Computational Fluid Dynamics Could Enable Individualized Surgical Treatment of Nasal Obstruction (A Preliminary Study)
Abstract
1. Introduction
2. Methods
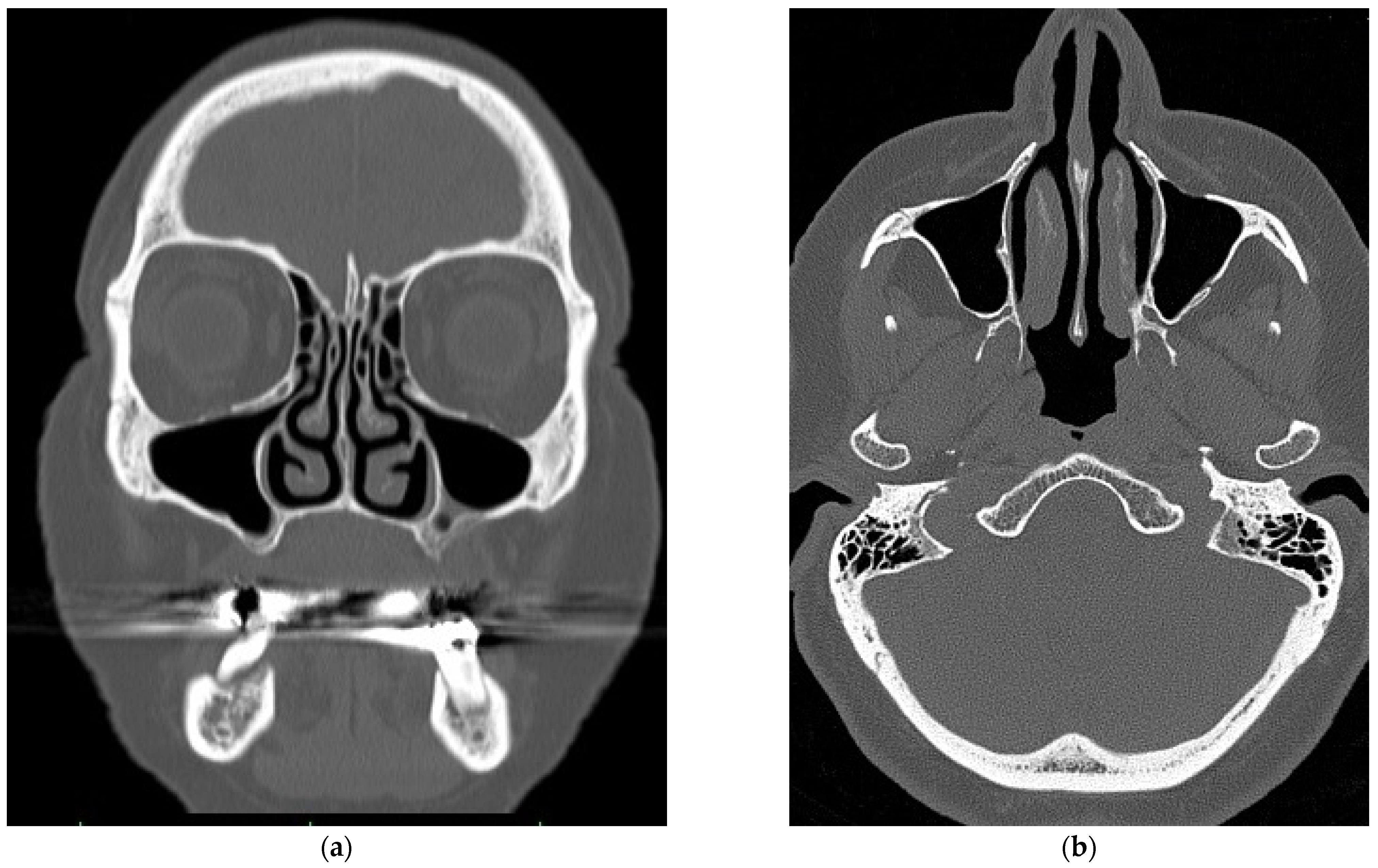
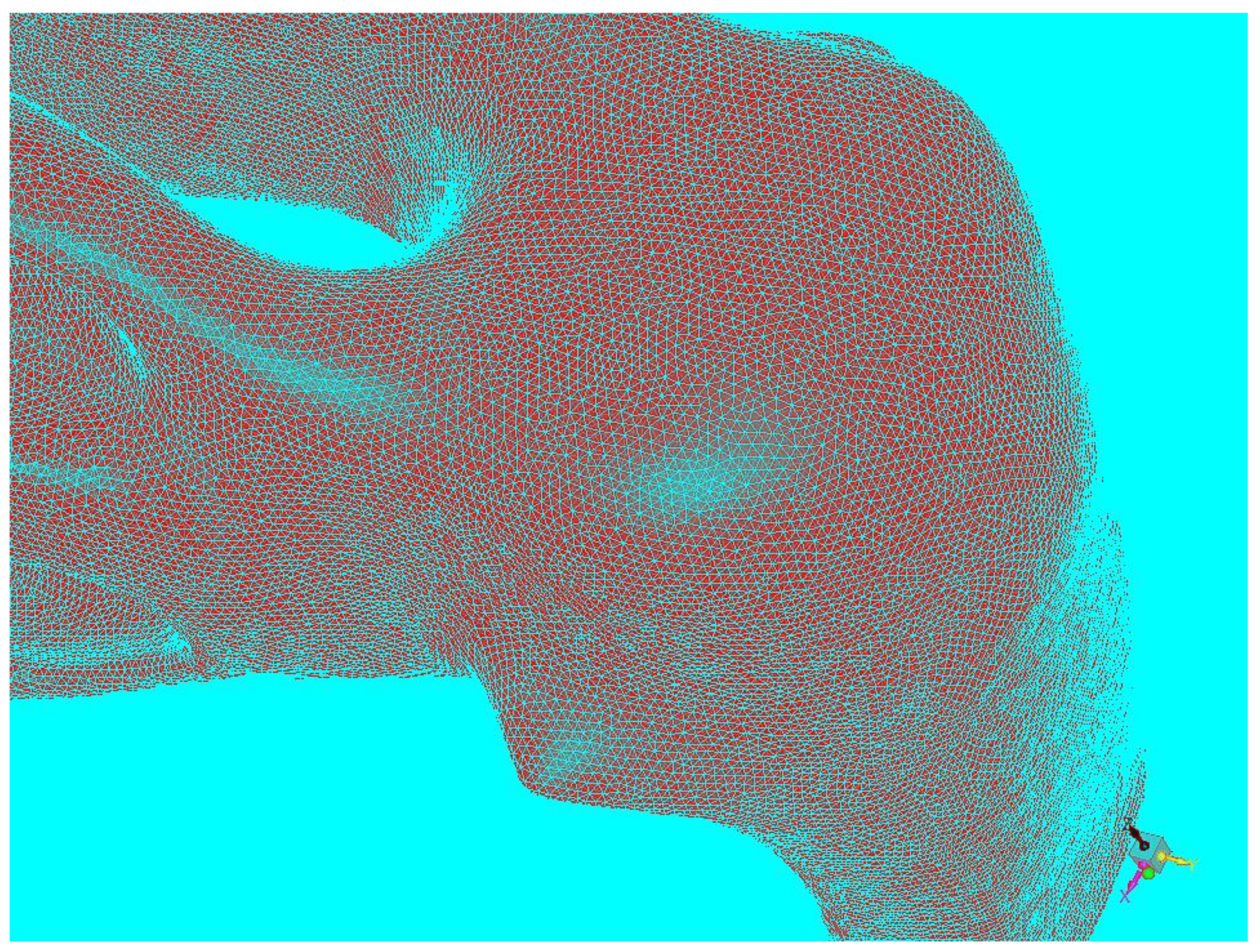
2.1. Virtual Model of the Nasal Cavity
2.2. Nasal Airflow Simulation
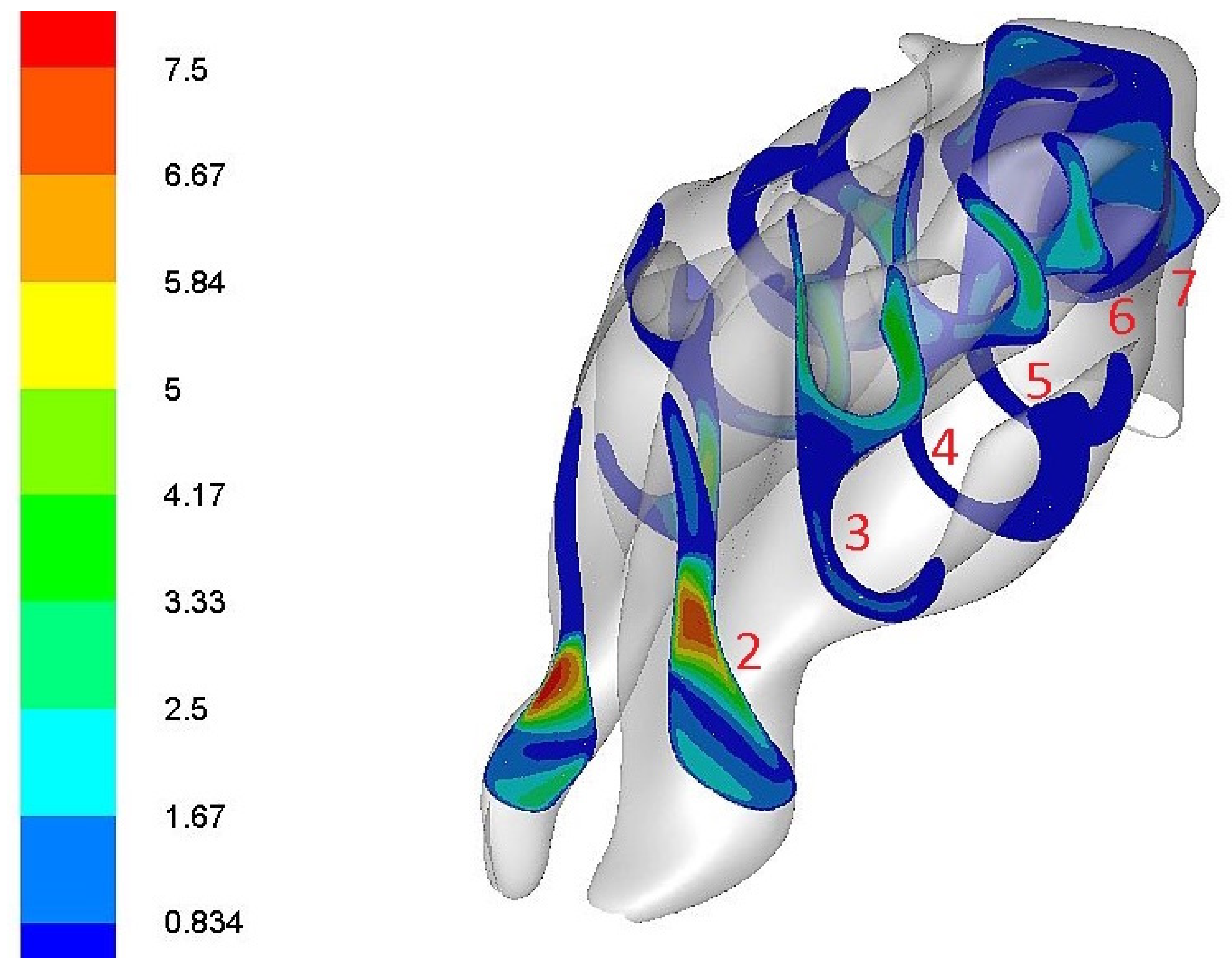
2.3. Locations of Measurements
- The nasal entrance (at 5 mm behind the entrance to the nasal cavity).
- The nasal valve (the plane that passed through the anterior edge of the lateral nasal cartilage).
- The front part of the nasal turbinates (at 5 mm behind the start of the lower turbinate),
- The middle part of the nasal turbinates (at 25 mm behind the start of the lower turbinate).
- The posterior part of the nasal turbinates (at 35 mm behind the start of the lower turbinate).
- The choana (the plane that passed through the choana, at 88 mm behind the nasal cavity entrance).
- The nasopharynx (the plane in the nasopharyngeal area, at 97 mm behind the nasal cavity entrance).
3. Results
3.1. The Main Routes of Nasal Airflow
3.2. The Greatest Resistance to Airflow
3.3. Highest Airflow Velocity
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chometon, F.; Ebbo, D.; Gillieron, P.; Koifman, P.; Lecomte, F.; Sorrel-Dejerine, N. A numerical simulation of the aerodynamics of the nasal cavity. Ann. Otolaryngol. Chir. Fac. 2000, 117, 98–104. [Google Scholar]
- Grützenmacher, S.; Lang, G.; Mlynski, G. Flow simulation, rhinoresistometry and acoustic rhinometry in nasal models before and after turbinate surgery. ORL 2003, 65, 341–347. [Google Scholar] [CrossRef]
- Liu, T.; Han, D.; Wang, J.; Tan, J.; Zang, H.; Wang, T.; Li, Y.; Cui, S. Effects of septal deviation on the airflow characteristics: Using computational fluid dynamics models. Acta Oto-Laryngol. 2012, 132, 290–298. [Google Scholar] [CrossRef]
- Önerci, T.; Shimzu, T.; Mlynski, R. Nasal Physiology and Pathophysiology of Nasal Disorders; Springer: Berlin/Heidelberg, Germany, 2013; Volume 280–300, pp. 323–356. [Google Scholar]
- Keyhani, K.; Scherrer, P.; Mozzel, M. Numerical simulation of airflow in the human nasal cavity. J. Biomech. Eng. 1995, 117, 429–441. [Google Scholar] [CrossRef]
- Leite, S.; Jain, R.; Douglas, R. The clinical implications of computerised fluid dynamics modelling in rhinology. Rhinology 2018, 56, 2–9. [Google Scholar] [CrossRef]
- Plášek, M.; Bojko, M.; Masárová, M.; Matoušek, P.; Komínek, P. The physiology and pathophysiology of the nasal airflow. Otorinolaryng A Foniat 2018, 67, 113–118. [Google Scholar]
- Lee, H.P.; Poh, H.J.; Chong, F.H.; Wang, D.Y. Changes of airflow pattern in inferior turbinate hypertrophy: A computational fluid dynamic model. Am. J. Rhinol. Allergy 2009, 23, 153–158. [Google Scholar] [CrossRef]
- Lee, T.S.; Goyal, P.; Li, C.; Zhao, K. Computational fluid dynamics to evaluate the effectiveness of inferior turbinate reduction techniques to improve nasal airflow. JAMA Facial Plast. Surg. 2018, 20, 263–270. [Google Scholar] [CrossRef]
- Bojko, M.; Kozdera, M.; Kozubkova, M. Investigation of viscous fluid flow in an eccentrically deposited annulus using CFD methods. EPJ Web Conf. 2013, 45, 01115. [Google Scholar] [CrossRef]
- Bojko, M.; Kocich, R. CFD analysis of the plate heat exchanger—Mathematical modelling of mass and heat transfer in serial connection with tubular heat exchanger. AIP Conf. Proc. 2016, 1745, 020002. [Google Scholar]
- Simmen, D.; Scherrer, J.L.; Moe, K.; Heinz, B. A dynamic and direct visualization model for the study of nasal airflow. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 1015–1021. [Google Scholar] [CrossRef]
- Mlynski, G.; Grützenmacher, S.; Plontke, S. A method for studying nasal airflow by means of fluid dynamics experiments. Z. Med. Phys. 2000, 10, 207–214. [Google Scholar] [CrossRef]
- Zhao, K.; Jiang, J. What is normal nasal airflow. A computational study of 22 healthy adults. Int. Forum Allergy Rhinol. 2014, 4, 435–446. [Google Scholar] [CrossRef]
- Zhao, K.; Dalton, P. The way the wind blows: Implications of modeling nasal airflow. Curr. Allergy Asthma Rep. 2007, 7, 117–125. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, H.; Gordon, B. Impacts of fluid dynamics simulation in study of nasal airflow physiology and pathophysiology in realistic human three-dimensional nose models. Clin. Exp. Otorhinolaryngol. 2012, 181, 181–187. [Google Scholar] [CrossRef]
- Tan, J.; Han, D.; Wang, J.; Liu, T.; Wang, T.; Zang, H.; Li, Y.; Wang, X. Numerical simulation of normal nasal cavity in Chinese adult: A computational flow dynamics model. Eur. Arch. Otorhinolaryngol. 2012, 269, 881–889. [Google Scholar] [CrossRef]
- Lee, K.B.; Jeon, Y.S.; Chung, S.K.; Kim, S.K. Effects of partial middle turbinectomy with varying resection volume and location on nasal functions and airflow characteristics by CFD. Comput. Biol. Med. 2016, 77, 214–221. [Google Scholar] [CrossRef]
- Leong, S.C.; Chen, X.B.; Lee, H.P.; Wang, D.Y. A review of the implications of computational fluid dynamics studies on nasal airflow and physiology. Rhinology 2010, 48, 139–145. [Google Scholar] [CrossRef]
- Schmidt, N.; Behrbohm, H.; Goubergrits, L.; Hildebrandt, T.; Brüning, J. Comparison of rhinomanometric and computational fluid dynamic assessment of nasal resistance with respect to measurement accuracy. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1519–1529. [Google Scholar] [CrossRef]
- Ormiskangas, J.; Valtonen, O.; Harju, T.; Rautiainen, M.; Kivekäs, I. Computational fluid dynamics assessed changes of nasal airflow after inferior turbinate surgery. Respir. Physiol. Neurobiol. 2022, 302, 103917. [Google Scholar] [CrossRef]
- Russel, S.M.; Frank-Ito, D.O. Gender Differences in Nasal Anatomy and Function Among Caucasians. Facial Plast. Surg. Aesthetic Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.; Menger, D.-J.; de Carvalho, H.C.; Spratley, J. An Overview of Computational Fluid Dynamics Preoperative Analysis of the Nasal Airway. Facial Plast. Surg. 2021, 37, 306–316. [Google Scholar] [CrossRef] [PubMed]


| Sections | Resistance (%) |
|---|---|
| 1–2 | 95 |
| 2–3 | 49.5 |
| 3–4 | 19.5 |
| 4–5 | 2.31 |
| 5–6 | 5.04 |
| 6–7 | 0.43 |
| Section | Maximal Velocity vmax (m/s) | Median Velocity vmed (m/s) |
|---|---|---|
| 1 | 11.8 | 3.09 |
| 2 | 8.34 | 2.09 |
| 3 | 4.64 | 1.21 |
| 4 | 3.70 | 0.69 |
| 5 | 2.99 | 0.79 |
| 6 | 1.71 | 0.62 |
| 7 | 1.14 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plášek, M.; Masárová, M.; Bojko, M.; Komínek, P.; Matoušek, P.; Formánek, M. Computational Fluid Dynamics Could Enable Individualized Surgical Treatment of Nasal Obstruction (A Preliminary Study). Diagnostics 2022, 12, 2642. https://doi.org/10.3390/diagnostics12112642
Plášek M, Masárová M, Bojko M, Komínek P, Matoušek P, Formánek M. Computational Fluid Dynamics Could Enable Individualized Surgical Treatment of Nasal Obstruction (A Preliminary Study). Diagnostics. 2022; 12(11):2642. https://doi.org/10.3390/diagnostics12112642
Chicago/Turabian StylePlášek, Marek, Michaela Masárová, Marián Bojko, Pavel Komínek, Petr Matoušek, and Martin Formánek. 2022. "Computational Fluid Dynamics Could Enable Individualized Surgical Treatment of Nasal Obstruction (A Preliminary Study)" Diagnostics 12, no. 11: 2642. https://doi.org/10.3390/diagnostics12112642
APA StylePlášek, M., Masárová, M., Bojko, M., Komínek, P., Matoušek, P., & Formánek, M. (2022). Computational Fluid Dynamics Could Enable Individualized Surgical Treatment of Nasal Obstruction (A Preliminary Study). Diagnostics, 12(11), 2642. https://doi.org/10.3390/diagnostics12112642





