Risk-Based Colposcopy for Cervical Precancer Detection: A Cross-Sectional Multicenter Study in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Cytology and HPV Testing
2.3. Colposcopy and Biopsy
2.4. Histological Diagnosis
2.5. Statistical Analysis
2.6. List of Risk-Based Strata Assessed
- Colposcopy and cytology (six subgroups)
- 1a.
- High-grade colp and HSIL+
- 1b.
- High-grade colp and <HSIL
- 1c.
- Low-grade colp and HSIL+
- 1d.
- Low-grade colp and <HSIL
- 1e.
- Normal/benign colp and HSIL+
- 1f.
- Normal/benign colp and <HSIL
- Colposcopy and HPV status (nine subgroups)
- 2a.
- High-grade colp and HPV16/18
- 2b.
- High-grade colp and non-16/18 hrHPV
- 2c.
- High-grade colp and hrHPV negative
- 2d.
- Low-grade colp and HPV16/18
- 2e.
- Low-grade colp and non-16/18 hrHPV
- 2f.
- Low-grade colp and hrHPV negative
- 2g.
- Normal/benign colp and HPV16/18
- 2h.
- Normal/benign colp and non-16/18 hrHPV
- 2i.
- Normal/benign colp and hrHPV negative
- Colposcopy, cytology and HPV status (18 subgroups)
- 3a.
- High-grade colp and HSIL+ and HPV16/18+
- 3b.
- High-grade colp and HSIL+ and non-16/18 hrHPV
- 3c.
- High-grade colp and HSIL+ and hrHPV negative
- 3d.
- High-grade colp and <HSIL and HPV16/18+
- 3e.
- High-grade colp and <HSIL and non-16/18 hrHPV
- 3f.
- High-grade colp and <HSIL and hrHPV negative
- 3g.
- Low-grade colp and HSIL+ and HPV16/18+
- 3h.
- Low-grade colp and HSIL+ and non-16/18 hrHPV
- 3i.
- Low-grade colp and HSIL+ and hrHPV negative
- 3j.
- Low-grade colp and <HSIL and HPV16/18+
- 3k.
- Low-grade colp and <HSIL and non-16/18 hrHPV
- 3l.
- Low-grade colp and <HSIL and hrHPV negative
- 3m.
- Normal/benign colp and HSIL+ and HPV16/18+
- 3n.
- Normal/benign colp and HSIL+ and non-16/18 hrHPV
- 3o.
- Normal/benign colp and HSIL+ and hrHPV negative
- 3p.
- Normal/benign colp and <HSIL and HPV16/18+
- 3q.
- Normal/benign colp and <HSIL and non-16/18 hrHPV
- 3r.
- Normal/benign colp and <HSIL and hrHPV negative
3. Results
3.1. Associations between Colposcopy, Cytology HPV Status, and Precancer Diagnosis
3.2. Cervical Precancer Risk Strata Based on Colposcopy and Cytology
3.3. Cervical Precancer Risk Strata Based on Colposcopy and HPV Status
3.4. Cervical Precancer Risk Strata Based on Colposcopy, Cytology, and HPV Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghebreyesus, T.A. WHO Director-General Calls for All Countries to Take Action to Help End the Suffering Caused by Cervical Cancer. Available online: https://www.who.int/news/item/18-05-2018-who-dg-calls-for-all-countries-to-take-action-to-help-end-the-suffering-caused-by-cervical-cancer (accessed on 13 January 2020).
- Xue, P.; Ng, M.T.A.; Qiao, Y. The challenges of colposcopy for cervical cancer screening in LMICs and solutions by artificial intelligence. BMC Med. 2020, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, A.; Roberts, J.M.; Garland, S.M.; Crescini, J.; Kaldor, J.M.; Machalek, D.A. Detection of high-grade cervical disease among women referred directly to colposcopy after a positive HPV screening test varies with age and cytology findings. Int. J. Cancer 2020, 147, 3068–3074. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.; Arbyn, M.; Parry-Smith, W.; De Bellis-Ayres, S.; Todd, R.; Redman, C.W.; Moss, E.L. Accuracy of colposcopy-directed punch biopsies: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Egemen, D.; Cheung, L.C.; Chen, X.; Demarco, M.; Perkins, R.B.; Kinney, W.; Poitras, N.; Befano, B.; Locke, A.; Guido, R.S.; et al. Risk Estimates Supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines. J. Low. Genit. Tract. Dis. 2020, 24, 132–143. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract. Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Schiffman, M.; Wentzensen, N.; Perkins, R.B.; Guido, R.S. An Introduction to the 2019 ASCCP Risk-Based Management Consensus Guidelines. J. Low. Genit. Tract. Dis. 2020, 24, 87–89. [Google Scholar] [CrossRef]
- Wentzensen, N.; Massad, L.S.; Mayeaux, E.J., Jr.; Khan, M.J.; Waxman, A.G.; Einstein, M.H.; Conageski, C.; Schiffman, M.H.; Gold, M.A.; Apgar, B.S.; et al. Evidence-Based Consensus Recommendations for Colposcopy Practice for Cervical Cancer Prevention in the United States. J. Low. Genit. Tract. Dis. 2017, 21, 216–222. [Google Scholar] [CrossRef]
- Wentzensen, N.; Schiffman, M.; Silver, M.I.; Khan, M.J.; Perkins, R.B.; Smith, K.M.; Gage, J.C.; Gold, M.A.; Conageski, C.; Einstein, M.H.; et al. ASCCP Colposcopy Standards: Risk-Based Colposcopy Practice. J. Low. Genit. Tract. Dis. 2017, 21, 230–234. [Google Scholar] [CrossRef]
- Wentzensen, N.; Walker, J.; Smith, K.; Gold, M.A.; Zuna, R.; Massad, L.S.; Liu, A.; Silver, M.I.; Dunn, S.T.; Schiffman, M. A prospective study of risk-based colposcopy demonstrates improved detection of cervical precancers. Am. J. Obs. Gynecol. 2018, 218, 604.e1–604.e8. [Google Scholar] [CrossRef]
- Solomon, D.; Davey, D.; Kurman, R.; Moriarty, A.; O’Connor, D.; Prey, M.; Raab, S.; Sherman, M.; Wilbur, D.; Wright, T., Jr.; et al. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA 2002, 287, 2114–2119. [Google Scholar] [CrossRef]
- Luesley, D.; Leeson, S. Colposcopy and Programme Management: Guidelines for the NHS Cervical Screening Programme, 2nd ed.; Public Health England: London, UK, 2010.
- Bornstein, J.; Bentley, J.; Bösze, P.; Girardi, F.; Haefner, H.; Menton, M.; Perrotta, M.; Prendiville, W.; Russell, P.; Sideri, M.; et al. 2011 colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy. Obs. Gynecol. 2012, 120, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Cree, I.A.; White, V.A.; Indave, B.I.; Lokuhetty, D. Revising the WHO classification: Female genital tract tumours. Histopathology 2020, 76, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Carreon, J.D.; Sherman, M.E.; Guillén, D.; Solomon, D.; Herrero, R.; Jerónimo, J.; Wacholder, S.; Rodríguez, A.C.; Morales, J.; Hutchinson, M.; et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: Results from a histological review of population-based cervical samples. Int. J. Gynecol. Pathol. 2007, 26, 441–446. [Google Scholar] [CrossRef]
- Herbert, A.; Arbyn, M.; Bergeron, C. Why CIN3 and CIN2 should be distinguished on histological reports. Cytopathology 2008, 19, 63–64. [Google Scholar] [CrossRef]
- Silver, M.I.; Andrews, J.; Cooper, C.K.; Gage, J.C.; Gold, M.A.; Khan, M.J.; Massad, L.S.; Parvu, V.; Perkins, R.B.; Schiffman, M.; et al. Risk of Cervical Intraepithelial Neoplasia 2 or Worse by Cytology, Human Papillomavirus 16/18, and Colposcopy Impression: A Systematic Review and Meta-analysis. Obs. Gynecol. 2018, 132, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, M.; Angeles, M.A.; Martí, C.; Henere, C.; Munmany, M.; Marimon, L.; Saco, A.; Rakislova, N.; Ordi, J.; Torné, A. Colposcopic Impression Has a Key Role in the Estimation of the Risk of HSIL/CIN3. Cancers 2021, 13, 1224. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Walker, J.L.; Gold, M.A.; Smith, K.M.; Zuna, R.E.; Mathews, C.; Dunn, S.T.; Zhang, R.; Moxley, K.; Bishop, E.; et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J. Clin. Oncol. 2015, 33, 83–89. [Google Scholar] [CrossRef]
- Schiffman, M.; Adrianza, M.E. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000, 44, 726–742. [Google Scholar] [CrossRef]
- Stoler, M.H.; Wright, T.C., Jr.; Parvu, V.; Vaughan, L.; Yanson, K.; Eckert, K.; Karchmer, T.; Kodsi, S.; Cooper, C.K. The Onclarity Human Papillomavirus Trial: Design, methods, and baseline results. Gynecol. Oncol. 2018, 149, 498–505. [Google Scholar] [CrossRef]
- Xue, P.; Tang, C.; Li, Q.; Li, Y.; Shen, Y.; Zhao, Y.; Chen, J.; Wu, J.; Li, L.; Wang, W.; et al. Development and validation of an artificial intelligence system for grading colposcopic impressions and guiding biopsies. BMC Med. 2020, 18, 406. [Google Scholar] [CrossRef]
- Jentschke, M.; Lehmann, R.; Drews, N.; Hansel, A.; Schmitz, M.; Hillemanns, P. Psychological distress in cervical cancer screening: Results from a German online survey. Arch. Gynecol. Obs. 2020, 302, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ohno, T.; Noguchi, W.; Matsuda, A.; Matsushima, E.; Kato, S.; Tsujii, H. Psychological distress and quality of life in cervical cancer survivors after radiotherapy: Do treatment modalities, disease stage, and self-esteem influence outcomes? Int. J. Gynecol. Cancer 2009, 19, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Number (%) |
|---|---|
| Age groups, y | |
| <30 | 989 (16.45) |
| 30–39 | 2261 (37.61) |
| 40–49 | 1672 (27.81) |
| >50 | 1090 (18.13) |
| Menopause | |
| No | 5065 (84.25) |
| Yes | 947 (15.75) |
| Gravidity | |
| None | 584 (9.71) |
| 1 | 1022 (17.00) |
| 2 | 1685 (28.03) |
| 3 | 1365 (22.70) |
| 4+ | 1356 (22.56) |
| Parity | |
| None | 944 (15.70) |
| 1 | 2588(43.05) |
| 2 | 1935 (32.19) |
| 3+ | 545 (9.06) |
| Cytology | |
| <HSIL | 5159 (85.81) |
| HSIL+ | 740 (12.31) |
| Not performed | 113 (1.88) |
| HPV status | |
| hrHPV negative | 889 (14.79) |
| Non-16/18 hrHPV | 3035 (50.48) |
| HPV16/18+ | 2049 (34.08) |
| Not performed | 39 (0.65) |
| Colposcopy impression | |
| Normal/benign | 1501 (24.97) |
| Low-grade | 3226 (53.66) |
| High-grade | 1285 (21.37) |
| Histological outcome | |
| <CIN2 | 4543 (75.57) |
| CIN2 | 881 (14.65) |
| CIN3 | 520 (8.65) |
| Cancer | 68 (1.13) |
| Risk Factors | CIN2+ | CIN3+ | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Cytology | ||||||||
| <HSIL | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| HSIL+ | 11.39 (9.57–13.55) | <0.001 | 3.54 (2.79–4.51) | <0.001 | 11.81 (9.77–14.29) | <0.001 | 2.87 (2.27–3.62) | <0.001 |
| HPV status | ||||||||
| hrHPV negative | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| Non-16/18 hrHPV | 3.12 (2.35–4.15) | <0.001 | 1.86 (1.29–2.67) | 0.001 | 2.68 (1.63–4.38) | <0.001 | 1.27 (0.72–2.22) | 0.41 |
| HPV16/18+ | 10.54 (7.95–13.97) | <0.001 | 4.91 (3.41–7.07) | <0.001 | 11.74 (7.27–18.96) | <0.001 | 3.65 (2.11–6.33) | <0.001 |
| Colposcopy | ||||||||
| Normal/benign | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| Low-grade | 5.02 (3.63–6.93) | <0.001 | 4.00 (2.87–5.58) | <0.001 | 11.72 (3.69–37.24) | <0.001 | 10.14 (3.18–32.40) | <0.001 |
| High-grade | 133.71 (95.63–186.95) | <0.001 | 70.03 (49.43–99.19) | <0.001 | 329.66 (105.62–1028.94) | <0.001 | 162.15 (51.37–511.79) | <0.001 |
| ID | Colposcopy | Cytology | Total, N | CIN2+, N | Risk | CIN3+, N | Risk |
|---|---|---|---|---|---|---|---|
| 1a | High-grade | HSIL+ | 519 | 462 | 0.8902 | 279 | 0.5376 |
| 1b | High-grade | <HSIL | 742 | 540 | 0.7278 | 224 | 0.3019 |
| 1c | Low-grade | HSIL+ | 187 | 63 | 0.3369 | 20 | 0.1070 |
| 1d | Low-grade | <HSIL | 2975 | 329 | 0.1106 | 53 | 0.0178 |
| 1f | Normal/benign | <HSIL | 1442 | 42 | 0.0291 | 3 | 0.0021 |
| 1e | Normal/benign | HSIL+ | 34 | 0 | 0 | 0 | 0 |
| ID | Colposcopy | HPV Status | Total, N | CIN2+, N | Risk | CIN3+, N | Risk |
|---|---|---|---|---|---|---|---|
| 2a | High-grade | HPV16/18 | 736 | 628 | 0.8533 | 355 | 0.4823 |
| 2b | High-grade | Non-16/18 hrHPV | 480 | 341 | 0.7104 | 131 | 0.2729 |
| 2c | High-grade | hrHPV negative | 54 | 36 | 0.6667 | 16 | 0.2963 |
| 2d | Low-grade | HPV16/18 | 993 | 212 | 0.2135 | 44 | 0.0443 |
| 2e | Low-grade | Non-16/18 hrHPV | 1942 | 176 | 0.0906 | 26 | 0.0134 |
| 2g | Normal/benign | HPV16/18 | 320 | 19 | 0.0594 | 1 | 0.0031 |
| 2f | Low-grade | hrHPV negative | 277 | 16 | 0.0578 | 2 | 0.0072 |
| 2h | Normal/benign | Non-16/18 hrHPV | 613 | 18 | 0.0294 | 2 | 0.0033 |
| 2i | Normal/benign | hrHPV negative | 558 | 5 | 0.0090 | 0 | 0 |
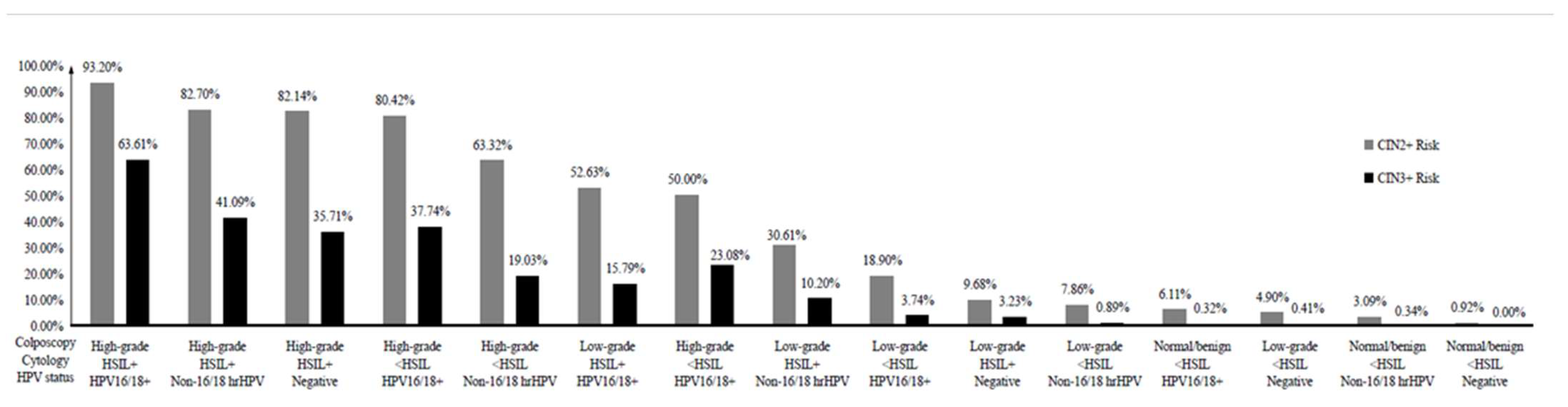
| ID | Colposcopy | Cytology | HPV Status | Total, N | CIN2+, N | Risk | CIN3+, N | Risk |
|---|---|---|---|---|---|---|---|---|
| 3a | High-grade | HSIL+ | HPV16/18+ | 294 | 274 | 0.9320 | 187 | 0.6361 |
| 3b | High-grade | HSIL+ | Non-16/18 hrHPV | 185 | 153 | 0.8270 | 76 | 0.4109 |
| 3c | High-grade | HSIL+ | hrHPV negative | 28 | 23 | 0.8214 | 10 | 0.3571 |
| 3d | High-grade | <HSIL | HPV16/18+ | 424 | 341 | 0.8042 | 160 | 0.3774 |
| 3e | High-grade | <HSIL | Non-16/18 hrHPV | 289 | 183 | 0.6332 | 55 | 0.1903 |
| 3g | Low-grade | HSIL+ | HPV16/18+ | 57 | 30 | 0.5263 | 9 | 0.1579 |
| 3f | High-grade | <HSIL | hrHPV negative | 26 | 13 | 0.50 | 6 | 0.2308 |
| 3h | Low-grade | HSIL+ | Non-16/18 hrHPV | 98 | 30 | 0.3061 | 10 | 0.1020 |
| 3j | Low-grade | <HSIL | HPV16/18+ | 910 | 172 | 0.1890 | 34 | 0.0374 |
| 3i | Low-grade | HSIL+ | hrHPV negative | 31 | 3 | 0.0968 | 1 | 0.0323 |
| 3k | Low-grade | <HSIL | Non-16/18 hrHPV | 1807 | 142 | 0.0786 | 16 | 0.0089 |
| 3p | Normal/benign | <HSIL | HPV16/18+ | 311 | 19 | 0.0611 | 1 | 0.0032 |
| 3l | Low-grade | <HSIL | hrHPV negative | 245 | 12 | 0.0490 | 1 | 0.0041 |
| 3q | Normal/benign | <HSIL | Non-16/18 hrHPV | 582 | 18 | 0.0309 | 2 | 0.0034 |
| 3r | Normal/benign | <HSIL | hrHPV negative | 541 | 5 | 0.0092 | 0 | 0 |
| 3n | Normal/benign | HSIL+ | Non-16/18 hrHPV | 17 | 0 | 0 | 0 | 0 |
| 3o | Normal/benign | HSIL+ | hrHPV negative | 16 | 0 | 0 | 0 | 0 |
| 3m | Normal/benign | HSIL+ | HPV16/18+ | None | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, P.; Seery, S.; Li, Q.; Jiang, Y.; Qiao, Y. Risk-Based Colposcopy for Cervical Precancer Detection: A Cross-Sectional Multicenter Study in China. Diagnostics 2022, 12, 2585. https://doi.org/10.3390/diagnostics12112585
Xue P, Seery S, Li Q, Jiang Y, Qiao Y. Risk-Based Colposcopy for Cervical Precancer Detection: A Cross-Sectional Multicenter Study in China. Diagnostics. 2022; 12(11):2585. https://doi.org/10.3390/diagnostics12112585
Chicago/Turabian StyleXue, Peng, Samuel Seery, Qing Li, Yu Jiang, and Youlin Qiao. 2022. "Risk-Based Colposcopy for Cervical Precancer Detection: A Cross-Sectional Multicenter Study in China" Diagnostics 12, no. 11: 2585. https://doi.org/10.3390/diagnostics12112585
APA StyleXue, P., Seery, S., Li, Q., Jiang, Y., & Qiao, Y. (2022). Risk-Based Colposcopy for Cervical Precancer Detection: A Cross-Sectional Multicenter Study in China. Diagnostics, 12(11), 2585. https://doi.org/10.3390/diagnostics12112585






