An Automatic Method for Assessing Spiking of Tibial Tubercles Associated with Knee Osteoarthritis
Abstract
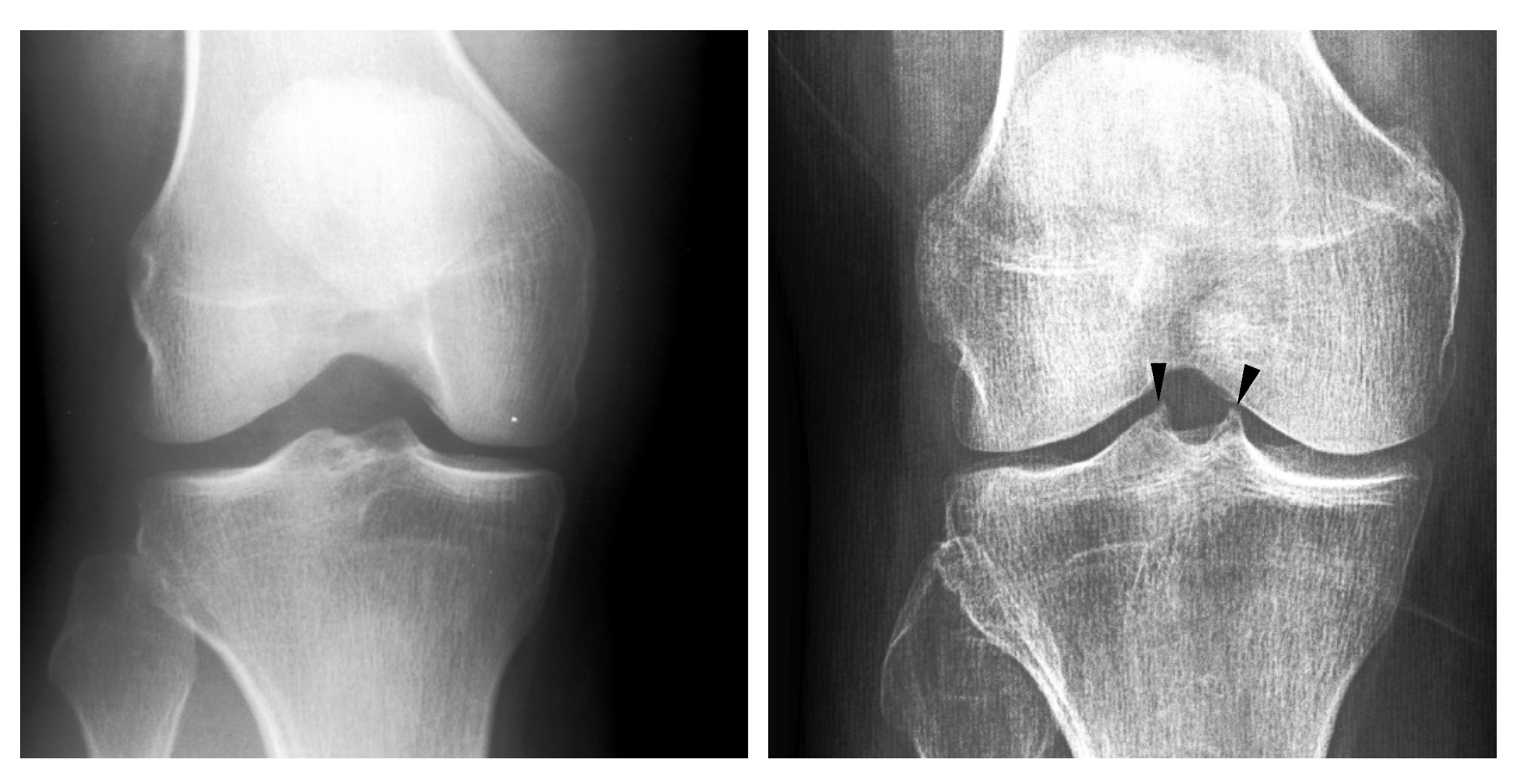
1. Introduction
- Grade 0: No radiological signs of OA.
- Grade 1: Doubtful JSN, possible osteophytic lipping.
- Grade 2: Definite osteophytes, possible JSN.
- Grade 3: Moderate multiple osteophytes, definite JSN, some sclerosis, possible deformity of bone ends.
- Grade 4: Large osteophytes, marked JSN, severe sclerosis, definite deformity of bone ends.
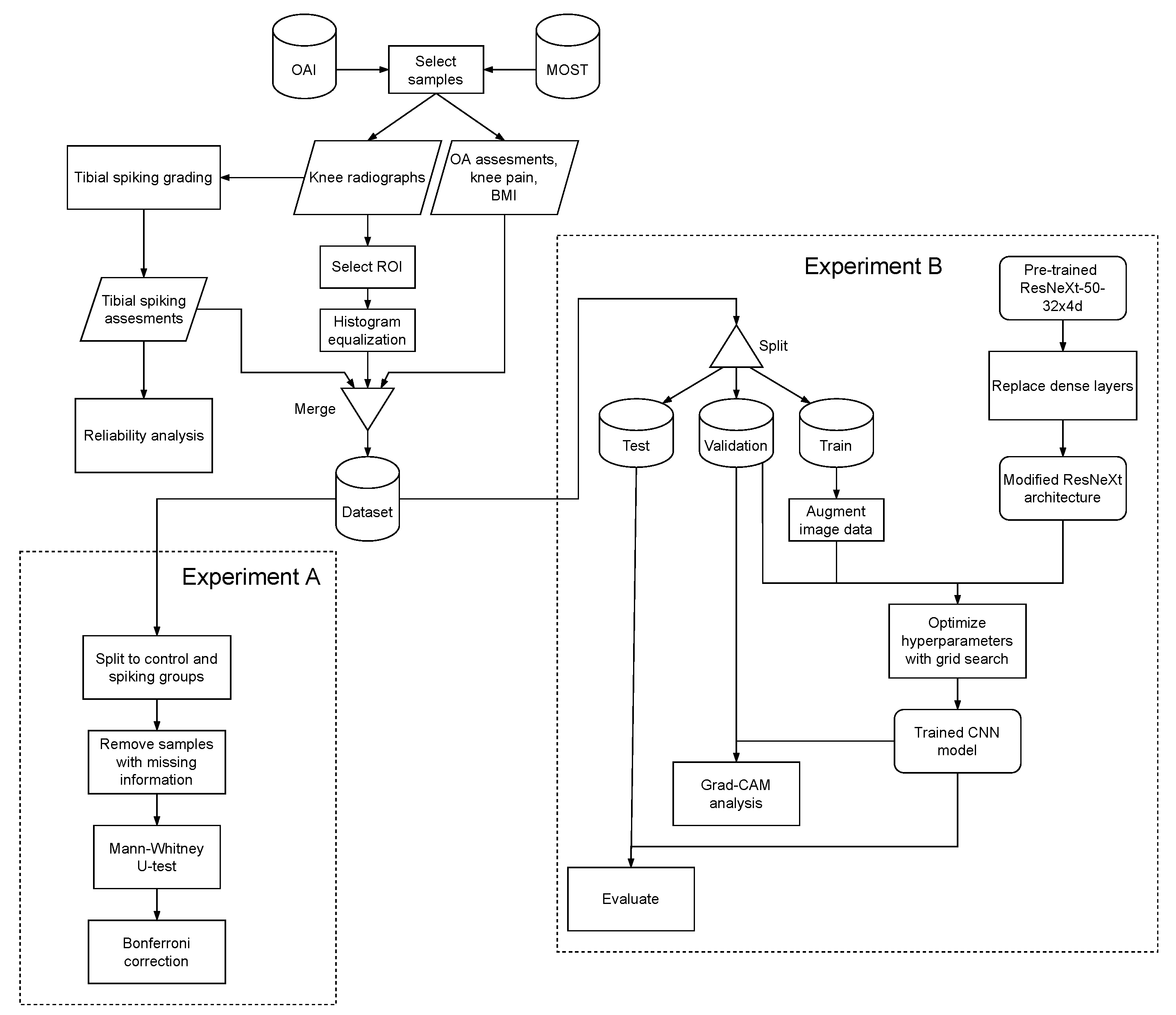
2. Materials and Methods
2.1. Radiographic Data
2.2. Data for Experiment A
2.3. Reliability
2.4. Experiment A
2.5. Data for Experiment B
2.6. Experiment B
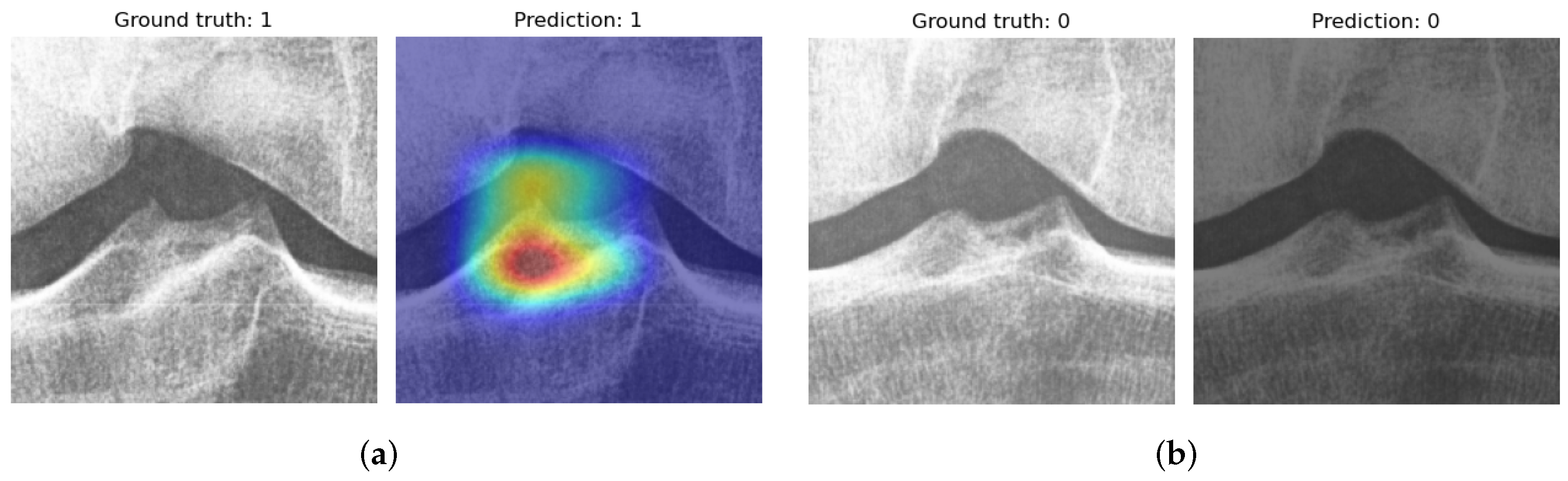
3. Results and Discussion
3.1. Reliability
3.2. Experiment A
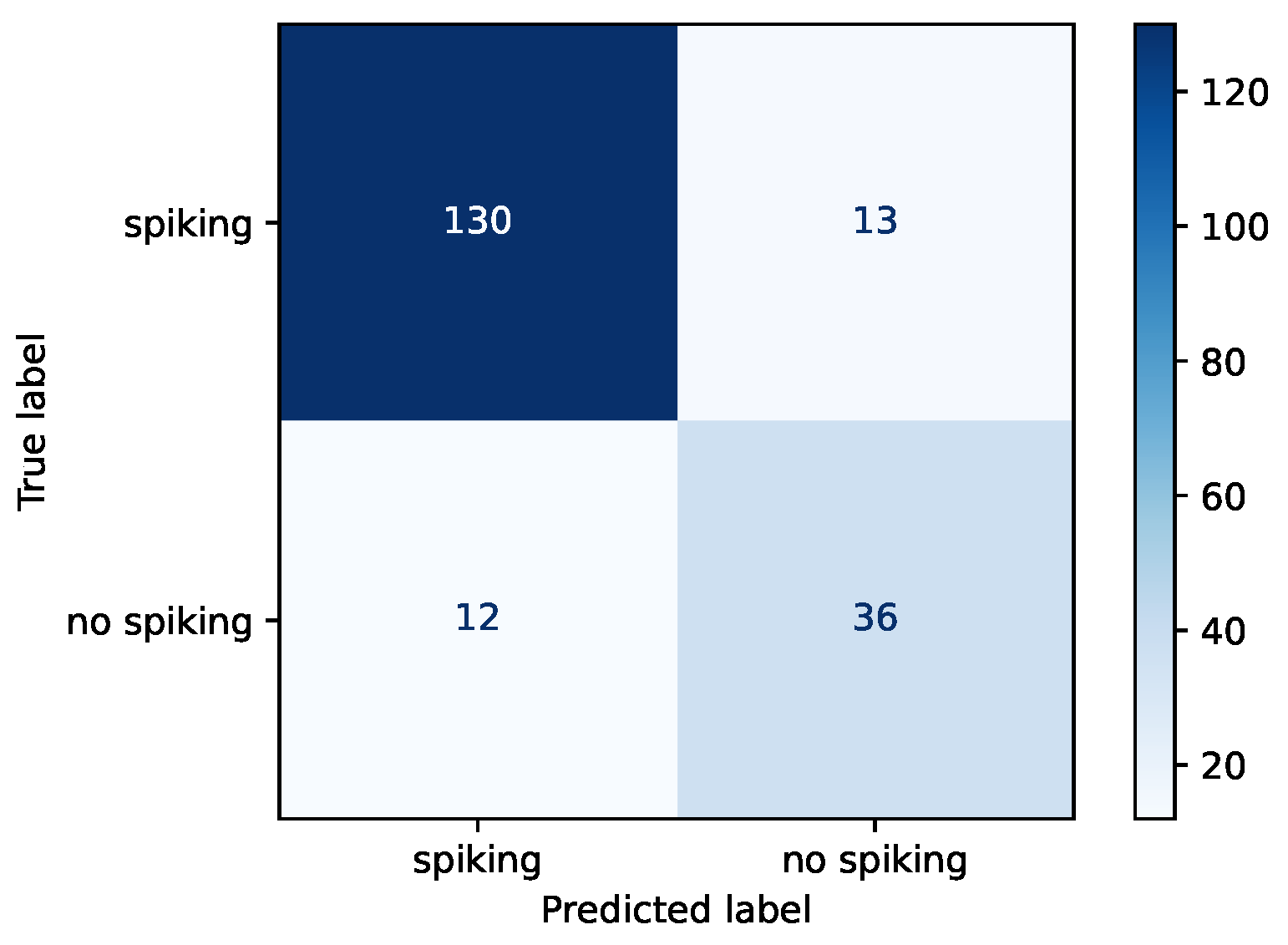
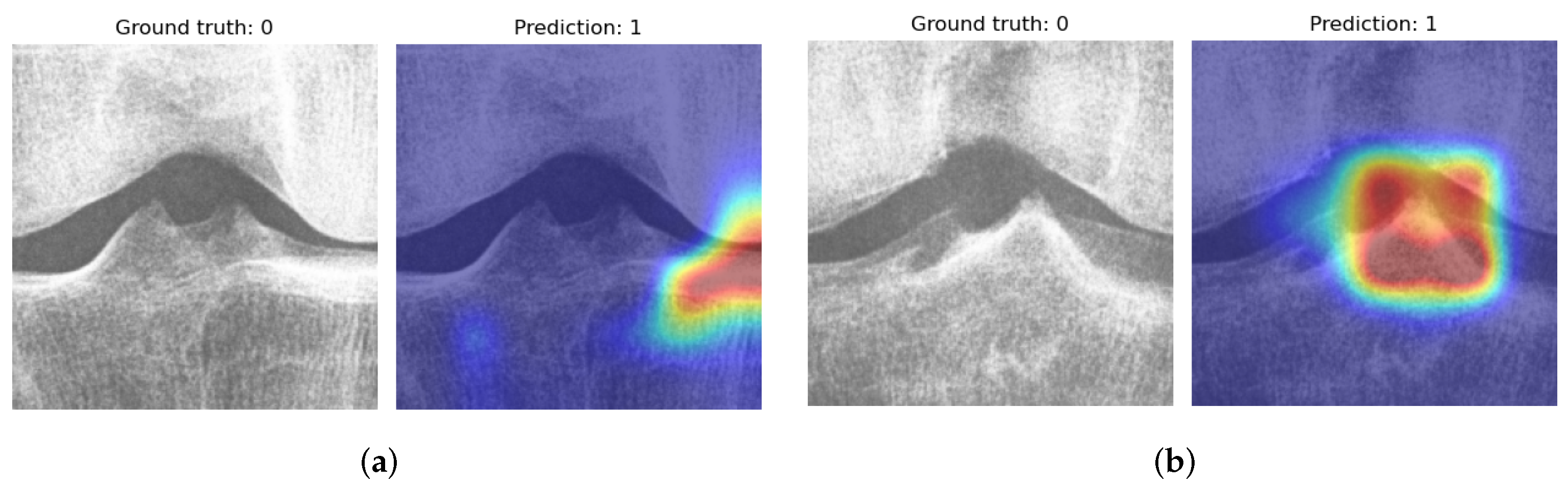
3.3. Experiment B
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Inacio, M.; Paxton, E.; Graves, S.; Namba, R.; Nemes, S. Projected increase in total knee arthroplasty in the United States—An alternative projection model. Osteoarthr. Cartil. 2017, 25, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Pamilo, K.J.; Haapakoski, J.; Sokka-Isler, T.; Remes, V.; Paloneva, J. Rapid rise in prevalence of knee replacements and decrease in revision burden over past 3 decades in Finland: A register-based analysis. Acta Orthop. 2022, 93, 382. [Google Scholar] [CrossRef] [PubMed]
- Swagerty, D.L., Jr.; Hellinger, D. Radiographic assessment of osteoarthritis. Am. Fam. Physician 2001, 64, 279. [Google Scholar]
- Guermazi, A.; Niu, J.; Hayashi, D.; Roemer, F.W.; Englund, M.; Neogi, T.; Aliabadi, P.; McLennan, C.E.; Felson, D.T. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: Population based observational study (Framingham Osteoarthritis Study). BMJ 2012, 345, e5339. [Google Scholar] [CrossRef]
- Hayashi, D.; Felson, D.; Niu, J.; Hunter, D.; Roemer, F.; Aliabadi, P.; Guermazi, A. Pre-radiographic osteoarthritic changes are highly prevalent in the medial patella and medial posterior femur in older persons: Framingham OA study. Osteoarthr. Cartil. 2014, 22, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; Hochberg, M.C. Methodological problems in the epidemiological study of osteoarthritis. Ann. Rheum. Dis. 1994, 53, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Gold, G. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthr. Cartil. 2007, 15, A1–A56. [Google Scholar] [CrossRef]
- Oka, H.; Muraki, S.; Akune, T.; Mabuchi, A.; Suzuki, T.; Yoshida, H.; Yamamoto, S.; Nakamura, K.; Yoshimura, N.; Kawaguchi, H. Fully automatic quantification of knee osteoarthritis severity on plain radiographs. Osteoarthr. Cartil. 2008, 16, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Shamir, L.; Ling, S.M.; Scott, W.W.; Bos, A.; Orlov, N.; Macura, T.J.; Eckley, D.M.; Ferrucci, L.; Goldberg, I.G. Knee X-ray image analysis method for automated detection of osteoarthritis. IEEE Trans. Biomed. Eng. 2008, 56, 407–415. [Google Scholar] [CrossRef]
- LeCun, Y.; Boser, B.; Denker, J.S.; Henderson, D.; Howard, R.E.; Hubbard, W.; Jackel, L.D. Backpropagation Applied to Handwritten Zip Code Recognition. Neural Comput. 1989, 1, 541–551. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-Level Classification of Skin Cancer with Deep Neural Networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Yi, P.H.; Wei, J.; Kim, T.K.; Sair, H.I.; Hui, F.K.; Hager, G.D.; Fritz, J.; Oni, J.K. Automated detection & classification of knee arthroplasty using deep learning. Knee 2020, 27, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.; McGuinness, K.; O’Connor, N.E.; Moran, K. Quantifying radiographic knee osteoarthritis severity using deep convolutional neural networks. In Proceedings of the 2016 23rd International Conference on Pattern Recognition (ICPR), Cancun, Mexico, 4–8 December 2016; pp. 1195–1200. [Google Scholar] [CrossRef]
- Yeoh, P.S.Q.; Lai, K.W.; Goh, S.L.; Hasikin, K.; Hum, Y.C.; Tee, Y.K.; Dhanalakshmi, S. Emergence of Deep Learning in Knee Osteoarthritis Diagnosis. Comput. Intell. Neurosci. 2021, 2021, 4931437. [Google Scholar] [CrossRef]
- Tiulpin, A.; Thevenot, J.; Rahtu, E.; Lehenkari, P.; Saarakkala, S. Automatic knee osteoarthritis diagnosis from plain radiographs: A deep learning-based approach. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Chen, P.; Gao, L.; Shi, X.; Allen, K.; Yang, L. Fully Automatic Knee Osteoarthritis Severity Grading Using Deep Neural Networks with a Novel Ordinal Loss. Comput. Med. Imaging Graph. 2019, 75, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, K.J.; Choi, D.; Lee, J.I.; Choi, H.G.; Lee, Y.S. Can Additional Patient Information Improve the Diagnostic Performance of Deep Learning for the Interpretation of Knee Osteoarthritis Severity. J. Clin. Med. 2020, 9, 3341. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D. A Textbook of Radiology and Imaging, 4th ed.; Churchill Livingstone: London, UK, 1987; p. 113. [Google Scholar]
- Reiff, D.; Heron, C.; Stoker, D. Spiking of the tubercles of the intercondylar eminence of the tibial plateau in osteoarthritis. Br. J. Radiol. 1991, 64, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.; Hart, D.J.; Doyle, D.V.; Spector, T.D. Spiking of the tibial tubercles–a radiological feature of osteoarthritis? Ann. Rheum. Dis. 1996, 55, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Unlu, Z.; Tarhan, S.; Goktan, C.; Tuzun, C. The correlation between magnetic resonance detected cartilage defects and spiking of tibial tubercles in osteoarthritis of the knee joint. Acta Medica Okayama 2006, 60, 207–214. [Google Scholar]
- Hayeri, M.R.; Shiehmorteza, M.; Trudell, D.J.; Heflin, T.; Resnick, D. Proximal tibial osteophytes and their relationship with the height of the tibial spines of the intercondylar eminence: Paleopathological study. Skelet. Radiol. 2010, 39, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Wirth, W.; Nevitt, M.C. Recent Advances in Osteoarthritis Imaging—The Osteoarthritis Initiative. Nat. Rev. Rheumatol. 2012, 8, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.A.; Nevitt, M.C.; Gross, K.D.; Gross, K.D.; Hietpas, J.; Glass, N.A.; Lewis, C.E.; Torner, J.C. The Multicenter Osteoarthritis Study: Opportunities for Rehabilitation Research. PM&R 2013, 5, 647–654. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Python Core Team. Python: A Dynamic, Open Source Programming Language; Python Core Team, 2015. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Pydicom/Pydicom; Pydicom 2.2.2; Version 2.2.2. Available online: https://github.com/pydicom/pydicom (accessed on 26 October 2022).
- Wada, K. Labelme: Image Polygonal Annotation with Python, 2022. Version 5.0.1. Available online: https://github.com/wkentaro/labelme (accessed on 26 October 2022).
- Bradski, G. The OpenCV Library, 2022. Version 4.5.5.64. Available online: https://github.com/opencv/opencv-python (accessed on 26 October 2022).
- Xie, S.; Girshick, R.; Dollár, P.; Tu, Z.; He, K. Aggregated Residual Transformations for Deep Neural Networks. arXiv 2016, arXiv:1611.05431. [Google Scholar]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Li, F.-F. ImageNet: A Large-Scale Hierarchical Image Database. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, CVPR09, Miami, FL, USA, 20–25 June 2009. [Google Scholar]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet Large Scale Visual Recognition Challenge. Int. J. Comput. Vis. 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Paszke, A.; Gross, S.; Massa, F.; Lerer, A.; Bradbury, J.; Chanan, G.; Killeen, T.; Lin, Z.; Gimelshein, N.; Antiga, L.; et al. PyTorch: An Imperative Style, High-Performance Deep Learning Library. In Proceedings of the Advances in Neural Information Processing Systems 32, Montreal, QC, Canada, 8–14 December 2019; Wallach, H., Larochelle, H., Beygelzimer, A., d’Alché-Buc, F., Fox, E., Garnett, R., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2019; pp. 8024–8035. [Google Scholar]
- Yosinski, J.; Clune, J.; Bengio, Y.; Lipson, H. How transferable are features in deep neural networks? In Proceedings of the Advances in Neural Information Processing Systems 27, Montreal, QC, Canada, 8–13 December 2014; Ghahramani, Z., Welling, M., Cortes, C., Lawrence, N., Weinberger, K., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2014; Volume 27. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 26 June 26–1 July 2016. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization. arXiv 2014, arXiv:1412.6980v9. [Google Scholar]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations From Deep Networks via Gradient-Based Localization. In Proceedings of the IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017. [Google Scholar]
- Fernandez, F.G. TorchCAM: Class Activation Explorer. 2020. Available online: https://github.com/frgfm/torch-cam (accessed on 26 October 2022).
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Gossec, L.; Jordan, J.; Mazzuca, S.; Lam, M.A.; Suarez-Almazor, M.; Renner, J.; Lopez-Olivo, M.; Hawker, G.; Dougados, M.; Maillefert, J. Comparative evaluation of three semi-quantitative radiographic grading techniques for knee osteoarthritis in terms of validity and reproducibility in 1759 X-rays: Report of the OARSI–OMERACT task force: Extended report. Osteoarthr. Cartil. 2008, 16, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Culvenor, A.G.; Engen, C.N.; Øiestad, B.E.; Engebretsen, L.; Risberg, M.A. Defining the presence of radiographic knee osteoarthritis: A comparison between the Kellgren and Lawrence system and OARSI atlas criteria. Knee Surgery Sport. Traumatol. Arthrosc. 2015, 23, 3532–3539. [Google Scholar] [CrossRef]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar]
- Alexander, C.J. Osteoarthritis: A review of old myths and current concepts. Skelet. Radiol. 1990, 19, 327–333. [Google Scholar] [CrossRef]

| KL0 | KL1 | KL2 | |
|---|---|---|---|
| OAI | 243 | 248 | 231 |
| MOST | 65 | 61 | 65 |
| Medial | Lateral | OR | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | ? | 0 | 1 | ? | 0 | 1 | |
| Train | 216 | 281 | 80 | 253 | 279 | 45 | 188 | 389 |
| Validation | 57 | 71 | 17 | 53 | 77 | 15 | 47 | 98 |
| Test | 66 | 108 | 17 | 59 | 116 | 16 | 48 | 143 |
| Total | 339 | 460 | 114 | 365 | 472 | 76 | 283 | 630 |
| Block | Output Size | Architecture | |
|---|---|---|---|
| Conv1 | , 64, stride 2 | ||
| Pool | max pool, stride 2 | ||
| BN1 | |||
| BN2 | |||
| BN3 | |||
| BN4 | |||
| global average pool | |||
| Dense | 2048, 2, softmax |
| Values | |
|---|---|
| Epochs | |
| Batch-size | |
| Learning-rate | |
| Step-size | |
| Gamma |
| Intra-Rater Reliability (Expert 1) | Intra-Rater Reliability (Expert 2) | Inter-Rater Reliability | |
|---|---|---|---|
| Medial | 0.61 (0.58–0.64) | 0.52 (0.50–0.54) | 0.34 (0.33–0.35) |
| Medial (o) | 0.78 (0.75–0.82) | 0.94 (0.92–0.96) | 0.59 (0.58–0.61) |
| Lateral | 0.59 (0.56–0.62) | 0.75 (0.73–0.76) | 0.55 (0.55–0.56) |
| Lateral (o) | 0.71 (0.67–0.74) | 1.00 (1.00–1.00) | 0.75 (0.74–0.76) |
| OR | 0.53 (0.50–0.57) | 0.69 (0.67–0.72) | 0.48 (0.47–0.49) |
| Spiking | Control | |
|---|---|---|
| KL-grade *** | 1.11 | 0.70 |
| WOMAC knee pain * | 2.14 | 1.62 |
| BMI *** | 29.09 | 27.46 |
| Medial JSN *** | 0.38 | 0.25 |
| Lateral JSN | 0.05 | 0.04 |
| Tibia medial osteophytes ** | 0.59 | 0.41 |
| Tibia lateral osteophytes *** | 0.40 | 0.21 |
| Femur medial osteophytes ** | 0.48 | 0.27 |
| Femur lateral osteophytes ** | 0.41 | 0.22 |
| Accuracy | Loss | Sensitivity | Specificity | Precision | |
|---|---|---|---|---|---|
| Train | 0.872 | 0.300 | 0.882 | 0.851 | 0.925 |
| Validation | 0.869 | 0.399 | 0.929 | 0.745 | 0.883 |
| Test | 0.869 | 0.314 | 0.909 | 0.750 | 0.915 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patron, A.; Annala, L.; Lainiala, O.; Paloneva, J.; Äyrämö, S. An Automatic Method for Assessing Spiking of Tibial Tubercles Associated with Knee Osteoarthritis. Diagnostics 2022, 12, 2603. https://doi.org/10.3390/diagnostics12112603
Patron A, Annala L, Lainiala O, Paloneva J, Äyrämö S. An Automatic Method for Assessing Spiking of Tibial Tubercles Associated with Knee Osteoarthritis. Diagnostics. 2022; 12(11):2603. https://doi.org/10.3390/diagnostics12112603
Chicago/Turabian StylePatron, Anri, Leevi Annala, Olli Lainiala, Juha Paloneva, and Sami Äyrämö. 2022. "An Automatic Method for Assessing Spiking of Tibial Tubercles Associated with Knee Osteoarthritis" Diagnostics 12, no. 11: 2603. https://doi.org/10.3390/diagnostics12112603
APA StylePatron, A., Annala, L., Lainiala, O., Paloneva, J., & Äyrämö, S. (2022). An Automatic Method for Assessing Spiking of Tibial Tubercles Associated with Knee Osteoarthritis. Diagnostics, 12(11), 2603. https://doi.org/10.3390/diagnostics12112603





