A Simplified Sanger Sequencing Method for Detection of Relevant SARS-CoV-2 Variants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Amplification Protocol
2.3. Purifications and Sequencing
2.4. Data Analysis
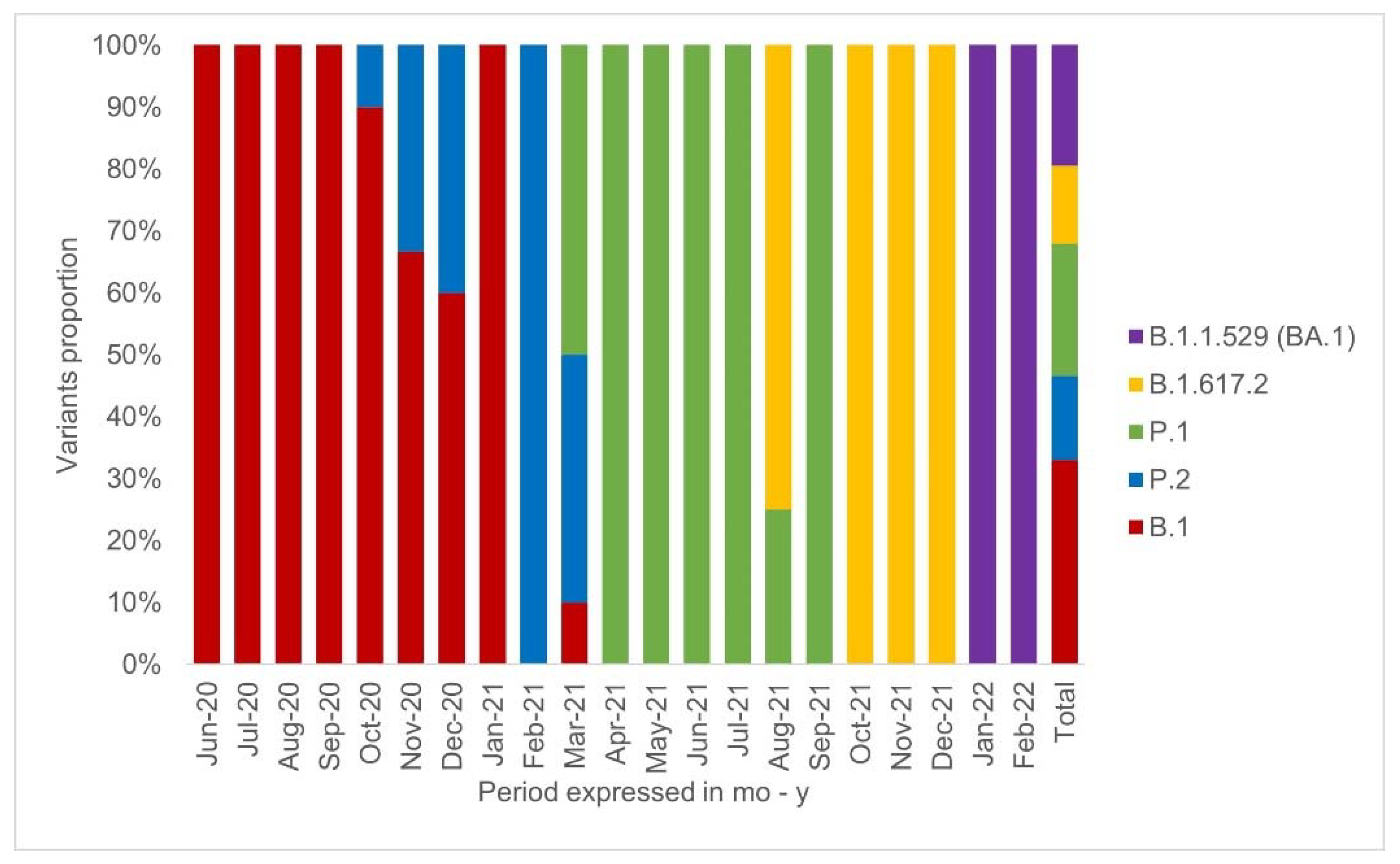
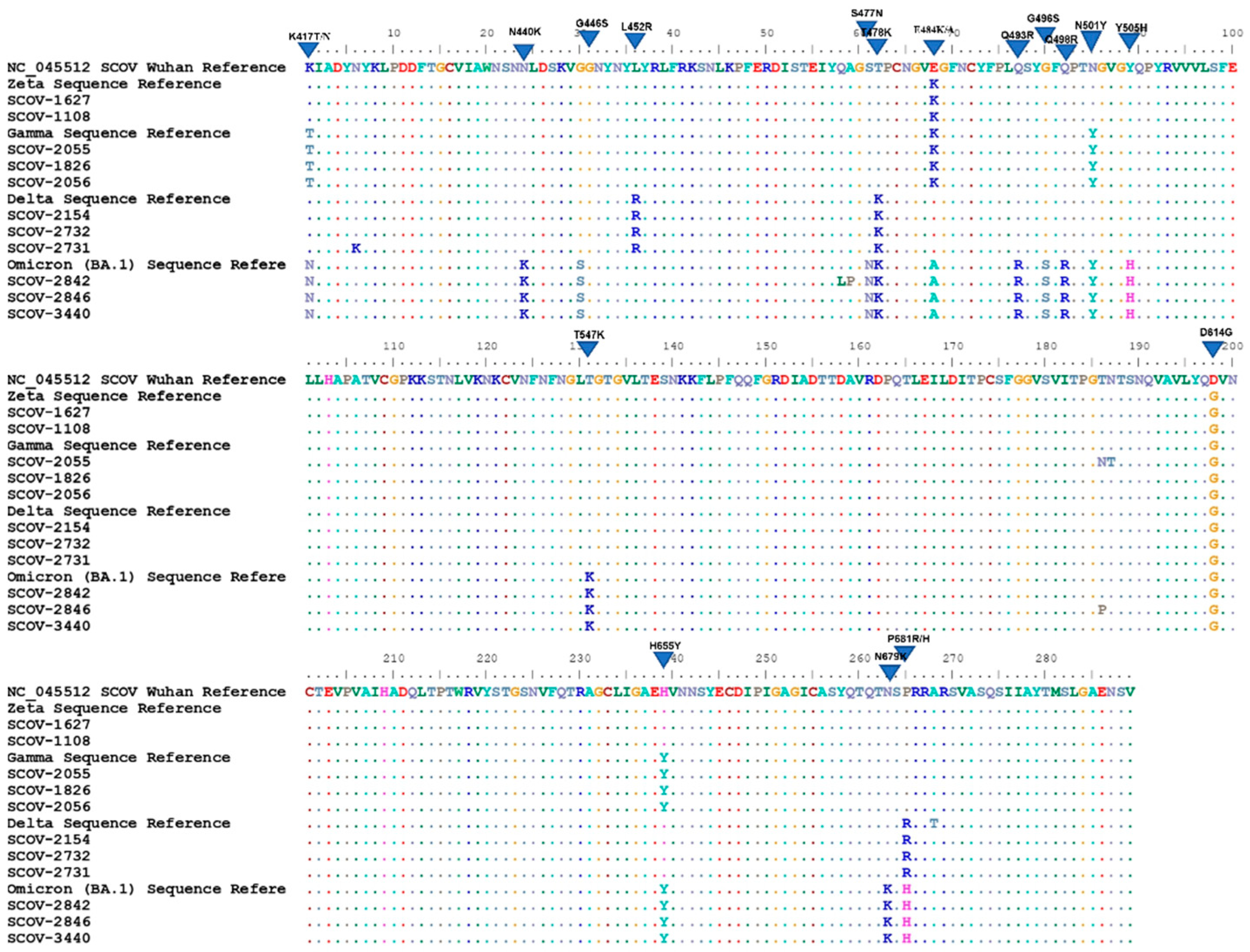
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef]
- Fujino, T.; Nomoto, H.; Kutsuna, S.; Ujiie, M.; Suzuki, T.; Sato, R.; Fujimoto, T.; Kuroda, M.; Wakita, T.; Ohmagari, N. Novel SARS-CoV-2 Variant in Travelers from Brazil to Japan. Emerg. Infect. Dis. 2021, 27, 1243–1245. [Google Scholar] [CrossRef]
- Chand, M.; Hopkins, S.; Dabrera, G.; Achison, C.; Barclay, W.; Ferguson, N.; Volz, E.; Loman, N.; Rambaut, A.; Barrett, J. Investigation of novel SARS-COV-2 variant Variant of Concern 202012/01. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959361/Technical_Briefing_VOC202012-2_Briefing_2.pdf (accessed on 10 May 2022).
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef]
- World Health Organization. Tracking SARS-CoV-2 variants [Internet]. 2022. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 29 May 2022).
- Tian, D.; Sun, Y.; Zhou, J.; Ye, Q. The Global Epidemic of the SARS-CoV-2 Delta Variant, Key Spike Mutations and Immune Escape. Front. Immunol. 2021, 12, 751778. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Munnink, B.B.O.; Worp, N.; Nieuwenhuijse, D.F.; Sikkema, R.S.; Haagmans, B.; Fouchier, R.A.M.; Koopmans, M. The next phase of SARS-CoV-2 surveillance: Real-time molecular epidemiology. Nat. Med. 2021, 27, 1518–1524. [Google Scholar] [CrossRef]
- GISAID Initiative [Internet]. Available online: https://www.gisaid.org/ (accessed on 23 March 2021).
- Salles, T.S.; Cavalcanti, A.C.; da Costa, F.B.; Dias, V.Z.; de Souza, L.M.; de Meneses, M.D.F.; da Silva, J.A.S.; Amaral, C.D.; Felix, J.R.; Pereira, D.A.; et al. Genomic surveillance of SARS-CoV-2 Spike gene by sanger sequencing. PLoS ONE 2022, 17, e0262170. [Google Scholar] [CrossRef]
- Vaz, S.N.; de Santana, D.S.; Netto, E.M.; Pedroso, C.; Wang, W.-K.; Santos, F.D.A.; Brites, C. Saliva is a reliable, non-invasive specimen for SARS-CoV-2 detection. Braz. J. Infect. Dis. 2020, 24, 422–427. [Google Scholar] [CrossRef]
- Shaibu, J.O.; Onwuamah, C.K.; James, A.B.; Okwuraiwe, A.P.; Amoo, O.S.; Salu, O.B.; Ige, F.A.; Liboro, G.; Odewale, E.; Okoli, L.C.; et al. Full length genomic sanger sequencing and phylogenetic analysis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Nigeria. PLoS ONE 2021, 16, e0243271. [Google Scholar] [CrossRef]
- Rambaut, A.; Holmes, E.C.; O’Toole, Á.; Hill, V.; McCrone, J.T.; Ruis, C.; du Plessis, L.; Pybus, O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020, 5, 1403–1407. [Google Scholar] [CrossRef]
- World Health Organization. Genomic Sequencing of SARS-CoV-2: A Guide to Implementation for Maximum Impact on Public Health; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Jørgensen, T.S.; Blin, K.; Kuntke, F.; Salling, H.K.; Michaelsen, T.Y.; Albertsen, M.; Larsen, H. A rapid, cost efficient and simple method to identify current SARS-CoV-2. medRxiv 2021. [Google Scholar] [CrossRef]
- Daniels, R.S.; Harvey, R.; Ermetal, B.; Xiang, Z.; Galiano, M.; Adams, L.; McCauley, J.W. A Sanger sequencing protocol for SARS-CoV-2 S-gene. Influ. Other Respir. Viruses 2021, 15, 707–710. [Google Scholar] [CrossRef]
- Bezerra, M.F.; Machado, L.C.; De Carvalho, V.D.C.V.; Docena, C.; Brandão-Filho, S.P.; Ayres, C.F.J.; Paiva, M.H.S.; Wallau, G.L. A Sanger-based approach for scaling up screening of SARS-CoV-2 variants of interest and concern. Infect. Genet. Evol. 2021, 92, 104910. [Google Scholar] [CrossRef]
- Lim, H.J.; Park, M.Y.; Jung, H.S.; Kwon, Y.; Kim, I.; Kim, D.K.; Yu, N.; Sung, N.; Lee, S.-H.; Park, J.E.; et al. Development of an efficient Sanger sequencing-based assay for detecting SARS-CoV-2 spike mutations. PLoS ONE 2021, 16, e0260850. [Google Scholar] [CrossRef]
- Tzou, P.L.; Ariyaratne, P.; Varghese, V.; Lee, C.; Rakhmanaliev, E.; Villy, C.; Yee, M.; Tan, K.; Michel, G.; Pinsky, B.A.; et al. Comparison of an In Vitro Diagnostic Next-Generation Sequencing Assay with Sanger Sequencing for HIV-1 Genotypic Resistance Testing. J. Clin. Microbiol. 2018, 56, e00105-18. [Google Scholar] [CrossRef]
- Lauring, A.S.; Hodcroft, E.B. Genetic Variants of SARS-CoV-2—What Do They Mean? JAMA 2021, 325, 529–531. [Google Scholar] [CrossRef]
- Cuevas, J.; Geller, R.; Garijo, R.; López-Aldeguer, J.; Sanjuán, R. Extremely High Mutation Rate of HIV-1 In Vivo. PLOS Biol. 2015, 13, e1002251. [Google Scholar] [CrossRef]
- LACEN-Ba. Boletim de Sequenciamento SARS-CoV-2; LACEN-Ba: Salvador, Brazil, 2022. [Google Scholar]
- Wang, L.; Cheng, G. Sequence analysis of the emerging SARS-CoV-2 variant Omicron in South Africa. J. Med Virol. 2021, 94, 1728–1733. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Sarkar, R. Mutational and phylogenetic analyses of the two lineages of the Omicron variant. J. Med. Virol. 2021, 94, 1777–1779. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. What Omicron’s BA.4 and BA.5 variants mean for the pandemic. Nature 2022, 606, 848–849. [Google Scholar] [CrossRef]
- World Health Organization. Statement on Omicron sublineage BA.2 [Internet]. 2022. Available online: https://www.who.int/news/item/22-02-2022-statement-on-omicron-sublineage-ba.2 (accessed on 29 May 2022).
- Singh, J.; Rahman, S.A.; Ehtesham, N.Z.; Hira, S.; Hasnain, S.E. SARS-CoV-2 variants of concern are emerging in India. Nat. Med. 2021, 27, 1131–1133. [Google Scholar] [CrossRef]
- Ko, K.; Takahashi, K.; Nagashima, S.; Bunthen, E.; Ouoba, S.; Hussain, R.A.; Akita, T.; Sugiyama, A.; Sakaguchi, T.; Tahara, H.; et al. Mass Screening of SARS-CoV-2 Variants using Sanger Sequencing Strategy in Hiroshima, Japan. Sci. Rep. 2022, 12, 2419. [Google Scholar] [CrossRef]
- Naveca, F.G.; Nascimento, V.; de Souza, V.C.; Corado, A.D.L.; Nascimento, F.; Silva, G.; Costa, Á.; Duarte, D.; Pessoa, K.; Mejía, M.; et al. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P.1 emergence. Nat. Med. 2021, 27, 1230–1238. [Google Scholar] [CrossRef]
- Ferrareze, P.A.G.; Franceschi, V.B.; Mayer, A.D.M.; Caldana, G.D.; Zimerman, R.A.; Thompson, C.E. E484K as an innovative phylogenetic event for viral evolution: Genomic analysis of the E484K spike mutation in SARS-CoV-2 lineages from Brazil. Infect. Genet. Evol. 2021, 93, 104941. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6; Erratum in Cell 2020, 183, 1735. [Google Scholar] [CrossRef]
- Fantini, J.; Yahi, N.; Colson, P.; Chahinian, H.; La Scola, B.; Raoult, D. The puzzling mutational landscape of the SARS-2-variant Omicron. J. Med Virol. 2022, 94, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.N.; Greaney, A.J.; Dingens, A.S.; Bloom, J.D. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. bioRxiv 2021, 22, 431683, Update in Cell Rep. Med. 2021, 2, 100255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature 2021, 602, 294–299. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med Virol. 2021, 94, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.; da Costa, C.; Nascimento, V.; Souza, V.; Corado, A.; Nascimento, F.; Costa, Á.; Duarte, D.; Silva, G.; Mejía, M.; et al. SARS-CoV-2 reinfection by the new Variant of Concern (VOC) P.1 in Amazonas, Brazil. Virological 2021. Available online: https://virological.org/t/sars-cov-2-reinfection-by-the-new-variant-of-concern-voc-p-1-in-amazonas-brazil/596 (accessed on 15 February 2022).
- Hoffmann, M.; Arora, P.; Groß, R.; Seidel, A.; Hörnich, B.F.; Hahn, A.S.; Krüger, N.; Graichen, L.; Hofmann-Winkler, H.; Kempf, A.; et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell 2021, 184, 2384–2393.e12. [Google Scholar] [CrossRef]

| Primer’s Name | Sequence | T.M | T.A | Position | |
|---|---|---|---|---|---|
| 1R | Primer foward 75 (a) | AGAGTCCAACCAACAGAATCTATTGT | 64.5° | 60° | 22.517–22.524 |
| Primer reverso 79 (a) | CATTTCATCTGTGAGCAAAGGTGG | 64.4° | 60° | 24.146–24.169 | |
| 2R | Primer forward CoV17 (b) | ATCTCTGCTTTACTAATGTCTATGC | 64.5° | 60° | 22.728–22.752 |
| Primer reverso 78 (a) | TGTGTACAAAAACTGCCATATTGCA | 64.5° | 60° | 23.823–23.847 | |
| SEQ | Primer foward 77 (a) | CCAGCAACTGTTTGTGGACCTA | 64.9° | 60° | 23.123–23.144 |
| Primer reverso 77 (a) | CAGCCCCTATTAAACAGCCTGC | 65.4° | 60° | 23.501–23.522 |
| Lineage | Zeta | Gama | Delta | Omicron | Alfa | Beta | Lambda | Eta | Iota | Kappa | Epsilon | Mu | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation | P.2 | P.1 | B.1.617.2 | BA.1 | BA.2 | BA.4 | BA.5 | B.1.1.7 | B.1.351 | C.37 | B.1.525 | B.1.526 | B.1.617.1 | B.1.427 | B.1.621 | |
| K417N | X | X | X | X | X | |||||||||||
| K417T | X | |||||||||||||||
| N440K | X | X | X | X | ||||||||||||
| G446S | X | X | X | X | ||||||||||||
| L452Q | X | |||||||||||||||
| L452R | X | X | X | X | X | |||||||||||
| S477N | X | X | X | X | X | |||||||||||
| T478K | X | X | X | X | X | |||||||||||
| E484A | X | X | X | X | ||||||||||||
| E484K | X | X | X | X | X | X | ||||||||||
| E484Q | X | |||||||||||||||
| F486V | X | X | ||||||||||||||
| F490S | X | |||||||||||||||
| Q493R | X | X | ||||||||||||||
| G496S | X | |||||||||||||||
| Q498R | X | X | X | X | ||||||||||||
| N501Y | X | X | X | X | X | X | X | X | ||||||||
| Y505H | X | X | X | X | ||||||||||||
| T547K | X | |||||||||||||||
| A570D | X | |||||||||||||||
| D614G | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| H655Y | X | X | X | X | X | |||||||||||
| Q677H | X | |||||||||||||||
| N679K | X | X | X | X | ||||||||||||
| P681H | X | X | X | X | X | X | ||||||||||
| P681R | X | X | ||||||||||||||
| A701V | X | X | ||||||||||||||
| T716I | X | |||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deminco, F.; Vaz, S.N.; Santana, D.S.; Pedroso, C.; Tadeu, J.; Stoecker, A.; Vieira, S.M.; Netto, E.; Brites, C. A Simplified Sanger Sequencing Method for Detection of Relevant SARS-CoV-2 Variants. Diagnostics 2022, 12, 2609. https://doi.org/10.3390/diagnostics12112609
Deminco F, Vaz SN, Santana DS, Pedroso C, Tadeu J, Stoecker A, Vieira SM, Netto E, Brites C. A Simplified Sanger Sequencing Method for Detection of Relevant SARS-CoV-2 Variants. Diagnostics. 2022; 12(11):2609. https://doi.org/10.3390/diagnostics12112609
Chicago/Turabian StyleDeminco, Felice, Sara N. Vaz, Daniele S. Santana, Celia Pedroso, Jean Tadeu, Andreas Stoecker, Sueli M. Vieira, Eduardo Netto, and Carlos Brites. 2022. "A Simplified Sanger Sequencing Method for Detection of Relevant SARS-CoV-2 Variants" Diagnostics 12, no. 11: 2609. https://doi.org/10.3390/diagnostics12112609
APA StyleDeminco, F., Vaz, S. N., Santana, D. S., Pedroso, C., Tadeu, J., Stoecker, A., Vieira, S. M., Netto, E., & Brites, C. (2022). A Simplified Sanger Sequencing Method for Detection of Relevant SARS-CoV-2 Variants. Diagnostics, 12(11), 2609. https://doi.org/10.3390/diagnostics12112609







