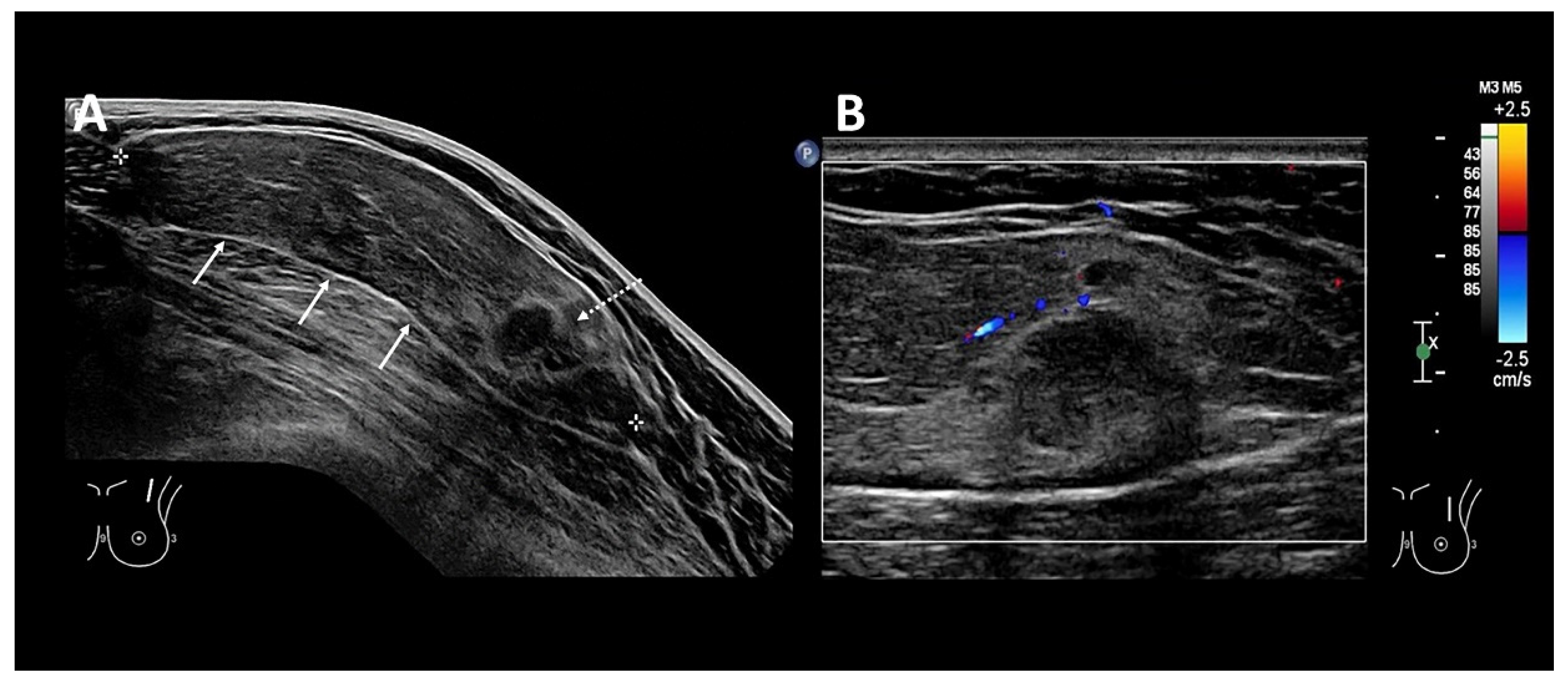
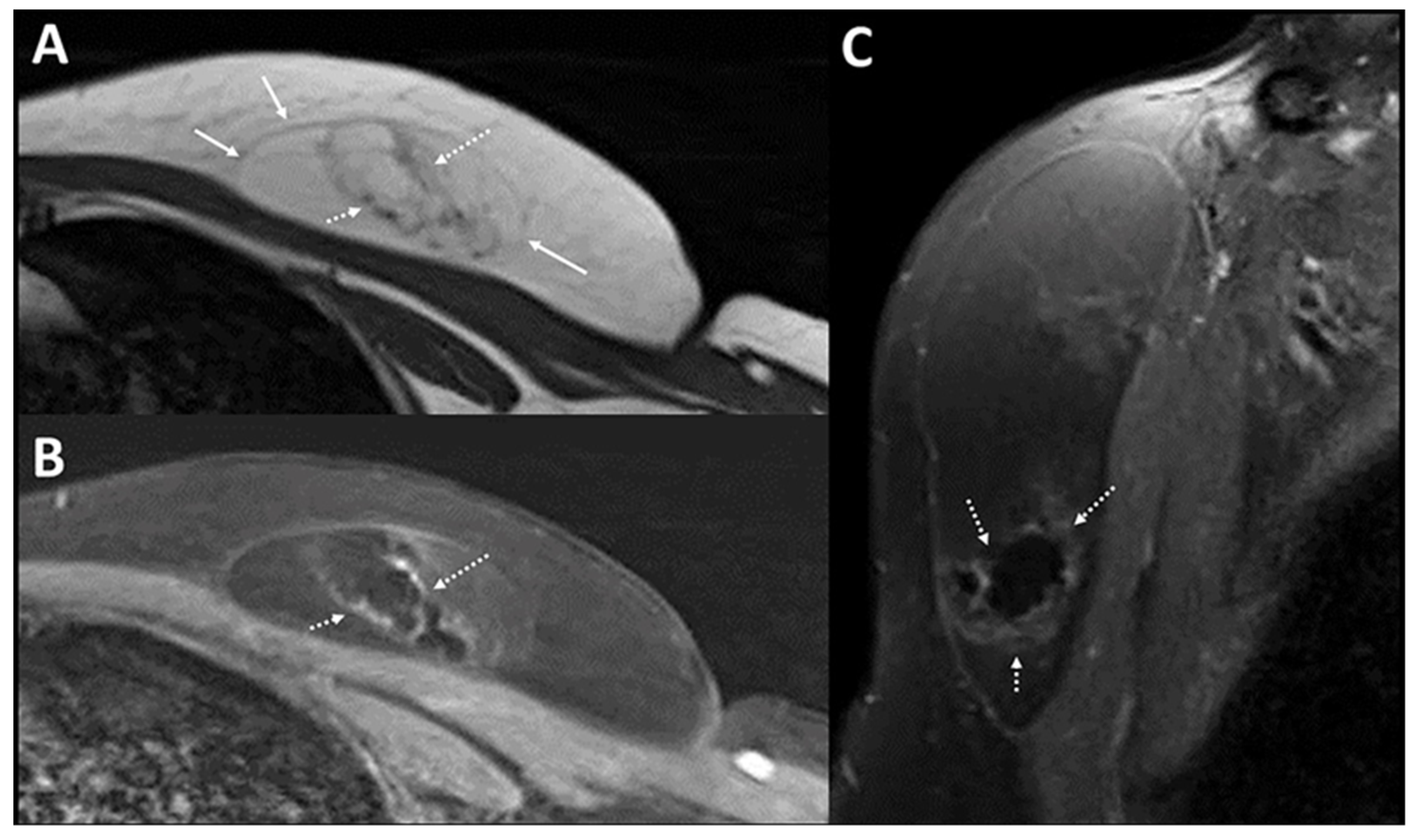
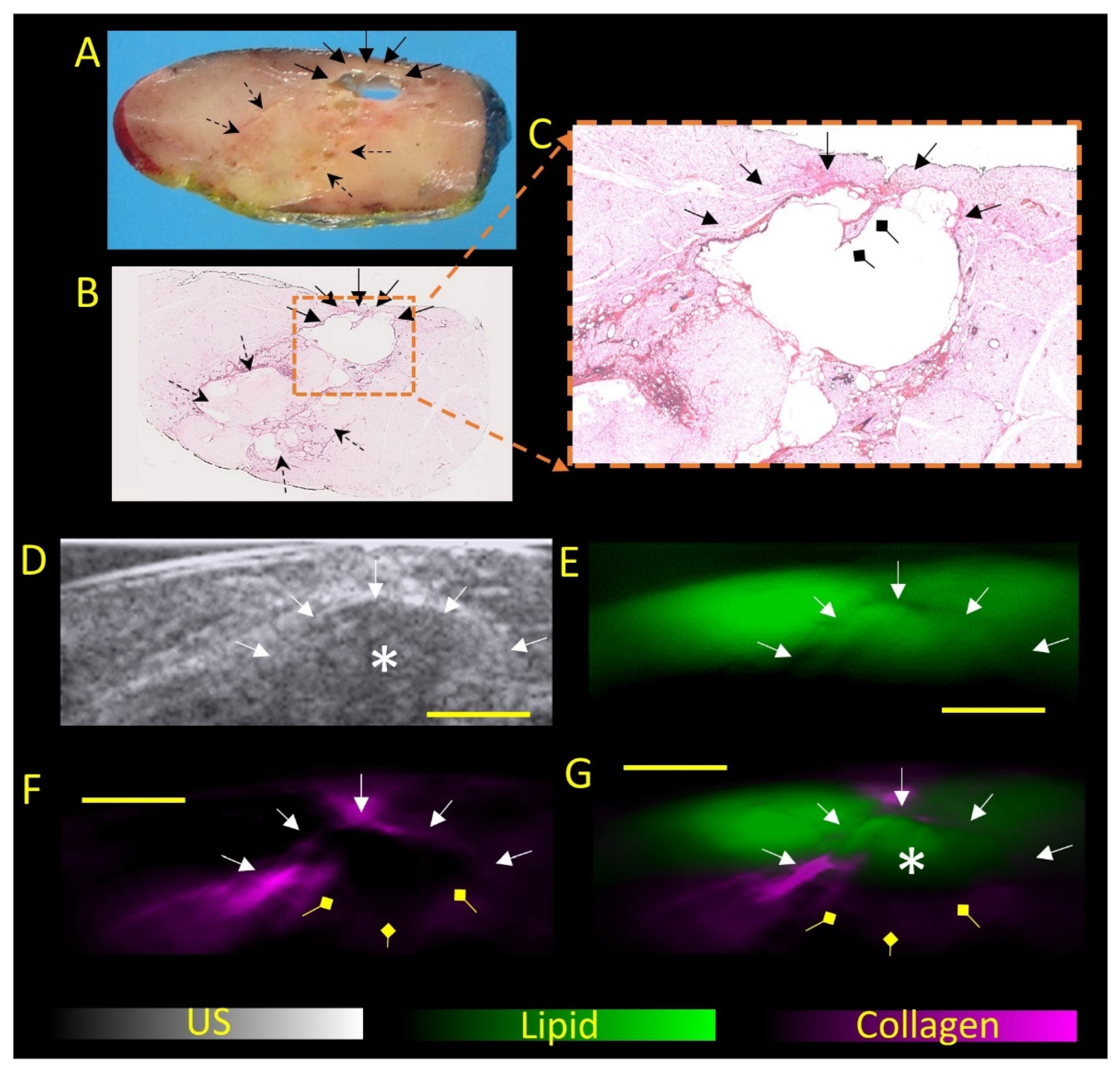
Photoacoustic Tomography Appearance of Fat Necrosis: A First-in-Human Demonstration of Biochemical Signatures along with Histological Correlation
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
Appendix A.1. Materials and Methods
References
- Genova, R.; Garza, R.F. Breast Fat Necrosis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chan, L.P.; Gee, R.; Keogh, C.; Munk, P.L. Imaging features of fat necrosis. AJR Am. J. Roentgenol. 2003, 181, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Taboada, J.L.; Stephens, T.W.; Krishnamurthy, S.; Brandt, K.R.; Whitman, G.J. The many faces of fat necrosis in the breast. AJR Am. J. Roentgenol. 2009, 192, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.A.; Somers, S.; Edmonson, R.D.; Sidhu, P.S. Subcutaneous fat necrosis: Hypoechoic appearance on sonography. J. Ultrasound Med. 2003, 22, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic tomography of blood oxygenation: A mini review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Diot, G.; Metz, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Goh, Y.; Moothanchery, M.; Attia, A.; Lim, H.Q.; Burton, N.C.; Qiu, Y.; Putti, T.C.; Chan, C.W.; Hartmann, M.; et al. Optoacoustic characterization of breast conserving surgery specimens—A pilot study. Photoacoustics 2020, 19, 100164. [Google Scholar] [CrossRef]
- Lei, P.; Hao, J.; Wang, L.; Wen, X.; Xiong, K.; Zhang, P.; Zhang, L.; Yang, S. Reliability assessment on intravascular photoacoustic imaging of lipid: Ex vivo animal and human sample validation. J. Biophotonics 2020, 13, e202000162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, S.; Ji, X.; Zhou, Q.; Xing, D. Characterization of lipid-rich aortic plaques by intravascular photoacoustic tomography: Ex vivo and in vivo validation in a rabbit atherosclerosis model with histologic correlation. J. Am. Coll. Cardiol. 2014, 64, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.; Balasundaram, G.; Tan, H.M.; Putti, T.C.; Tang, S.W.; Ng, C.W.Q.; Buhari, S.A.; Fang, E.; Moothanchery, M.; Bi, R.; et al. Biochemical “decoding” of breast ultrasound images with optoacoustic tomography fusion: First-in-human display of lipid and collagen signals on breast ultrasound. Photoacoustics 2022, 27, 100377. [Google Scholar] [CrossRef]
- Regensburger, A.P.; Fonteyne, L.M.; Jüngert, J.; Wagner, A.L.; Gerhalter, T.; Nagel, A.M.; Heiss, R.; Flenkenthaler, F.; Qurashi, M.; Neurath, M.F.; et al. Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, Y.; Balasundaram, G.; Tan, H.M.; Putti, T.C.; Ng, C.W.Q.; Fang, E.; Bi, R.; Tang, S.W.; Buhari, S.A.; Hartman, M.; et al. Photoacoustic Tomography Appearance of Fat Necrosis: A First-in-Human Demonstration of Biochemical Signatures along with Histological Correlation. Diagnostics 2022, 12, 2456. https://doi.org/10.3390/diagnostics12102456
Goh Y, Balasundaram G, Tan HM, Putti TC, Ng CWQ, Fang E, Bi R, Tang SW, Buhari SA, Hartman M, et al. Photoacoustic Tomography Appearance of Fat Necrosis: A First-in-Human Demonstration of Biochemical Signatures along with Histological Correlation. Diagnostics. 2022; 12(10):2456. https://doi.org/10.3390/diagnostics12102456
Chicago/Turabian StyleGoh, Yonggeng, Ghayathri Balasundaram, Hui Min Tan, Thomas Choudary Putti, Celene Wei Qi Ng, Eric Fang, Renzhe Bi, Siau Wei Tang, Shaik Ahmad Buhari, Mikael Hartman, and et al. 2022. "Photoacoustic Tomography Appearance of Fat Necrosis: A First-in-Human Demonstration of Biochemical Signatures along with Histological Correlation" Diagnostics 12, no. 10: 2456. https://doi.org/10.3390/diagnostics12102456
APA StyleGoh, Y., Balasundaram, G., Tan, H. M., Putti, T. C., Ng, C. W. Q., Fang, E., Bi, R., Tang, S. W., Buhari, S. A., Hartman, M., Chan, C. W., Lim, Y. T., Olivo, M., & Quek, S. T. (2022). Photoacoustic Tomography Appearance of Fat Necrosis: A First-in-Human Demonstration of Biochemical Signatures along with Histological Correlation. Diagnostics, 12(10), 2456. https://doi.org/10.3390/diagnostics12102456






