Incorporation of Tumor-Free Distance and Other Alternative Ultrasound Biomarkers into a Myometrial Invasion-Based Model Better Predicts Lymph Node Metastasis in Endometrial Cancer: Evidence and Future Prospects
Abstract
1. Introduction
2. Materials and Methods
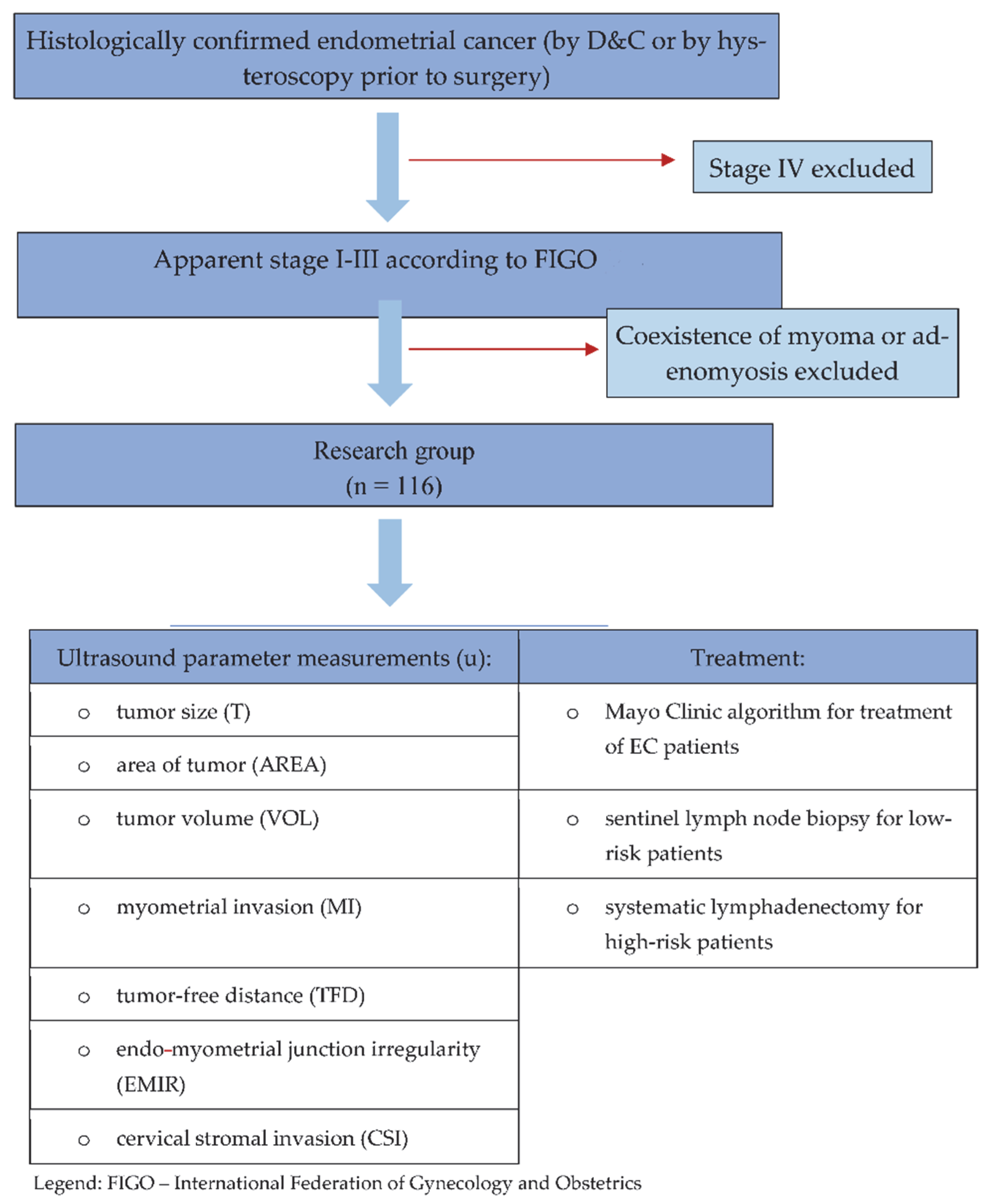
2.1. Study Design and Participants
2.2. Ultrasound Examination
2.3. Surgery, Including Lymph Nodes Procedure
2.4. Histopathology
2.5. Statistical Analysis
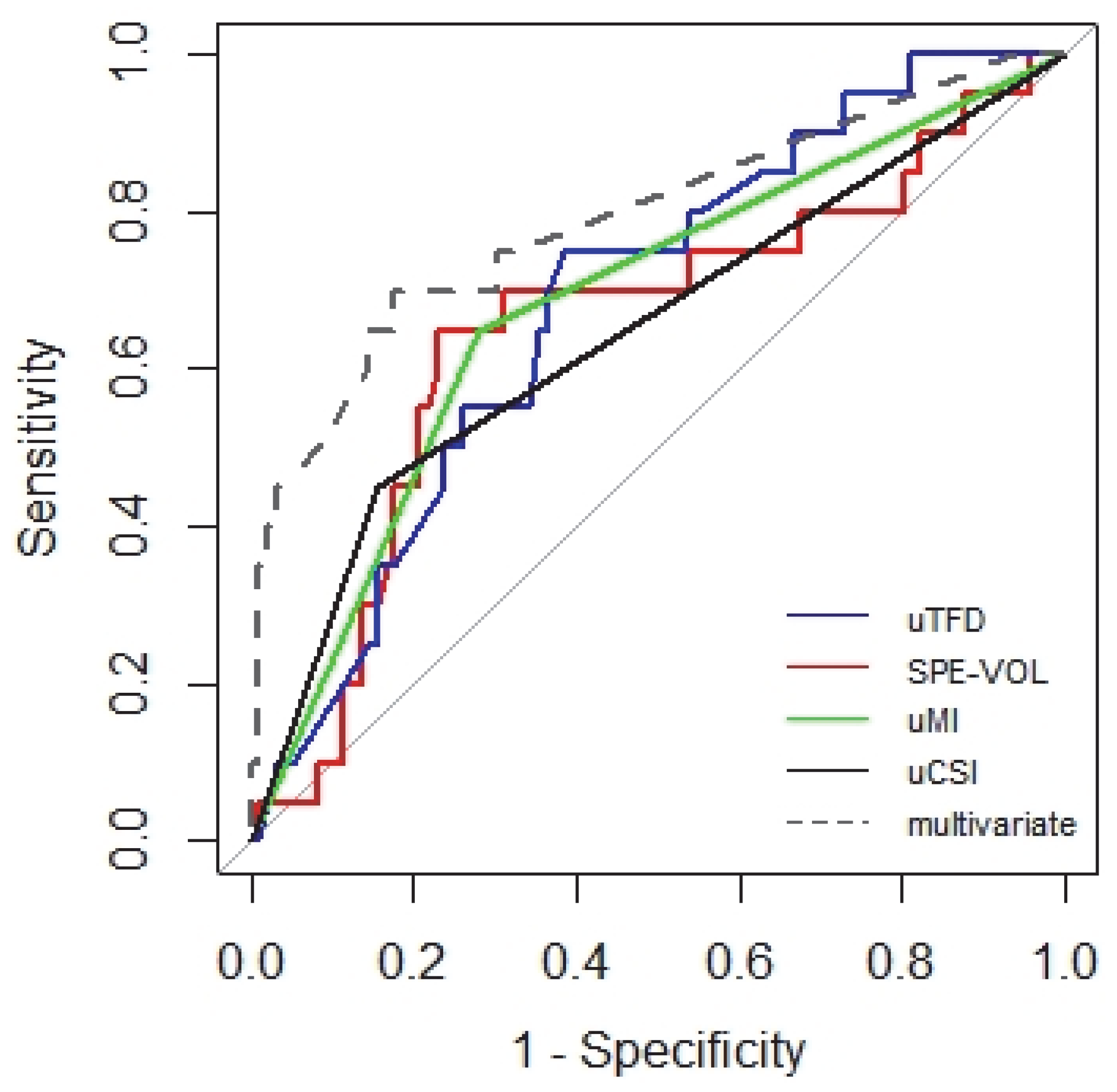
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C. ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi33–vi38. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Dobrotwir, A.; McNally, O.; Abu-Rustum, N.R.; Narayan, K. Role of imaging in the routine management of endometrial cancer. Int. J. Gynecol. Obstet. 2018, 143 (Suppl. 2), 109–117. [Google Scholar] [CrossRef]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer: A gynecologic oncology group study. Cancer 1987, 60 (Suppl. 8), 2035–2041. [Google Scholar] [CrossRef]
- Lee, K.R.; Vacek, P.M.; Belinson, J.L. Traditional and nontraditional histopathologic predictors of recurrence in uterine endometrioid adenocarcinoma. Gynecol. Oncol. 1994, 54, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Black, D.; Soslow, R.A. Difficulties in assessing the depth of myometrial invasion in endometrial carcinoma. Int. J. Gynecol. Pathol. 2007, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.M.; Lawrence, W.D. Endometrial adenocarcinoma with variable-level myometrial involvement limited to adenomyosis: A clinicopathologic study of 23 cases. Gynecol. Oncol. 1990, 37, 401–407. [Google Scholar] [CrossRef]
- Murray, S.K.; Young, R.H.; Scully, R.E. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: A study of their frequency, associated diagnostic problems, and prognostic significance. Int. J. Gynecol. Pathol. 2003, 22, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis: Use of FIGO Grading and Genomic Subcategories in Clinical Practice: Recommendations of the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S64–S74. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial car-cinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Schink, J.C.; Rademaker, A.W.; Miller, D.S.; Lurain, J.R. Tumor size in endometrial cancer. Cancer 1991, 67, 2791–2794. [Google Scholar] [CrossRef]
- Karlsson, B.; Norström, A.; Granberg, S.; Wikland, M. The use of endovaginal ultrasound to diagnose invasion of endometrial carcinoma. Ultrasound Obstet. Gynecol. 1992, 2, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Choi, H.J.; Kang, S.; Kim, J.; Nam, J.; Watari, H.; Tamakoshi, A.; Sakuragi, N. Clinical significance of tumor volume in endometrial cancer: A Japan-Korea cooperative study. Gynecol. Oncol. 2013, 131, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Galaal, K.A.; Patel, A.; Fisher, A.; Nayar, A.; Cross, P.; Naik, R. Tumour-free distance from serosa is a better prognostic indicator than depth of invasion and percentage myometrial invasion in endometrioid endometrial cancer. BJOG 2012, 119, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.M.; Connor, G.P.; Broste, S.K.; Krawisz, B.R.; Johnson, K.K. Prognostic significance of gross myometrial invasion with endometrial cancer. Obstet. Gynecol. 1996, 88, 394–398. [Google Scholar] [CrossRef]
- Naftalin, J.; Jurkovic, D. The endometrial-myometrial junction: A fresh look at a busy crossing. Ultrasound Obstet. Gynecol. 2009, 34, 1–11. [Google Scholar] [CrossRef]
- Singh, N.; Hirschowitz, L.; Zaino, R.; Alvarado-Cabrero, I.; Duggan, M.A.; Ali-Fehmi, R.; Euscher, E.; Hecht, J.L.; Horn, L.; Ioffe, O.; et al. Pathologic Prognostic Factors in Endometrial Carcinoma (Other Than Tumor Type and Grade). Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S93–S113. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLO-BOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Liro, M.; Śniadecki, M.; Wycinka, E.; Wojtylak, S.; Brzeziński, M.; Stańczak, A.; Wydra, D. Ultrasound measurement of tumor-free distance from the serosal surface as the alternative to measuring the depth of myometrial invasion in predicting lymph node metastases in endometrial cancer. Diagnostics 2021, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997, 277, 9256. [Google Scholar]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Dowdy, S.C.; Keeney, G.L.; Long, H.J.; Lesnick, T.G.; Podratz, K.C. High-risk endometrial cancer subgroups: Candidates for target-based adjuvant therapy. Gynecol. Oncol. 2004, 95, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Dowdy, S.C.; Cliby, W.A.; Gostout, B.S.; Jones, M.B.; Wilson, T.O.; Podratz, K.C. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol. Oncol. 2008, 109, 11–18. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Eijkemans, M.J.; Harrell, F.E., Jr.; Habbema, J.D. Prognostic modelling with logistic regression analysis: A comparison of selection and estimation methods in small data sets. Stat. Med. 2000, 30, 1059–1079. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41, published correction appears in Ann. Oncol. 2017, 28 (Suppl. 4), iv167–iv168. [Google Scholar] [CrossRef]
- Antonsen, S.L.; Jensen, L.N.; Loft, A.; Berthelsen, A.K.; Costa, J.; Tabor, A.; Qvist, I.; Hansen, M.R.; Fisker, R.; Søgaard Andersen, E.; et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer—A multicenter prospective comparative study. Gynecol. Oncol. 2013, 128, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Koplay, M.; Dogan, N.U.; Erdogan, H.; Sivri, M.; Erol, C.; Nayman, A.; Karabagli, P.; Paksoy, Y.; Celik, C. Diagnostic efficacy of diffusion-weighted MRI for pre-operative assessment of myometrial and cervical invasion and pelvic lymph node metastasis in endometrial carcinoma. J. Med. Imaging Radiat. Oncol. 2014, 58, 538–648. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, M.; Crivellaro, C.; Buda, A.; Guerra, L.; Fruscio, R.; Elisei, F.; Dolci, C.; Cuzzocrea, M.; Milani, R.; Messa, C. Staging of High-Risk Endometrial Cancer With PET/CT and Sentinel Lymph Node Mapping. Clin. Nucl. Med. 2015, 40, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Naftalin, J.; Nunes, N.; Hoo, W.; Arora, R.; Jurkovic, D. Endometrial cancer and ultrasound: Why measuring endometrial thickness is sometimes not enough. Ultrasound Obstet. Gynecol. 2012, 39, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Frühauf, F.; Zikan, M.; Semeradova, I.; Dundr, P.; Nemejcova, K.; Dusek, L.; Cibula, D.; Fischerova, D. The Diagnostic Accuracy of Ultrasound in Assessment of Myometrial Invasion in Endometrial Cancer: Subjective Assessment versus Objective Techniques. Biomed. Res. Int. 2017, 2017, 1318203. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Orozco, R.; Martinez-Astorquiza Corral, T.; Juez, L.; Utrilla-Layna, J.; Mínguez, J.A.; Jurado, M. Transvaginal ultrasound for preoperative assessment of myometrial invasion in patients with endometrial cancer: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2015, 46, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.S.E.; Epstein, E.; Testa, A.C.; Fischerova, D.; Valentin, L.; Sladkevicius, P.; Franchi, D.; Frühauf, F.; Fruscio, R.; Haak, L.A.; et al. Ultrasound-based risk model for preoperative prediction of lymph-node metastases in women with endometrial cancer: Model-development study. Ultrasound Obstet. Gynecol. 2020, 56, 443–452. [Google Scholar] [CrossRef]
- Verbakel, J.Y.; Mascilini, F.; Wynants, L.; Fischerova, D.; Testa, A.C.; Franchi, D.; Frühauf, F.; Cibula, D.; Lindqvist, P.G.; Fruscio, R.; et al. Validation of ultrasound strategies to assess tumor extension and to predict high-risk endometrial cancer in women from the prospective IETA (International Endometrial Tumor Analysis)-4 cohort. Ultrasound Obstet. Gynecol. 2020, 55, 115–124. [Google Scholar] [CrossRef]
- Alcazar, J.L.; Pineda, L.; Martinez-Astorquiza Corral, T.; Orozco, R.; Utrilla-Layna, J.; Juez, L.; Jurado, M. Transvaginal/transrectal ultrasound for assessing myometrial invasion in endometrial cancer: A comparison of six different approaches. J. Gynecol. Oncol. 2015, 26, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.; Møller, C.; Rydbjerg, S.; Hansen, E.S.; Ørtoft, G. An ultrasound algorithm for identification of endometrial cancer. Ultrasound Obstet. Gynecol. 2014, 43, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yamashita, T.; Ishikawa, M. Regulation of tumor invasion by HOXB13 gene overexpressed in human endometrial cancer. Oncol. Rep. 2005, 13, 721–726. [Google Scholar] [CrossRef]
- Gibson, D.A.; Saunders, P.T. Endocrine disruption of oestrogen action and female reproductive tract cancers. Endocr.-Relat. Cancer 2014, 21, T13–T31. [Google Scholar] [CrossRef]
- Epstein, E.; Fischerova, D.; Valentin, L.; Testa, A.C.; Franchi, D.; Sladkevicius, P.; Frühauf, F.; Lindqvist, P.G.; Mascilini, F.; Fruscio, R.; et al. Ultrasound characteristics of endometrial cancer as defined by International Endometrial Tumor Analysis (IETA) consensus nomenclature: Prospective multicenter study. Ultrasound Obstet. Gynecol. 2018, 51, 818–828, published correction appears in Ultrasound Obstet. Gynecol. 2018, 52, 684. [Google Scholar] [CrossRef]
- Mitamura, T.; Watari, H.; Todo, Y.; Kato, T.; Konno, Y.; Hosaka, M.; Sakuragi, N. Lymphadenectomy can be omitted for low-risk endometrial cancer based on preoperative assessments. J. Gynecol. Oncol. 2014, 25, 301–305. [Google Scholar] [CrossRef]
- Imai, K.; Kato, H.; Katayama, K.; Nakanishi, K.; Kawano, A.; Iura, A.; Konnai, K.; Onose, R.; Hirahara, F.; Miyagi, E. A preoperative risk-scoring system to predict lymph node metastasis in endometrial cancer and stratify patients for lymphadenectomy. Gynecol. Oncol. 2016, 142, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Watari, H.; Okamoto, K.; Hareyama, H.; Minobe, S.; Kato, H.; Sakuragi, N. Tumor volume successively reflects the state of disease progression in endometrial cancer. Gynecol. Oncol. 2013, 129, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Trujillo, A.; Martínez-Serrano, M.J.; Martínez-Román, S.; Martí, C.; Buñesch, L.; Nicolau, C.; Pahisa, J. Preoperative Assessment of Myometrial Invasion in Endometrial Cancer by 3D Ultrasound and Diffusion-Weighted Magnetic Resonance Imaging: A Comparative Study. Int. J. Gynecol. Cancer 2016, 26, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Berretta, R.; Merisio, C.; Piantelli, G.; Rolla, M.; Giordano, G.; Melpignano, M.; Nardelli, G.B. Preoperative transvaginal ultrasonography and intraoperative gross examination for assessing myometrial invasion by endometrial cancer. J. Ultrasound Med. 2008, 27, 349–355. [Google Scholar] [CrossRef]
- Fischerova, D.; Frühauf, F.; Zikan, M.; Pinkavova, I.; Kocián, R.; Dundr, P.; Nemejcova, K.; Dusek, L.; Cibula, D. Factors affecting sonographic preoperative local staging of endometrial cancer. Ultrasound Obstet. Gynecol. 2014, 43, 575–585. [Google Scholar] [CrossRef]
- Hertel, J.D.; Huettner, P.C.; Pfeifer, J.D. Lymphovascular space invasion in microcystic elongated and fragmented (MELF)-pattern well-differentiated endometrioid adenocarcinoma is associated with a higher rate of lymph node metastasis. Int. J. Gynecol. Pathol. 2014, 33, 127–134. [Google Scholar] [CrossRef]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]

| Variable | Characteristic | Value |
|---|---|---|
| Age at diagnosis (range) | Mean +/− SD (range) | 63 +/− 8.3 (40–85) |
| FIGO stage * | Number (%) | |
| Ia | 69 (59) | |
| Ib | 35 (30) | |
| II | 5 (5) | |
| III | 7 (6) | |
| Histologic type | Number (%) | |
| Endometrioid | 82 (71) | |
| Endometrioid with epithelial differentiation | 20 (17) | |
| Serous carcinoma | 11 (9) | |
| Carcinosarcoma | 3 (3) | |
| Grade | Number (%) | |
| 1 | 41 (36) | |
| 2 | 45 (39) | |
| 3 | 28 (25) | |
| Lymph node rocedure | Number (%) | |
| SLNB only | 70 (60) | |
| LND (+SLNB) | 46 (40) | |
| Lymph nodes extracted | Number | 1298 |
| SLNB cases | Number (%) | 313 (24) |
| LND (+SLNB) cases | Number (%) | 985 (76) |
| Lymph nodes metastases | Number of patients (%) | 20 (17) |
| Distribution of positive nodes | Number (%) | 34/1298 (2.62) |
| Obturator | 19 (7 SLN) | |
| Iliac | 13 (2 SLN) | |
| Para-aortic | 2 | |
| Risk grouping according to initial risk | Number (%) | Number of patients with metastatic nodes (%) |
| Low | 86 (74) | 8 (24) |
| High | 30 (26) | 12 (40) |
| Ultrasound Variable | Characteristic | Value |
|---|---|---|
| T | Number (%) | |
| ≤2 cm | 76 (66%) | |
| >2 cm | 40 (34%) | |
| AREA [cm2] | Mean ± SD (range) | 7.49 ± 9.77 (0.161–67) |
| SPE-VOL [cm3] | Mean ± SD (range) | 17.00 ± 26.93 (0.033–127) |
| TFD [mm] | Mean ± SD (range) | 7.39 ± 4.83 (0.3–22) |
| uMI | Number (%) | |
| <50% | 76 (66%) | |
| ≥50% | 40 (34%) | |
| EMIR | Number (%) | 44 (38%) |
| CSI | Number (%) | 24 (20.7%) |
| Model | Covariate | Est. | Std. Error | p-Value | AIC | ACC | AUC (95% CI) | p-Value (LRT) |
|---|---|---|---|---|---|---|---|---|
| A | (Intercept) | −1.79 | 0.3 | 0 | ||||
| uSPE-VOL | 0.01 | 0 | 0.143 | 108.67 | 0.75 | 0.652 (0.507–0.796) | 0.159 | |
| B | (Intercept) | −1.87 | 0.32 | 0 | 108.18 | 0.767 | 0.646 (0.499–0.794) | 0.115 |
| uAREA | 0.03 | 0.02 | 0.11 | |||||
| C | (Intercept) | −0.50 | 0.44 | 0.254 | 102.68 | 0.638 | 0.683 (0.563–0.803) | 0.005 |
| uTFD | −0.17 | 0.07 | 0.012 | |||||
| D | (Intercept) | −2.68 | 0.49 | 0 | 103.86 | 0.767 | 0.689 (0.538–0.840) | |
| bs(uSPE-VOL)1 | 6.81 | 2.28 | 0.003 | |||||
| bs(uSPE-VOL)2 | −3.48 | 2.94 | 0.236 | 0.013 | ||||
| bs(uSPE-VOL)3 | 2.36 | 1.78 | 0.186 | |||||
| E | (Intercept) | −2.61 | 0.61 | 0 | 106.23 | 0.784 | 0.671 (0.520–0.821) | |
| bs(uAREA)1 | 5.18 | 4.37 | 0.215 | |||||
| bs(uAREA)2 | −0.01 | 13.98 | 1 | 0.038 | ||||
| bs(uAREA)3 | −5.84 | 38.32 | 0.878 | |||||
| F | (Intercept) | −0.62 | 0.93 | 0.506 | 106.32 | 0.638 | 0.683 (0.563–0.803) | |
| bs(uTFD)1 | −1.37 | 4.14 | 0.74 | |||||
| bs(uTFD)2 | −0.58 | 6.49 | 0.929 | 0.04 | ||||
| bs(uTFD)3 | −8.04 | 11.92 | 0.5 | |||||
| G | (Intercept) | −2.29 | 0.4 | 0 | 101.17 | 0.707 | 0.684 (0.568–0.801) | |
| uMI | 1.56 | 0.52 | 0.003 | 0.002 | ||||
| H | (Intercept) | −2.23 | 0.4 | 0 | 103.34 | 0.672 | 0.664 (0.547–0.781) | |
| uEMIR | 1.36 | 0.52 | 0.009 | 0.007 | ||||
| I | (Intercept) | −2.00 | 0.32 | 0 | 103.11 | 0.776 | 0.647 (0.529–0.765) | |
| uCSI | 1.49 | 0.53 | 0.005 | 0.06 | ||||
| J | (Intercept) | −3.43 | 0.82 | 0 | 104.02 | 0.69 | 0.654 (0.535–0.773) | 0.01 |
| Size | 1.29 | 0.51 | 0.011 | |||||
| K | (Intercept) | −2.62 | 0.44 | 0 | 94.81 | 0.802 | 0.791 (0.673–0.91) | |
| uMI:bs(uTFD)1 | −13.24 | 4.74 | 0.005 | |||||
| uMI:bs(uTFD)2 | −54.62 | 27.47 | 0.046 | |||||
| uMI:bs(uTFD)3 | 121.59 | 60.64 | 0.044 | 0.005 | ||||
| uCSI:bs(uSPE-VOL)1 | 10.09 | 3.51 | 0.004 | 0.006 | ||||
| uCSI:bs(uSPE-VOL)2 | −15.04 | 9.3 | 0.105 | |||||
| uCSI:bs(uSPE-VOL)3 | 9.39 | 9.07 | 0.301 |
| Model | Covariate | Est. | Std. Error | p-Value | AIC | ACC | AUC (95% CI) | p-Value (LRT) |
|---|---|---|---|---|---|---|---|---|
| A′ | (Intercept) uSPE-VOL | −0.13 −0.01 | 0.48 0.01 | 0.786 0.509 | 49.62 | 0.618 | 0.479 (0.268–0.689) | 0.501 |
| B’ | (Intercept) uAREA | −0.12 −0.01 | 0.53 0.03 | 0.827 0.550 | 49.69 | 0.618 | 0.475 (0.265–0.685) | 0.536 |
| C′ | (Intercept) uTFD | 0.12 −0.09 | 0.58 0.08 | 0.836 0.316 | 49.01 | 0.588 | 0.571 (0.375–0.768) | 0.304 |
| D′ | (Intercept) | −1.04 | 0.77 | 0.176 | 50.22 | 0.765 | 0.686 (0.483–0.889) | |
| bs(uSPE.VOL)1 | 5.11 | 3.12 | 0.101 | |||||
| bs(uSPE.VOL)2 | −4.68 | 3.24 | 0.148 | 0.278 | ||||
| bs(uSPE.VOL)3 | 0.87 | 1.91 | 0.650 | |||||
| E′ | (Intercept) | −0.84 | 0.82 | 0.307 | 52.09 | 0.765 | 0.643 (0.423–0.863) | |
| bs(uAREA)1 | 2.863.28 | 3.84 | 0.394 | |||||
| bs(uAREA)2 | −3.11 | 6.09 | 0.610 | 0.577 | ||||
| bs(uAREA)3 | −3.58 | 9.19 | 0.697 | |||||
| F′ | (Intercept) | −0.14 | 0.77 | 0.855 | 52.57 | 0.618 | 0.600 (0.404–0.796) | |
| bs(uTFD)1 | 0.05 | 3.42 | 0.989 | |||||
| bs(uTFD)2 | 0.30 | 3.56 | 0.933 | 0.683 | ||||
| bs(uTFD)3 | −2.51 | 3.49 | 0.472 | |||||
| G′ | (Intercept) | −0.81 | 0.60 | 0.177 | 49.11 | 0.559 | 0.582 (0.416–0.748) | |
| uMI | 0.72 | 0.74 | 0.335 | 0.328 | ||||
| H′ | (Intercept) | −0.69 | 0.55 | 0.206 | 49.38 | 0.559 | 0.571 (0.399–0.744) | |
| uEMIR | 0.59 | 0.71 | 0.411 | 0.407 | ||||
| I′ | (Intercept) | −0.69 | 0.55 | 0.206 | 49.38 | 0.559 | 0.571 (0.399–0.743) | |
| uCSI | 0.59 | 0.71 | 0.411 | 0.407 | ||||
| J′ | (Intercept) Size | −0.48 0.07 | 1.36 0.77 | 0.726 0.928 | 50.06 | 0.471 | 0.507 (0.347–0.667) | 0.928 |
| K′ | (Intercept) | −0.89 | 0.45 | 0.048 | 45.19 | 0.706 | 0.675 (0.517–0.833) | . |
| uCSI:uMI | 1.74 | 0.82 | 0.035 | 0.027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liro, M.; Śniadecki, M.; Wycinka, E.; Wojtylak, S.; Brzeziński, M.; Jastrzębska, J.; Wydra, D. Incorporation of Tumor-Free Distance and Other Alternative Ultrasound Biomarkers into a Myometrial Invasion-Based Model Better Predicts Lymph Node Metastasis in Endometrial Cancer: Evidence and Future Prospects. Diagnostics 2022, 12, 2604. https://doi.org/10.3390/diagnostics12112604
Liro M, Śniadecki M, Wycinka E, Wojtylak S, Brzeziński M, Jastrzębska J, Wydra D. Incorporation of Tumor-Free Distance and Other Alternative Ultrasound Biomarkers into a Myometrial Invasion-Based Model Better Predicts Lymph Node Metastasis in Endometrial Cancer: Evidence and Future Prospects. Diagnostics. 2022; 12(11):2604. https://doi.org/10.3390/diagnostics12112604
Chicago/Turabian StyleLiro, Marcin, Marcin Śniadecki, Ewa Wycinka, Szymon Wojtylak, Michał Brzeziński, Joanna Jastrzębska, and Dariusz Wydra. 2022. "Incorporation of Tumor-Free Distance and Other Alternative Ultrasound Biomarkers into a Myometrial Invasion-Based Model Better Predicts Lymph Node Metastasis in Endometrial Cancer: Evidence and Future Prospects" Diagnostics 12, no. 11: 2604. https://doi.org/10.3390/diagnostics12112604
APA StyleLiro, M., Śniadecki, M., Wycinka, E., Wojtylak, S., Brzeziński, M., Jastrzębska, J., & Wydra, D. (2022). Incorporation of Tumor-Free Distance and Other Alternative Ultrasound Biomarkers into a Myometrial Invasion-Based Model Better Predicts Lymph Node Metastasis in Endometrial Cancer: Evidence and Future Prospects. Diagnostics, 12(11), 2604. https://doi.org/10.3390/diagnostics12112604







