% vs. 16.7%), AI-assisted colposcopy was able to detect diseases with a similar PPV as that of a skilled physician (42.9% vs. 41.8%). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allahqoli, L.; Laganà, A.S.; Mazidimoradi, A.; Salehiniya, H.; Günther, V.; Chiantera, V.; Karimi Goghari, S.; Ghiasvand, M.M.; Rahmani, A.; Momenimovahed, Z.; et al. Diagnosis of Cervical Cancer and Pre-Cancerous Lesions by Artificial Intelligence: A Systematic Review. Diagnostics 2022, 12, 2771. https://doi.org/10.3390/diagnostics12112771
Allahqoli L, Laganà AS, Mazidimoradi A, Salehiniya H, Günther V, Chiantera V, Karimi Goghari S, Ghiasvand MM, Rahmani A, Momenimovahed Z, et al. Diagnosis of Cervical Cancer and Pre-Cancerous Lesions by Artificial Intelligence: A Systematic Review. Diagnostics. 2022; 12(11):2771. https://doi.org/10.3390/diagnostics12112771
Chicago/Turabian StyleAllahqoli, Leila, Antonio Simone Laganà, Afrooz Mazidimoradi, Hamid Salehiniya, Veronika Günther, Vito Chiantera, Shirin Karimi Goghari, Mohammad Matin Ghiasvand, Azam Rahmani, Zohre Momenimovahed, and et al. 2022. "Diagnosis of Cervical Cancer and Pre-Cancerous Lesions by Artificial Intelligence: A Systematic Review" Diagnostics 12, no. 11: 2771. https://doi.org/10.3390/diagnostics12112771
APA StyleAllahqoli, L., Laganà, A. S., Mazidimoradi, A., Salehiniya, H., Günther, V., Chiantera, V., Karimi Goghari, S., Ghiasvand, M. M., Rahmani, A., Momenimovahed, Z., & Alkatout, I. (2022). Diagnosis of Cervical Cancer and Pre-Cancerous Lesions by Artificial Intelligence: A Systematic Review. Diagnostics, 12(11), 2771. https://doi.org/10.3390/diagnostics12112771







