Strumal Carcinoid Tumor of the Ovary: Report of Rare Occurrence with Review of Literature
Abstract
1. Background
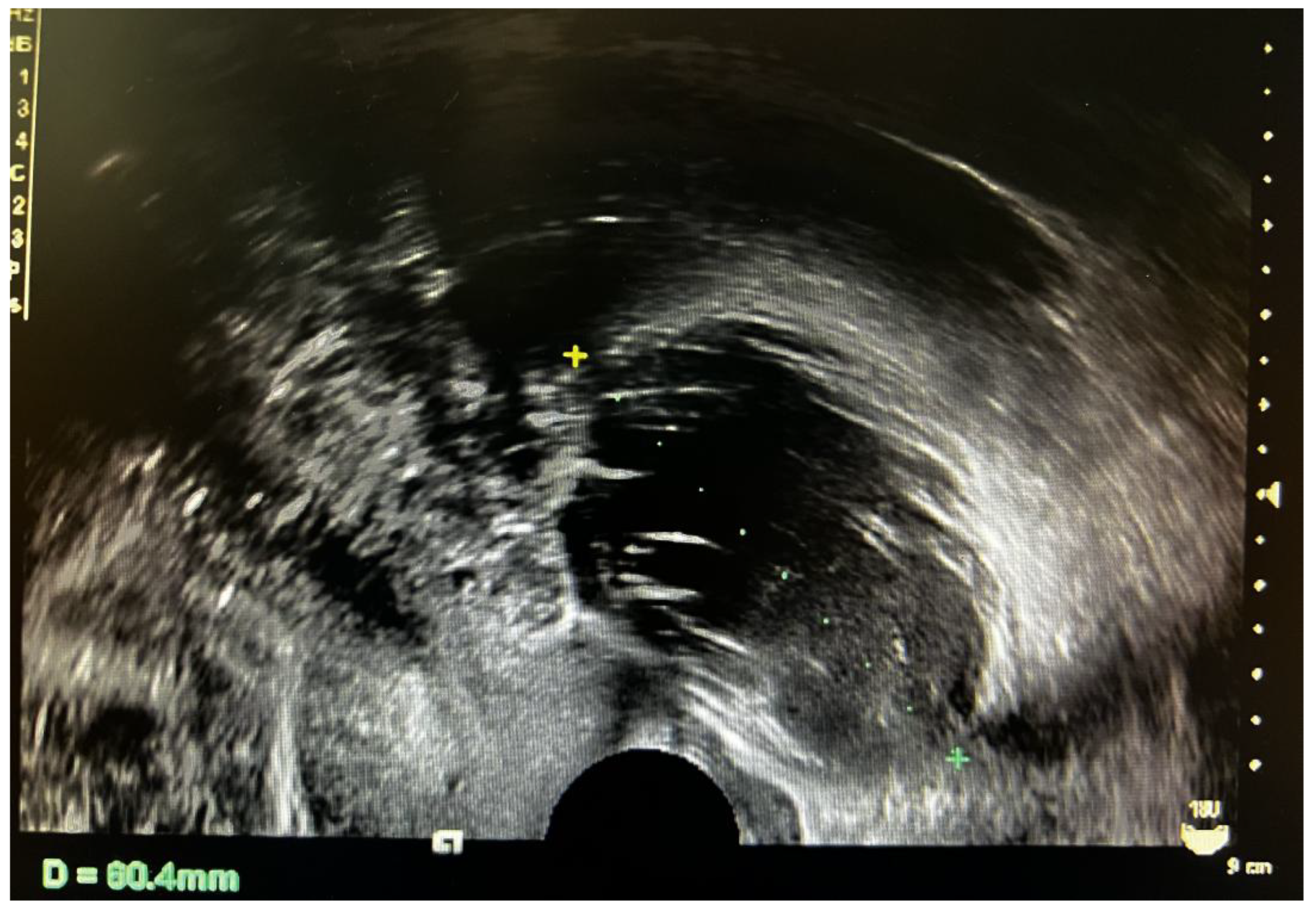
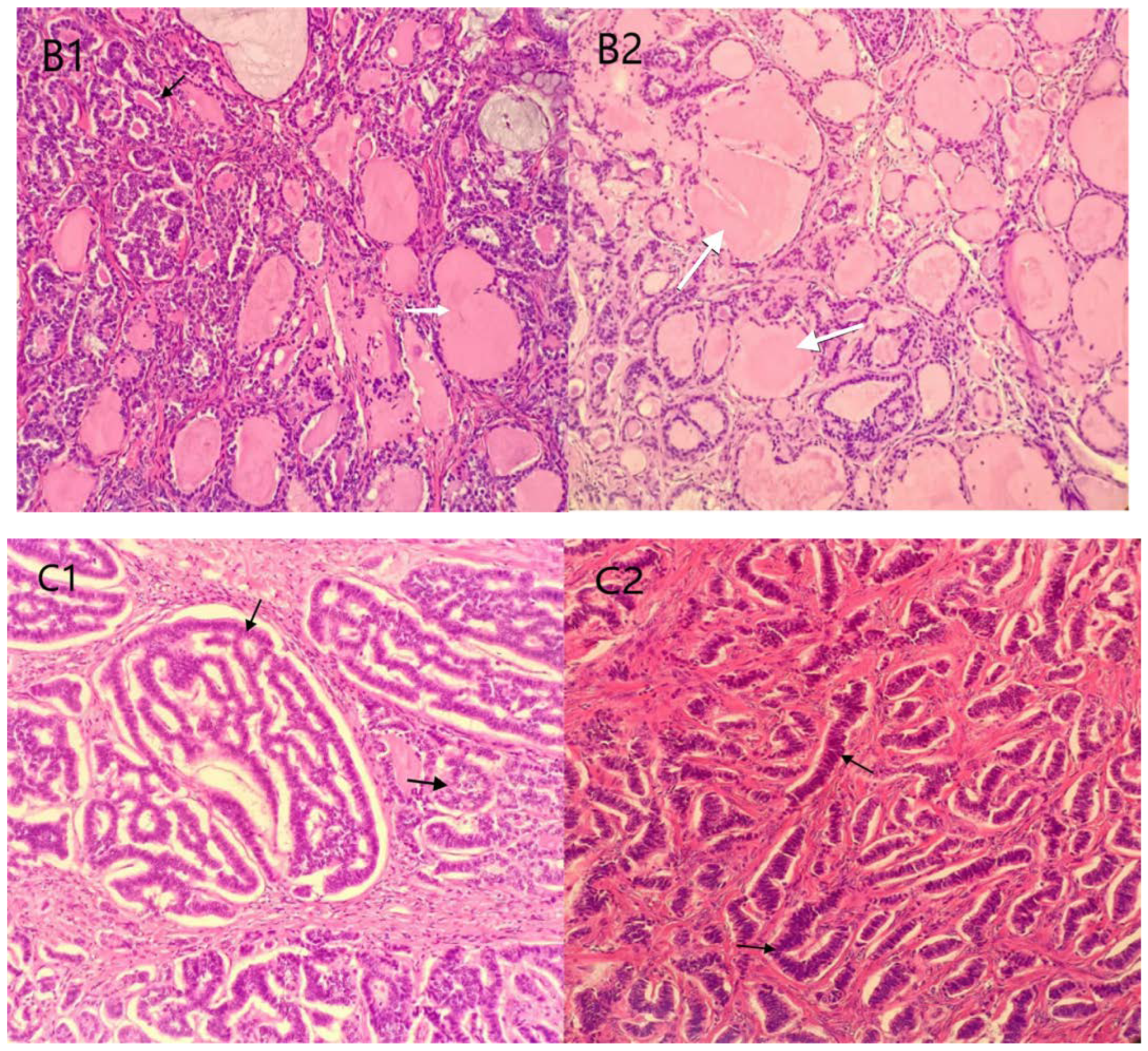
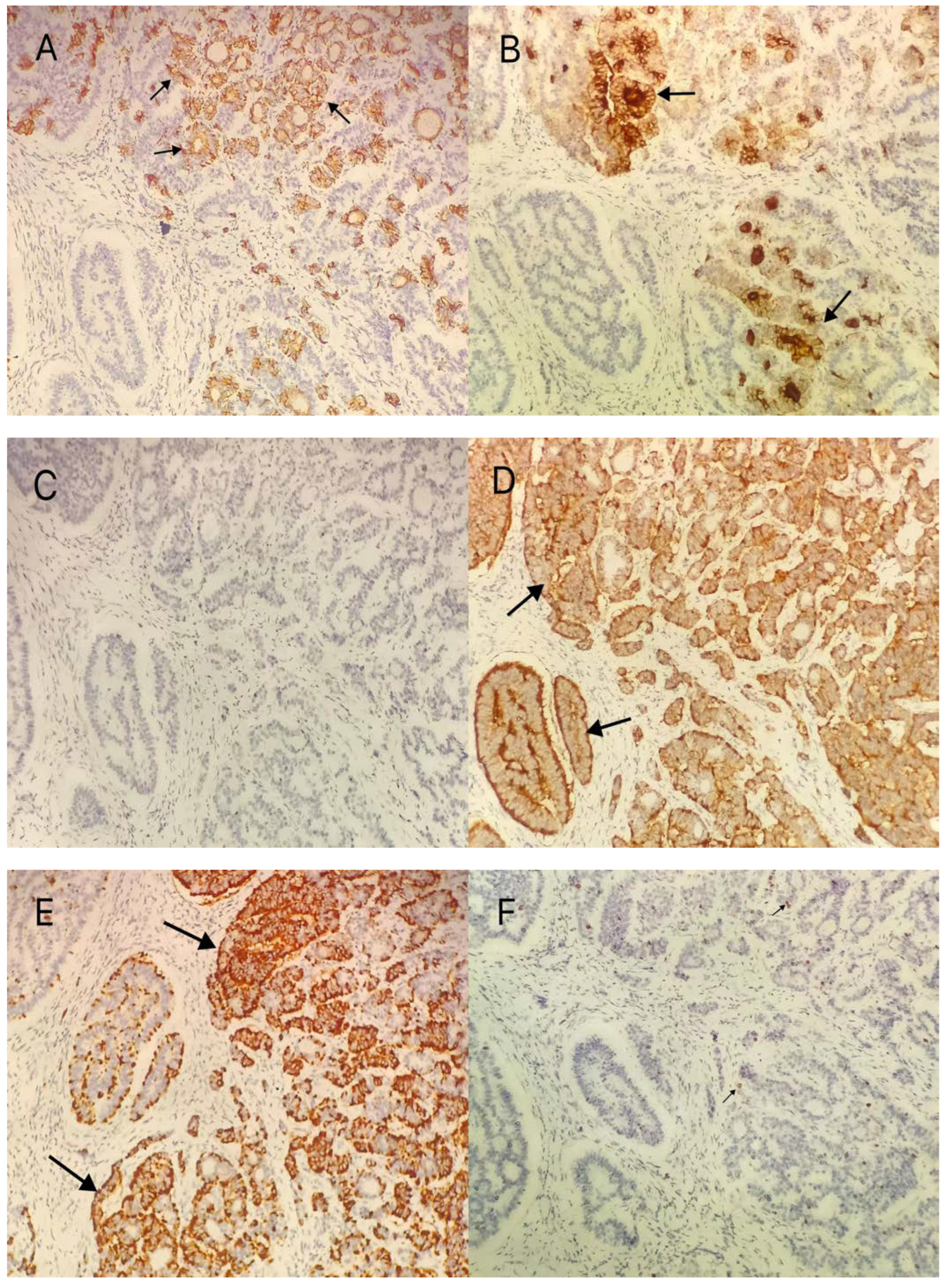
2. Case Presentation
3. Discussion and Review of Literature
3.1. Clinical Characteristics
3.2. Imaging Characteristics
3.3. Pathological Characteristics
3.4. Immunohistochemical Methods
3.5. Operative Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, B.; Suneja, A.; Vaid, N.B.; Bhatia, A. Primary ovarian carcinoid tumor simulating virilizing tumor of the ovary: A rare entity. Indian J. Cancer 2014, 51, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, T.S.; Robinson, R.A.; Samuelson, M.I. Pure Primary Ovarian Carcinoid Tumor. Int. J. Gynecol. Pathol. 2021, 40, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, J. Introduction of WHO classification of tumours of female reproductive organs, fourth edition. Zhonghua Bing Li Xue Za Zhi 2014, 43, 649–650. [Google Scholar] [PubMed]
- Robboy, S.J.; Scully, R.E. Strumal carcinoid of the ovary: An analysis of 50 cases of a distinctive tumor composed of thyroid tissue and carcinoid. Cancer 1980, 46, 2019–2034. [Google Scholar] [CrossRef]
- Borghese, M.; Razzore, P.; Ferrero, A.; Daniele, L.; Mariani, L.L.; Sgro, L.G. Metastatic Bilateral Strumal Carcinoid: A Case Report and Review of the Literature. Anticancer Res. 2019, 39, 5053–5056. [Google Scholar] [CrossRef]
- Takatori, E.; Shoji, T.; Miura, J.; Takeuchi, S.; Yoshizaki, A.; Sugiyama, T. Case of peptide-YY-producing strumal carcinoid of the ovary: A case report and review. J. Obstet. Gynaecol. Res. 2012, 38, 1266–1270. [Google Scholar] [CrossRef]
- Muller, K.E.; Tafe, L.J.; Gonzalez, J.L.; West, L.A.; Schned, A.R. Ovarian strumal carcinoid producing peptide YY associated with severe constipation: A case report and review of the literature. Int. J. Gynecol. Pathol. 2015, 34, 30–35. [Google Scholar] [CrossRef]
- Erdenebaatar, C.; Yamaguchi, M.; Saito, F.; Motooka, C.; Tashiro, H.; Katabuchi, H. An Ovarian Carcinoid Tumor with Peptide YY-Positive Insular Component: A Case Report and Review of the Literature. Int. J. Gynecol. Pathol. 2016, 35, 362–368. [Google Scholar] [CrossRef]
- Ge, H.J.; Bi, R.; Cheng, Y.F.; Chang, B.; Yu, L.; Tang, S.X. Clinicopathologic analysis of primary carcinoid of the ovary. Zhonghua Bing Li Xue Za Zhi 2018, 47, 517–521. [Google Scholar]
- Zhu, R.; Xue, X.W.; Luo, Y.F.; Wu, H.W.; Huo, Z. Primary carcinoid of ovary: A clinicopathologic analysis of 17 cases. Zhonghua Bing Li Xue Za Zhi 2018, 47, 339–343. [Google Scholar]
- Yan, F.; Zhou, Q.; Lin, Y.; Yu, C.; Chang, H.; Li, Y. Clinicopathological analysis of primary carcinoid tumour of the ovary arising in mature cystic teratomas. J. Int. Med. Res. 2021, 49, 3000605211034666. [Google Scholar] [CrossRef] [PubMed]
- Turla, A.; Zamparini, M.; Milione, M.; Grisanti, S.; Amoroso, V.; Pedersini, R. Ovarian Strumal Carcinoid: Case Report, Systematic Literature Review and Pooled Analysis. Front. Endocrinol. 2022, 13, 871210. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.K.; Kwon, B.S.; Kim, Y.H.; Lee, N.K.; Choi, K.U.; Suh, D.S. Peptide YY producing strumal carcinoid tumor of the ovary in a postmenopausal woman: A rare cause of chronic constipation. Obstet. Gynecol. Sci. 2017, 60, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Macháleková, K.; Kolníková, G.; Redecha, M.; Žúbor, P.; Kajo, K. Strumal carcinoid of the ovary—Report of two cases and review of literature. Ceska. Gynekol. 2018, 83, 452–457. [Google Scholar] [PubMed]
- Antovska, V.S.; Trajanova, M.; Krstevska, I.; Gosheva, I.; Chelebieva, J.; Prodanova, I. Ovarian Strumal Carcinoid Tumour: Case Report. Open Access Maced. J. Med. Sci. 2018, 6, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Zhang, W.; Zhou, L.; Sun, X.; Jia, G. Strumal carcinoid tumor of the ovary: A rare case report. Medicine 2019, 98, e18009. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Arimoto, T.; Sandoh, K.; Okano, K.; Ebisu, Y.; Ito, H. Imprint cytology of strumal carcinoid of the ovary: A case report with immunocytochemical analysis. Diagn. Cytopathol. 2019, 47, 218–221. [Google Scholar] [CrossRef]
- Mohammed, S.Y.; Anelo, O.M.; Khan, F. Strumal Carcinoid Presenting as Large Pelvic Mass: A Rare Case and Review of Literature. Cureus 2021, 13, e20494. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Yang, H. Clinicopathological observation of mature teratoma with malignant transformation, a single center retrospective study. Indian J. Pathol. Microbiol. 2022, 65, 369–373. [Google Scholar]
- Baruah, U.; Tak, A.; Kakoti, L.; Barmon, D. Carcinoid ovary with synchronous carcinoid tumour of the appendix: Report of a rare occurrence with review of literature. BMJ Case Rep. 2022, 15, e248869. [Google Scholar] [CrossRef]
- Cagino, K.; Levitan, D.; Schatz-Siemers, N.; Zarnegar, R.; Chapman-Davis, E.; Holcomb, K.; Frey, M.K. Multiple malignant transformations of an ovarian mature cystic teratoma. Ecancermedicalscience 2020, 14, 1009. [Google Scholar] [CrossRef]
- Matsuda, K.; Maehama, T.; Kanazawa, K. Strumal carcinoid tumor of the ovary: A case exhibiting severe constipation associated with PYY. Gynecol. Oncol. 2002, 87, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Leniček, T.; Tomas, D.; Soljačić-Vraneš, H.; Kraljević, Z.; Klarić, P.; Kos, M.; Kos, M. Strumal carcinoid of the ovary: Report of two cases. Acta Clin. Croat. 2012, 51, 649–653. [Google Scholar] [PubMed]
- Hiett, A.K.; Sonek, J.D.; Guy, M.; Reid, T.J. Performance of IOTA Simple Rules, Simple Rules risk assessment, ADNEX model and O-RADS in differentiating between benign and malignant adnexal lesions in North American women. Ultrasound Obstet. Gynecol. 2022, 59, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Haba, R.; Kushida, Y.; Kadota, K.; Katsuki, N.; Miyai, Y. Cytopathologic characteristics of the primary strumal carcinoid tumor of the ovary: A case report with emphasis on differential diagnostic considerations. Diagn. Cytopathol. 2013, 41, 812–816. [Google Scholar] [CrossRef]
- Hsu, W.W.; Mao, T.L.; Chen, C.H. Primary ovarian mucinous carcinoid tumor: A case report and review of literature. Taiwan J. Obstet. Gynecol. 2019, 58, 570–573. [Google Scholar] [CrossRef]
- Baker, P.M.; Oliva, E.; Young, R.H.; Talerman, A.; Scully, R.E. Ovarian mucinous carcinoids including some with a carcinomatous component: A report of 17 cases. Am. J. Surg. Pathol. 2001, 25, 557–568. [Google Scholar] [CrossRef]
- Theurer, S.; Ingenwerth, M.; Herold, T.; Herrmann, K.; Schmid, K.W. Immunohistochemical Profile and 47-Gene Next-Generation Sequencing (NGS) Solid Tumor Panel Analysis of a Series of 13 Strumal Carcinoids. Endocr. Pathol. 2020, 31, 101–107. [Google Scholar] [CrossRef]
- Kurabayashi, T.; Minamikawa, T.; Nishijima, S.; Tsuneki, I.; Tamura, M.; Yanase, T. Primary strumal carcinoid tumor of the ovary with multiple bone and breast metastases. J. Obstet. Gynaecol. Res. 2010, 36, 567–571. [Google Scholar] [CrossRef]
- Metwally, I.H.; Elalfy, A.F.; Awny, S.; Elzahaby, I.A.; Abdelghani, R.M. Primary ovarian carcinoid: A report of two cases and a decade registry. J. Egypt Natl. Cancer Inst. 2016, 28, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Baloch, Z.W.; LiVolsi, V.A. Pathology of Struma Ovarii: A Report of 96 Cases. Endocr. Pathol. 2015, 26, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Armes, J.E.; Ostör, A.G. A case of malignant strumal carcinoid. Gynecol. Oncol. 1993, 51, 419–423. [Google Scholar] [CrossRef] [PubMed]


| Authors (Year) | Patients (N) | Clinical Manifestations | Mean Size | Classification | Immunohistochemical |
|---|---|---|---|---|---|
| Takatori E et al. (2012) [6] | 6 | abdominal mass (2) Constipation (1) | 11 | Trabecular | CgA+ Syn+ TG+ PYY+ |
| Muller KE et al. (2014) [7] | 14 | Constipation (13) | 9.9 | Strumal (5) Trabecular (4) Strumal+Trabecular (5) | PYY++ (10) |
| Erdenebaatar C et al. (2015) [8] | 20 | Constipation (18) | 9.7 | Strumal (8) Trabecular (6) Strumal + Trabecular (5) | PYY++ |
| Ge HJ et al. (2018) [9] | 15 | abdominal mass (3) colporrhagia (4) abdominal pain (1) Constipation (1) | 8.8 | Insular (3) Trabecular (1) Strumal (15) Mucinous (1) | Syn: + (14/15); CgA: + (10/15); CD56: + (9/15) Ki—67: <2% |
| Zhu R et al. (2018) [10] | 17 | abdominal pain (3) | 8.2 | Insular (4) Trabecular (4) Insular+Trabecular (2) Strumal (1) Mucinous (6) | NSE, Syn: +(17/17) CgA: + (10/17) Ki—67: ≤ 2% (17/17) |
| Yan F et al. (2021) [11] | 4 | abdominal mass (2) | 8.9 | Insular (1) Trabecular (1) Strumal (2) | CK, Syn, CgA and CD56: ++ TTF—1: − Ki67: 2% |
| Turla A et al. (2022) [12] | 111 | abdominal mass (41) abdominal pain (49) Constipation (54) | 8 | Trabecular (61) Insular + trabecular (44) Insular (5) | NEC, CgA, Syn, CD56: + (111) TTF—1: + (101) TG: +(98) |
| Authors (Year) | Patients (N) | Clinical Manifestations | Mean Size | Tumor Maker | Classification | Immunohistochemical | Concomitant Tumors |
|---|---|---|---|---|---|---|---|
| Noh HK et al. (2017) [13] | 1 | Constipation | 6.4 | Normal | Strumal | Syn, PAX8: + PYY+ | Absent |
| Macháleková K et al. (2018) [14] | 2 | Absent | 6.5 7.5 | CA125:45 IU/mL | Trabecular Trabecular+Mucinous | Syn, CK7: + CgA: + (50%) Ki—67: < 2% | Mature cystic teratoma |
| Antovska VS et al. (2018) [15] | 1 | Absent | 6 | Normal | Insular+Trabecular | Syn, NSE, CD56: ++ CgA: + Ki—67: 10% | Mature cystic teratoma |
| Borghese M et al. (2019) [5] | 1 | Absent | 4 | Normal | Trabecular | Syn, NSE, CD56: + Tg:+/−, CDX2: +/− Ki—67: < 2% | Mature cystic teratoma /metastasis |
| Chai W et al. (2019) [16] | 1 | Abdominal mass | 8.8 | CA125: 768.10 IU/mL | Insular | Syn, CgA, NSE: + | Absent |
| Ishida M et al. (2019) [17] | 1 | Absent | 5 | Normal | Trabecular | Syn: ++ | Absent |
| Mohammed SY et al. (2021) [18] | 1 | Abdominal pain Anorexia Frequent urination | 24 | CA125:105 IU/mL; CEA: 6.4 ng/mL | Insular+Trabecular | Syn, CgA: + | Mature cystic teratoma |
| Turla A et al. (2022) [12] | 1 | Abdominal pain Constipation | 4 | Normal | absent | Syn, CgA, CD56: + CK7: + Ki—67: 2% | Absent |
| Yuan Y et al. (2022) [19] | 1 | Absent | 9 | absent | Strumal | CD56, NSE, PSAP, CDX2: + AE1/AE3: + | Mature cystic teratoma |
| Upasana Baruah et al. (2022) [20] | 1 | Abdominal distension | 15 | CA125:944 IU/mL; CA199:944 ng/dL | Strumal | Syn, CD56, PAX8: + Ki—67 < 3% | Mature cystic teratoma /Appendix carcinoid |
| Cagino K et al. (2022) [21] | 1 | Abdominal pain Colporrhagia | 10 | absent | Insular | NCE, Syn, CgA: + | Mature cystic teratoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.-P.; Yang, A.-Q.; Jin, L. Strumal Carcinoid Tumor of the Ovary: Report of Rare Occurrence with Review of Literature. Diagnostics 2022, 12, 2706. https://doi.org/10.3390/diagnostics12112706
Shen L-P, Yang A-Q, Jin L. Strumal Carcinoid Tumor of the Ovary: Report of Rare Occurrence with Review of Literature. Diagnostics. 2022; 12(11):2706. https://doi.org/10.3390/diagnostics12112706
Chicago/Turabian StyleShen, Li-Ping, An-Qiang Yang, and Lei Jin. 2022. "Strumal Carcinoid Tumor of the Ovary: Report of Rare Occurrence with Review of Literature" Diagnostics 12, no. 11: 2706. https://doi.org/10.3390/diagnostics12112706
APA StyleShen, L.-P., Yang, A.-Q., & Jin, L. (2022). Strumal Carcinoid Tumor of the Ovary: Report of Rare Occurrence with Review of Literature. Diagnostics, 12(11), 2706. https://doi.org/10.3390/diagnostics12112706






