Concordance Rate of Colposcopy in Detecting Cervical Intraepithelial Lesions
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
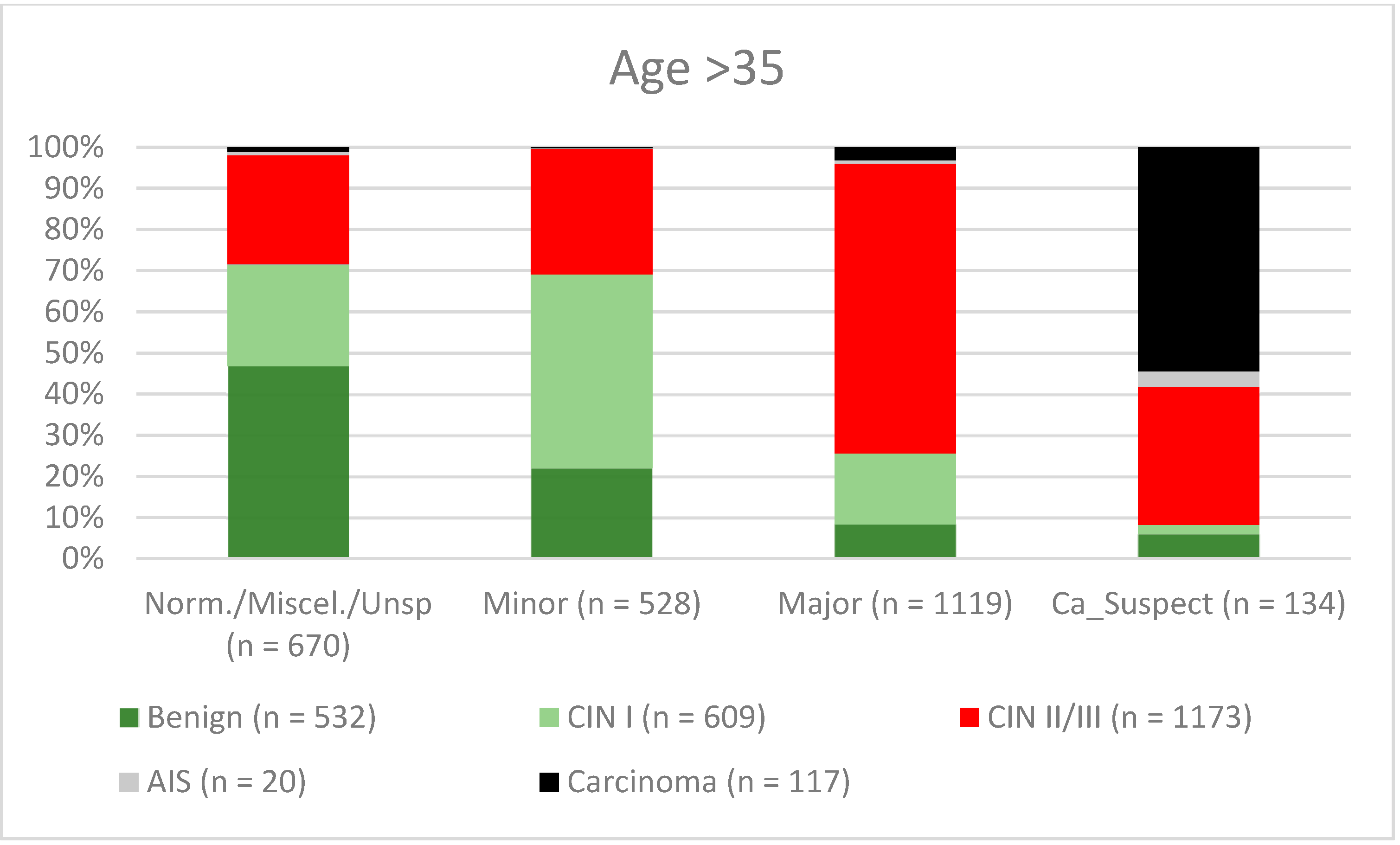
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buskwofie, A.; David-West, G.; Clare, C.A. A Review of Cervical Cancer: Incidence and Disparities. J. Natl. Med. Assoc. 2020, 112, 229–232. [Google Scholar] [CrossRef]
- Beckmann, M.W.; Stübs, F.A.; Koch, M.C.; Mallmann, P.; Dannecker, C.; Dietl, A.; Sevnina, A.; Mergel, F.; Lotz, L.; Hack, C.C.; et al. Diagnosis, Therapy and Follow-up of Cervical Cancer. Guideline of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL, May 2021)—Part 1 with Recommendations on Epidemiology, Screening, Diagnostics and Therapy. Geburtshilfe Frauenheilkd 2022, 82, 139–180. [Google Scholar] [CrossRef]
- Fehm, T.; Stübs, F.A.; Koch, M.C.; Mallmann, P.; Dannecker, C.; Dietl, A.; Sevnina, A.; Mergel, F.; Lotz, L.; Ehret, A.; et al. Diagnosis, Therapy and Follow-up of Cervical Cancer. Guideline of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL, May 2021)—Part 2 with Recommendations on Psycho-oncology, Rehabilitation, Follow-up, Recurrence, Palliative Therapy and Healthcare Facilities. Geburtshilfe Frauenheilkd 2022, 82, 181–205. [Google Scholar]
- Beckmann, M.W.; Stuebs, F.A.; Vordermark, D.; Koch, M.C.; Horn, L.C.; Fehm, T. The Diagnosis, Treatment, and Aftercare of Cervical Carcinoma. Dtsch. Arztebl. Int. 2021, 118, 806–812. [Google Scholar]
- Krebs_in_Deutschland_2027/2018. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2021/krebs_in_deutschland_2021.pdf?__blob=publicationFile (accessed on 13 August 2022).
- Stuebs, F.A.; Schulmeyer, C.E.; Mehlhorn, G.; Gass, P.; Kehl, S.; Renner, S.K.; Renner, S.P.; Geppert, C.; Adler, W.; Hartmann, A.; et al. Accuracy of colposcopy-directed biopsy in detecting early cervical neoplasia: A retrospective study. Arch. Gynecol. Obstet. 2019, 299, 525–532. [Google Scholar] [CrossRef]
- Stuebs, F.A.; Gass, P.; Dietl, A.K.; Schulmeyer, C.E.; Adler, W.; Geppert, C.; Hartmann, A.; Knöll, A.; Beckmann, M.W.; Koch, M.C. Human papilloma virus genotype distribution in women with premalignant or malignant lesions of the uterine cervix. Arch. Gynecol. Obstet. 2021, 304, 751–758. [Google Scholar] [CrossRef]
- Revathidevi, S.; Murugan, A.K.; Nakaoka, H.; Inoue, I.; Munirajan, A.K. APOBEC: A molecular driver in cervical cancer pathogenesis. Cancer Lett. 2021, 496, 104–116. [Google Scholar] [CrossRef]
- Beckmann, M.W.; Quaas, J.; Bischofberger, A.; Kämmerle, A.; Lux, M.P.; Wesselmann, S. Establishment of the Certification System “Gynaecological Dysplasia” in Germany. Geburtshilfe Frauenheilkd 2014, 74, 860–867. [Google Scholar] [CrossRef]
- Najib, F.S.; Hashemi, M.; Shiravani, Z.; Poordast, T.; Sharifi, S.; Askary, E. Diagnostic Accuracy of Cervical Pap Smear and Colposcopy in Detecting Premalignant and Malignant Lesions of Cervix. Indian J. Surg. Oncol. 2020, 11, 453–458. [Google Scholar] [CrossRef]
- Schneider, V. Criticism of the Pap Smear as a Diagnostic Tool in Cervical Cancer Screening. Acta Cytol. 2017, 61, 338–344. [Google Scholar] [CrossRef]
- Nam, K. Colposcopy at a turning point. Obstet. Gynecol. Sci. 2018, 61, 1–6. [Google Scholar] [CrossRef]
- Stuebs, F.A.; Koch, M.C.; Dietl, A.K.; Adler, W.; Geppert, C.; Hartmann, A.; Knöll, A.; Beckmann, M.W.; Mehlhorn, G.; Schulmeyer, C.E.; et al. Cytology and High-Risk Human Papillomavirus Test for Cervical Cancer Screening Assessment. Diagnostics 2022, 12, 1748. [Google Scholar] [CrossRef]
- Baum, M.E.; Rader, J.S.; Gibb, R.K.; McAlister, R.P.; Powell, M.A.; Mutch, D.G.; Gao, F.; Wright, J.D. Colposcopic accuracy of obstetrics and gynecology residents. Gynecol. Oncol. 2006, 103, 966–970. [Google Scholar] [CrossRef]
- Petousis, S.; Christidis, P.; Margioula-Siarkou, C.; Sparangis, N.; Athanasiadis, A.; Kalogiannidis, I. Discrepancy between colposcopy, punch biopsy and final histology of cone specimen: A prospective study. Arch. Gynecol. Obstet. 2018, 297, 1271–1275. [Google Scholar] [CrossRef]
- Karimi-Zarchi, M.; Peighmbari, F.; Karimi, N.; Rohi, M.; Chiti, Z. A Comparison of 3 Ways of Conventional Pap Smear, Liquid-Based Cytology and Colposcopy vs Cervical Biopsy for Early Diagnosis of Premalignant Lesions or Cervical Cancer in Women with Abnormal Conventional Pap Test. Int. J. Biomed. Sci. 2013, 9, 205–210. [Google Scholar]
- Olaniyan, O.B. Validity of colposcopy in the diagnosis of early cervical neoplasia—A review. Afr. J. Reprod. Health 2002, 6, 59–69. [Google Scholar] [CrossRef]
- Mousavi, A.S.; Fakour, F.; Gilani, M.M.; Behtash, N.; Ghaemmaghami, F.; Karimi Zarchi, M. A prospective study to evaluate the correlation between Reid colposcopic index impression and biopsy histology. J. Low Genit. Tract Dis. 2007, 11, 147–150. [Google Scholar] [CrossRef]
- Arbyn, M.; Kyrgiou, M.; Simoens, C.; O Raifu, A.; Koliopoulos, G.; Martin-Hirsch, P.; Prendiville, W.; Paraskevaidis, E. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: Meta-analysis. BMJ 2008, 337, a1284. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Koliopoulos, G.; Martin-Hirsch, P.; Arbyn, M.; Prendiville, W.; Paraskevaidis, E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: Systematic review and meta-analysis. Lancet 2006, 367, 489–498. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Athanasiou, A.; Kalliala, I.E.J.; Paraskevaidi, M.; Mitra, A.; Martin-Hirsch, P.P.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst. Rev. 2017, 11, Cd012847. [Google Scholar] [CrossRef]
- Bornstein, J.; Bentley, J.; Bösze, P.; Girardi, F.; Haefner, H.; Menton, M.; Perrotta, M.; Prendiville, W.; Russell, P.; Sideri, M.; et al. 2011 colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet. Gynecol. 2012, 120, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Quaas, J.; Reich, O.; Kuppers, V. Explanation and Use of the Rio 2011 Colposcopy Nomenclature of the IFCPC (International Federation for Cervical Pathology and Colposcopy): Comments on the general colposcopic assessment of the uterine cervix: Adequate/inadequate; squamocolumnar junction; transformation zone. Geburtshilfe Frauenheilkd 2014, 74, 1090–1092. [Google Scholar] [PubMed]
- Quaas, J.; Reich, O.; Tirri, B.F.; Küppers, V. Explanation and Use of the Colposcopy Terminology of the IFCPC (International Federation for Cervical Pathology and Colposcopy) Rio 2011. Geburtshilfe Frauenheilkd 2013, 73, 904–907. [Google Scholar] [CrossRef]
- Loopik, D.L.; Doucette, S.; Bekkers, R.L.; Bentley, J.R. Regression and Progression Predictors of CIN2 in Women Younger than 25 Years. J. Low Genit. Tract Dis. 2016, 20, 213–217. [Google Scholar] [CrossRef]
- Massad, L.S.; Collins, Y.C. Strength of correlations between colposcopic impression and biopsy histology. Gynecol. Oncol. 2003, 89, 424–428. [Google Scholar] [CrossRef]
- Ruan, Y.; Liu, M.; Guo, J.; Zhao, J.; Niu, S.; Li, F. Evaluation of the accuracy of colposcopy in detecting high-grade squamous intraepithelial lesion and cervical cancer. Arch. Gynecol. Obstet. 2020, 302, 1529–1538. [Google Scholar] [CrossRef]
- Mustafa, R.A.; Santesso, N.; Khatib, R.; Mustafa, A.A.; Wiercioch, W.; Kehar, R.; Gandhi, S.; Chen, Y.; Cheung, A.; Hopkins, J.; et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int. J. Gynaecol. Obstet. 2016, 132, 259–265. [Google Scholar] [CrossRef]
- Hopman, E.H.; Kenemans, P.; Helmerhorst, T.J. Positive predictive rate of colposcopic examination of the cervix uteri: An overview of literature. Obstet. Gynecol. Surv. 1998, 53, 97–106. [Google Scholar] [CrossRef]
- Ferris, D.G.; Litaker, M.S. Colposcopy quality control by remote review of digitized colposcopic images. Am. J. Obstet. Gynecol. 2004, 191, 1934–1941. [Google Scholar] [CrossRef]
- Beschluss des Gemeinsamen Bundesausschusses über eine Änderung der Krebsfrüherkennungs-Richtlinie und eine Änderung der Richtlinie für organisierte Krebsfrüherkennungsprogramme: Programm zur Früherkennung von Zervixkarzinomen 2018. Available online: https://www.g-ba.de/downloads/39-261-3597/2018-11-22_oKFE-RL_Zervixkarzinom.pdf (accessed on 22 November 2018).
- Bujan Rivera, J.; Klug, S.J. Cervical cancer screening in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2018, 61, 1528–1535. [Google Scholar] [CrossRef]
- Hillemanns, P.; Mallmann, P.; Beckmann, M.W. New Screening Proposals: The Federal Joint Commission Defines the Parameters for Cervical Cancer Screening from 2018: Statement of the Gynecology Oncology Working Group (AGO). Geburtshilfe Frauenheilkd 2016, 76, 145–146. [Google Scholar] [PubMed]
- Hillemanns, P.; Friese, K.; Dannecker, C.; Klug, S.; Seifert, U.; Iftner, T.; Hädicke, J.; Löning, T.; Horn, L.; Schmidt, D.; et al. Prevention of Cervical Cancer: Guideline of the DGGG and the DKG (S3 Level, AWMF Register Number 015/027OL, December 2017)—Part 1 with Introduction, Screening and the Pathology of Cervical Dysplasia. Geburtshilfe Frauenheilkd 2019, 79, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Hillemanns, P.; Friese, K.; Dannecker, C.; Klug, S.; Seifert, U.; Iftner, T.; Hädicke, J.; Löning, T.; Horn, L.; Schmidt, D.; et al. Prevention of Cervical Cancer: Guideline of the DGGG and the DKG (S3 Level, AWMF Register Number 015/027OL, December 2017)—Part 2 on Triage, Treatment and Follow-up. Geburtshilfe Frauenheilkd 2019, 79, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Hillemanns, P.; Tempfer, C.; Beckmann, M.W.; Küppers, V.; Quaas, J. Statement of the AGO and AG-CPC on the Aftercare/Follow-up for Surgical Procedures of the Lower Genital Tract after the Introduction of a New Cancer Screening Guideline. Geburtshilfe Frauenheilkd 2020, 80, 809–812. [Google Scholar] [CrossRef]
- Barnes, B.; Kraywinkel, K.; Nowossadeck, E.; Schönfeld, I.; Starker, A.; Wienecke, A.; Wolf, U. RKI-Bericht zum Krebsgeschehen in Deutschland 2016. 2016. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebsgeschehen/Kr-ebsgeschehe (accessed on 5 October 2022).
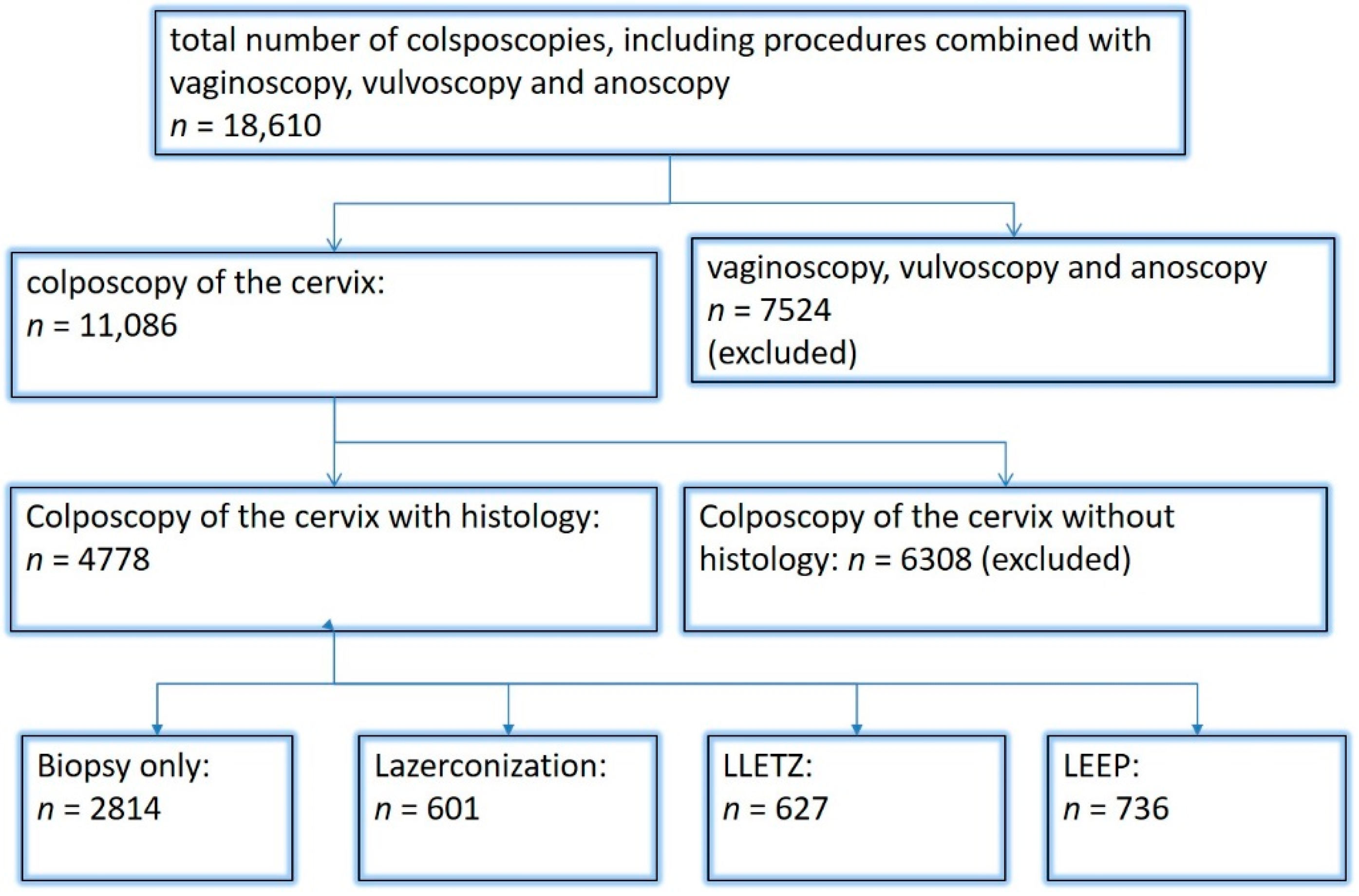
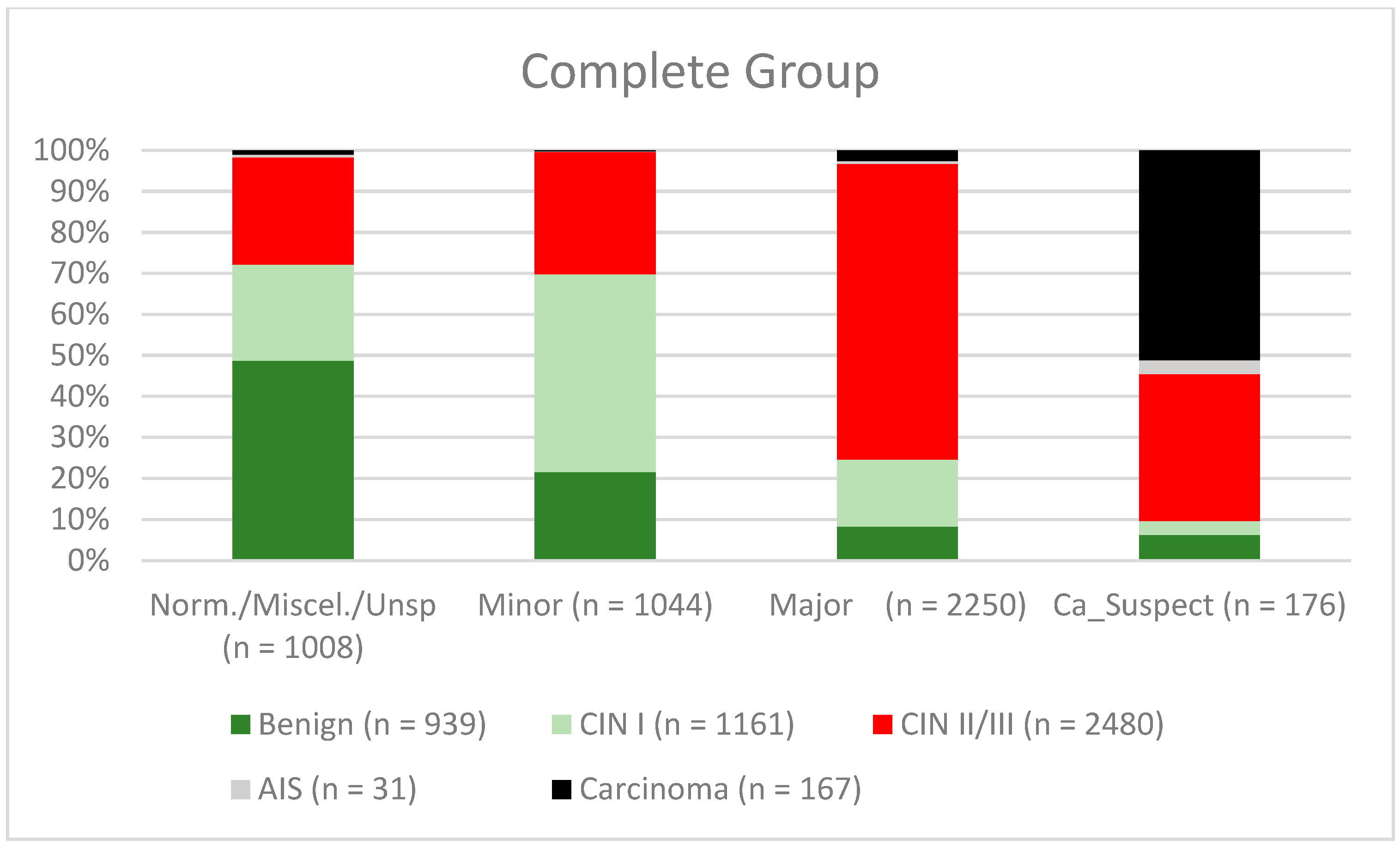
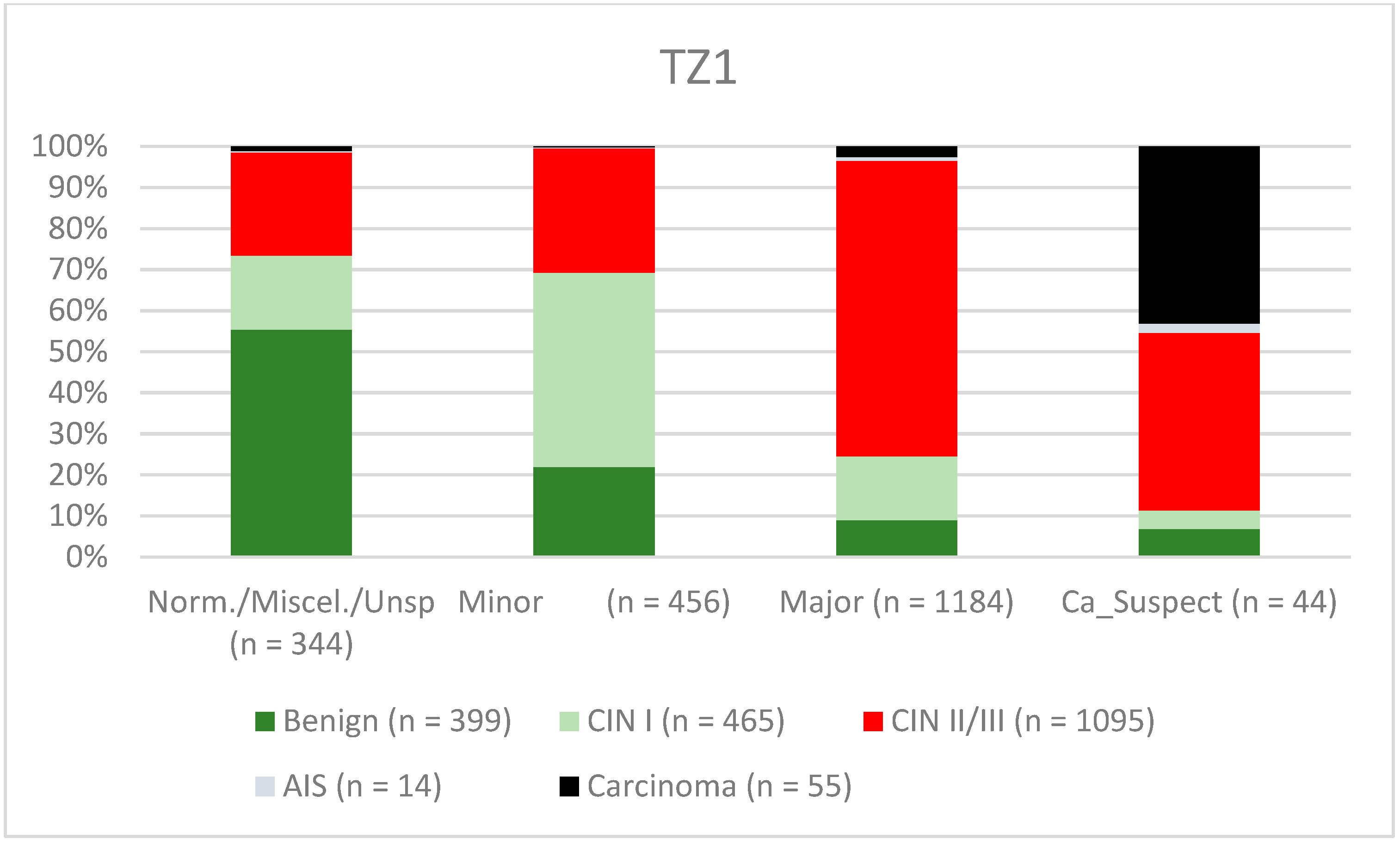
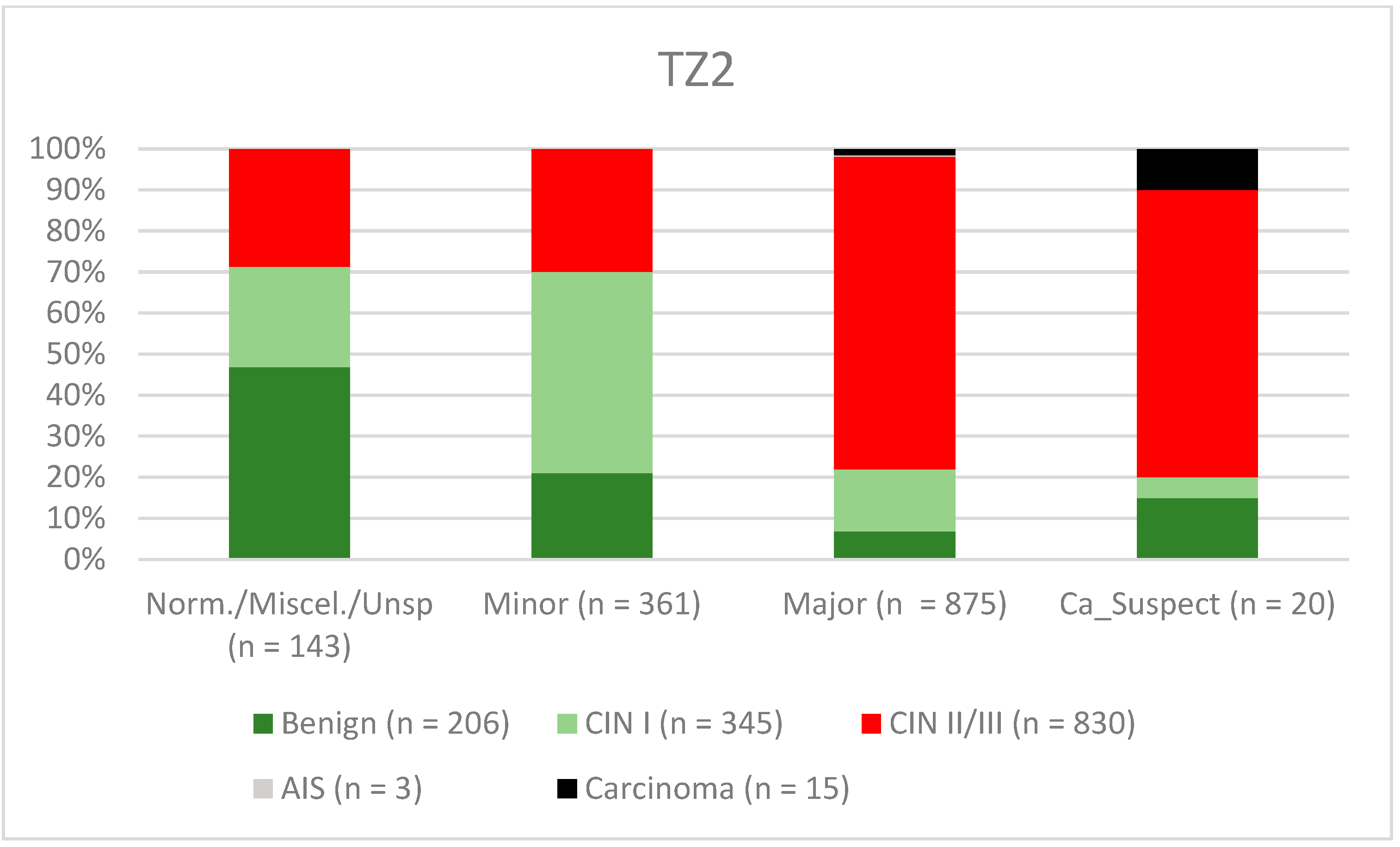
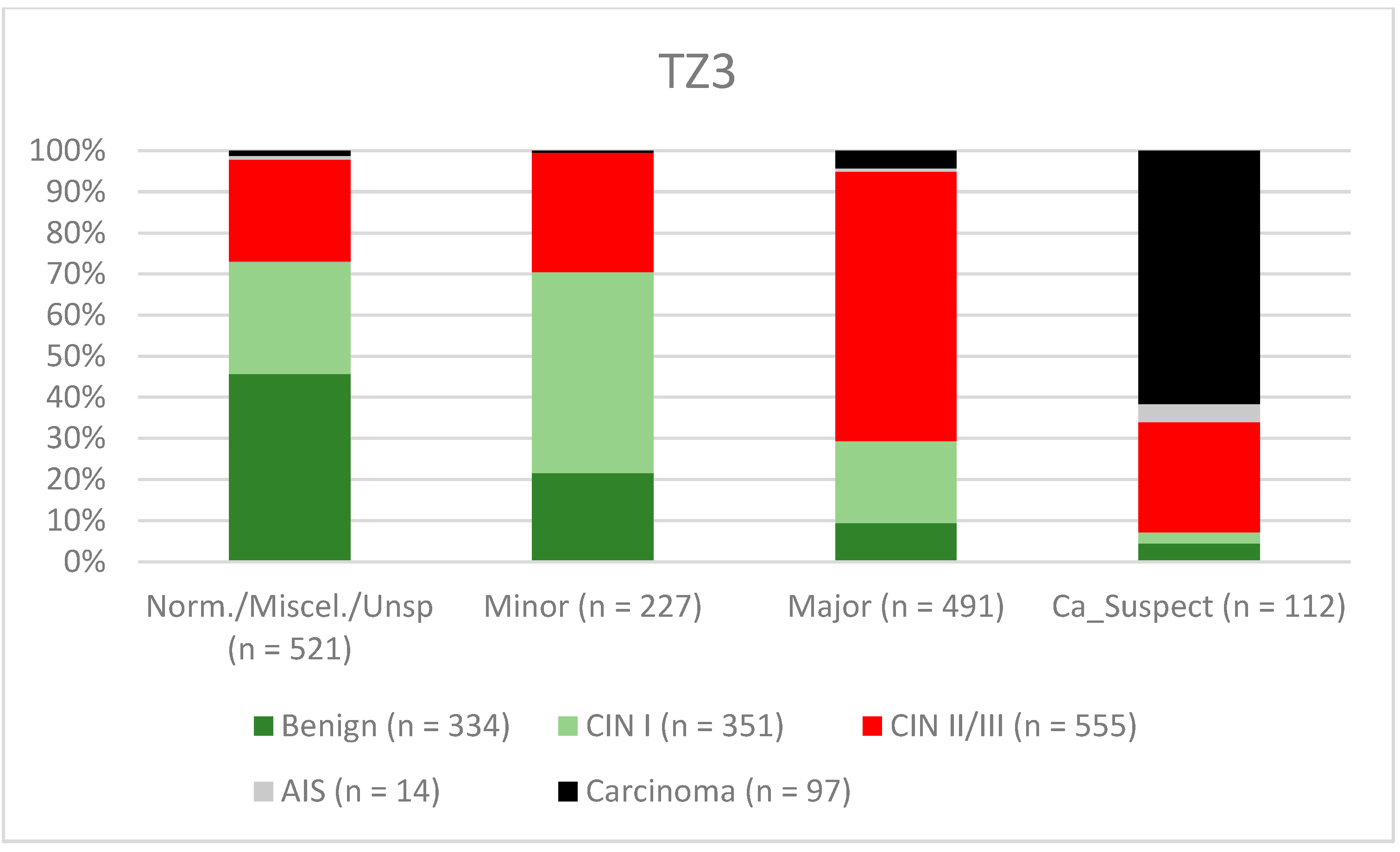
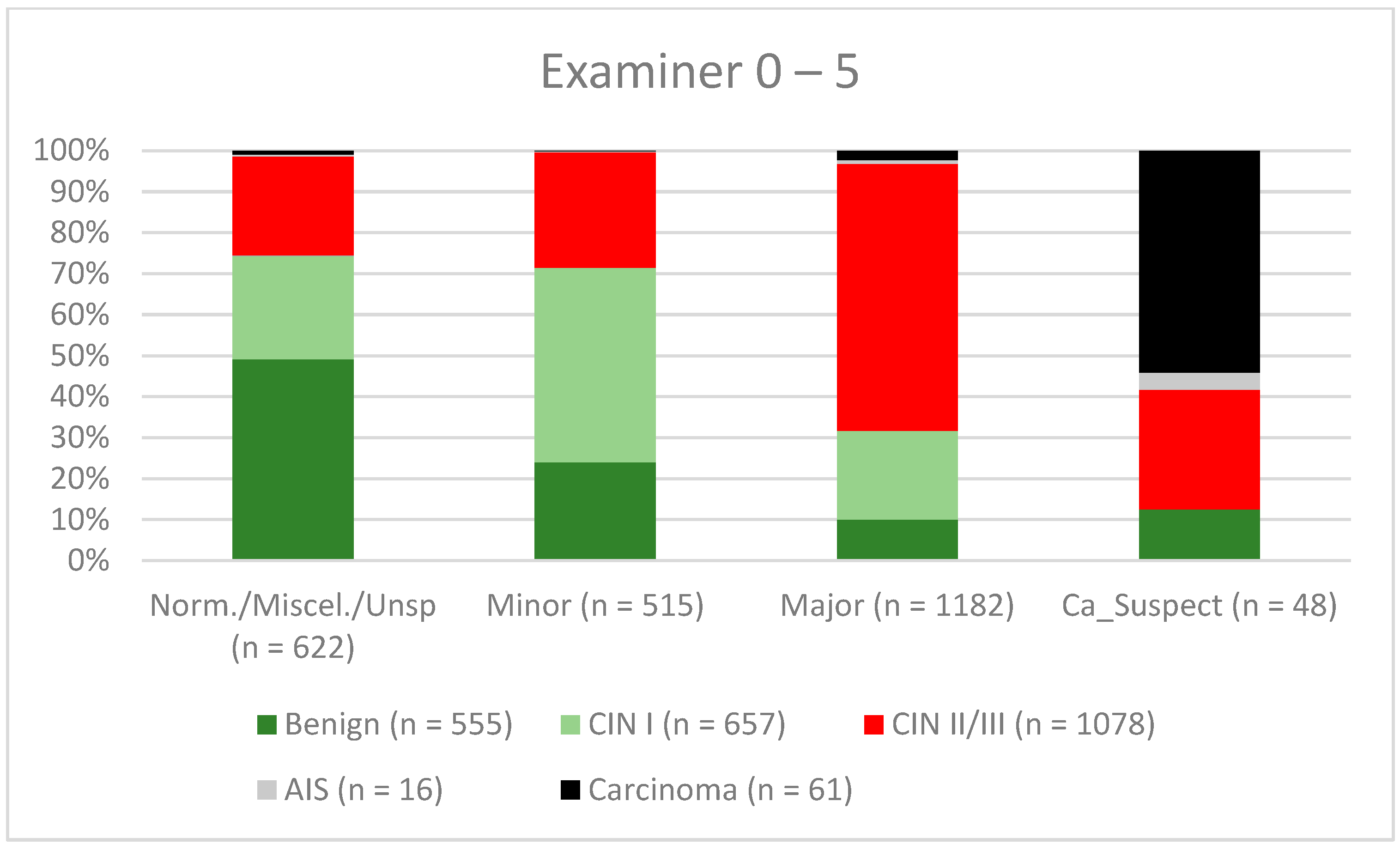
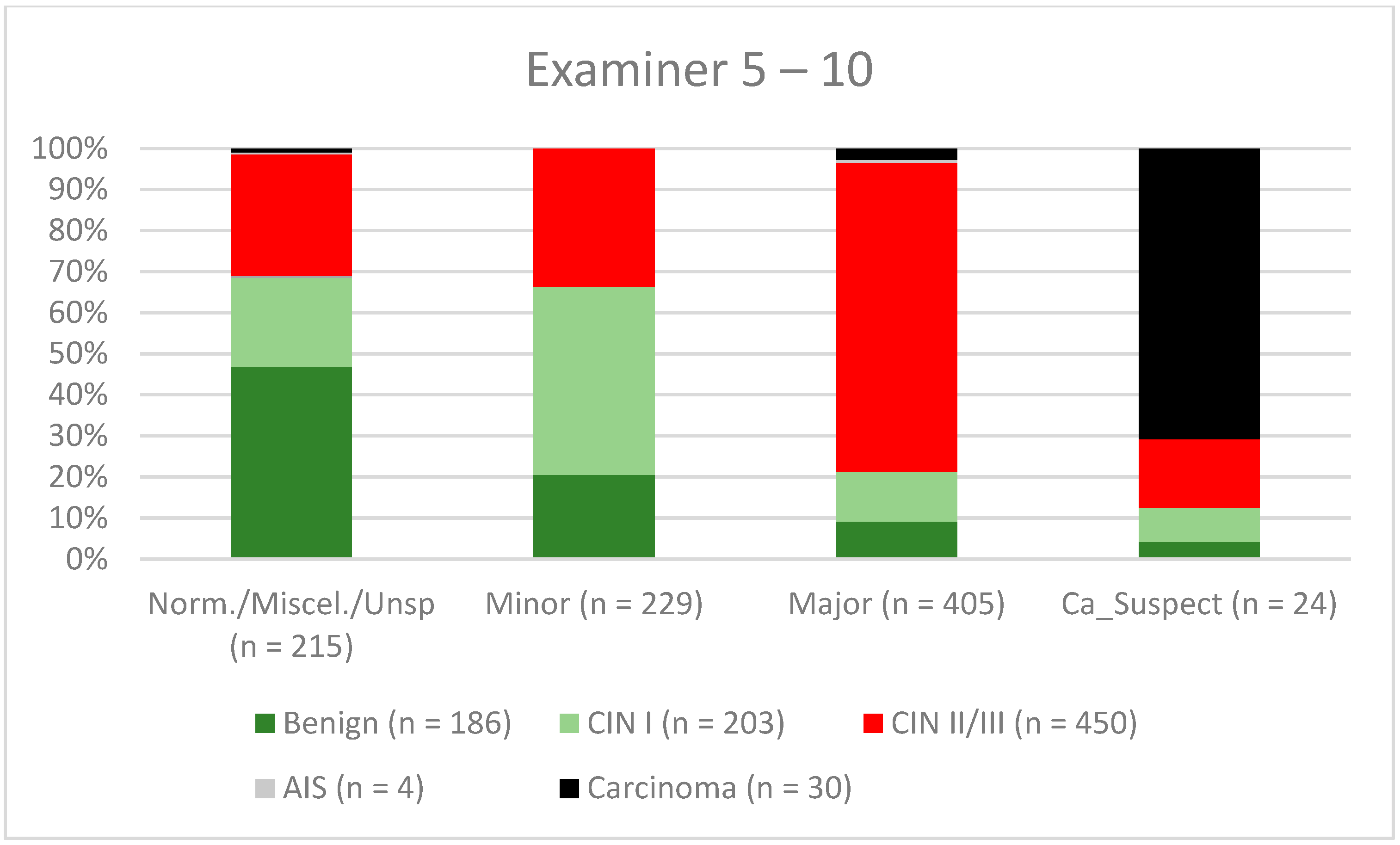
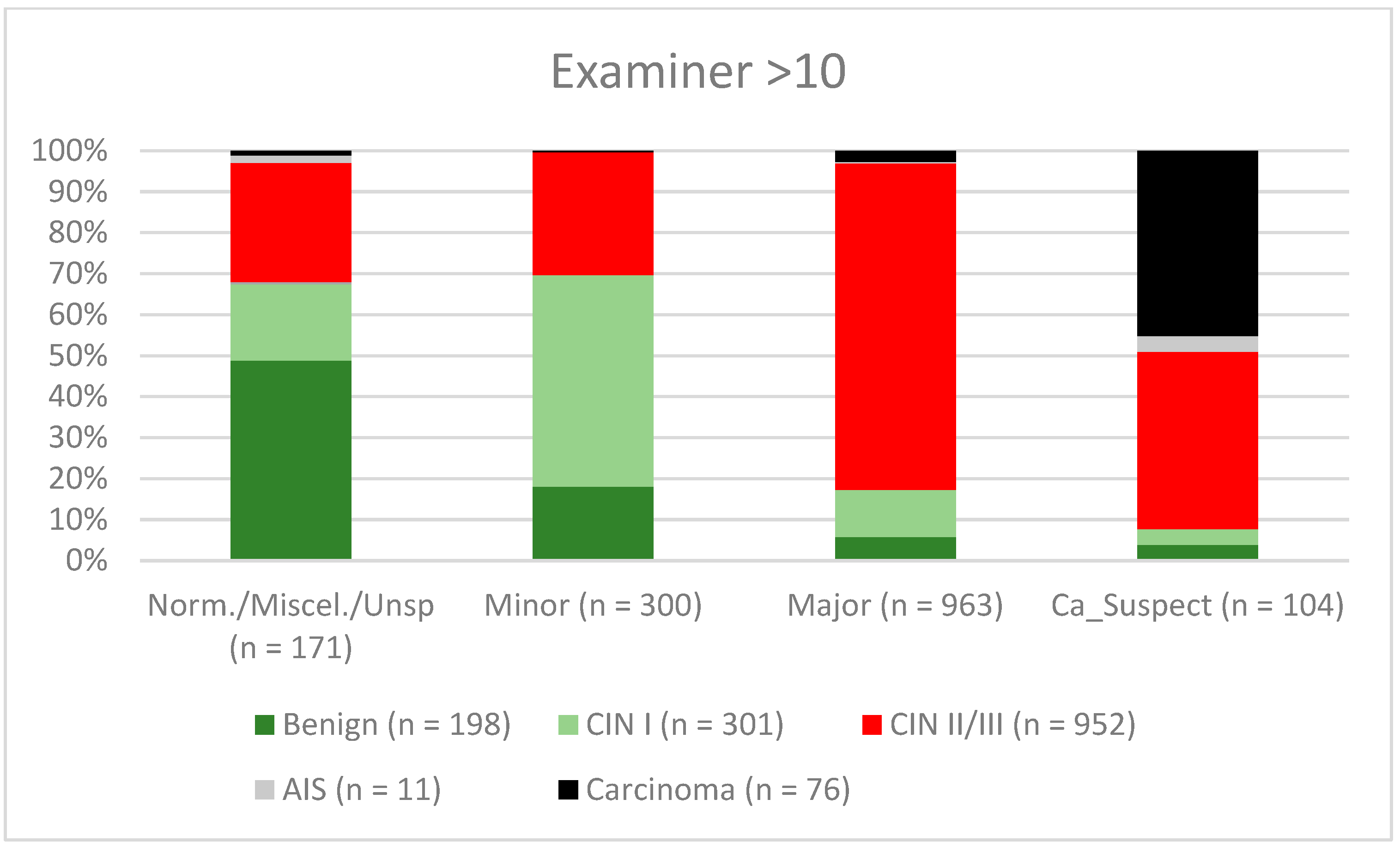

| Findings/Histology (n = 4778) | Benign (n = 939) | CIN I (n = 1161) | CIN II/III (n = 2480) | AIS (n = 31) | Carcinoma (n = 167) |
|---|---|---|---|---|---|
| (p < 0.001) | (p < 0.001) | Reference | (p < 0.001) | ||
| Normal/miscellaneous/unspecific (n = 1008) | 491 (52.3%) | 236 (20.3%) | 264 (10.6%) | 7 (22.6%) | 10 (6%) |
| Minor (n = 1044) | 225 (24%) | 504 (43.4%) | 312 (12.6%) | 1 (3.2%) | 2 (1.2%) |
| Major (n = 2550) | 212 (22.6%) | 415 (35.7%) | 1841 (74.2%) | 17 (54.8%) | 65 (38.9%) |
| Suspicious for cancer (n = 176) | 11 (1.2%) | 6 (0.5%) | 63 (2.5%) | 6 (19.4%) | 90 (53.9%) |
| Sensitivity (95 CI) | Specificity (95 CI) | PPV (95 CI) | NPV (95 CI) | |
|---|---|---|---|---|
| Complete group | 77.8% (76.12–79.31%) | 69.3% (67.31–71.30%) | 76.4% (74.74–77.96%) | 71.0% (68.94–72.91%) |
| TZ1 | 80.1% (77.66–82.33) | 65.7% (62.47–68.9) | 75.9% (73.4–78.26) | 71.0% (67.72–74.12) |
| TZ2 | 82.4% (79.7–84.93) | 64.4% (60.27–68.43) | 78.1% (75.25–80.77) | 70.4% (66.24–74.39) |
| TZ3 | 67.7% (64.02–71.26) | 77.8% (74.51–80.87) | 74.8% (71.13–78.21) | 71.3% (67.87–74.48) |
| Examiner 0–5 | 73.5% (70.86–76.03) | 68.6% (65.87–71.17) | 69.0% (66.36–71.6) | 73.1% (70.41–75.65) |
| Examiner 5–10 | 70.2% (65.89–74.24) | 77.1% (72.62–81.2) | 79.2% (75.05–82.95) | 67.6% (62.99–71.9) |
| Examiner >10 | 86.0% (83.77–88.08) | 65.1% (60.77–69.31) | 83.7% (81.34–85.86) | 69.2% (64.76–73.3) |
| Age 0–34 | 82.3% (80.18–84.3) | 63.8% (60.68–66.86) | 76.4% (74.19–78.59) | 71.7% (68.51–74.66) |
| Age >35 | 73.0% (70.48–75.37) | 74.0% (71.32–76.5) | 76.3% (73.84–78.63) | 70.5% (67.78–73.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuebs, F.A.; Dietl, A.K.; Behrens, A.; Adler, W.; Geppert, C.; Hartmann, A.; Knöll, A.; Beckmann, M.W.; Mehlhorn, G.; Schulmeyer, C.E.; et al. Concordance Rate of Colposcopy in Detecting Cervical Intraepithelial Lesions. Diagnostics 2022, 12, 2436. https://doi.org/10.3390/diagnostics12102436
Stuebs FA, Dietl AK, Behrens A, Adler W, Geppert C, Hartmann A, Knöll A, Beckmann MW, Mehlhorn G, Schulmeyer CE, et al. Concordance Rate of Colposcopy in Detecting Cervical Intraepithelial Lesions. Diagnostics. 2022; 12(10):2436. https://doi.org/10.3390/diagnostics12102436
Chicago/Turabian StyleStuebs, Frederik A., Anna K. Dietl, Annika Behrens, Werner Adler, Carol Geppert, Arndt Hartmann, Antje Knöll, Matthias W. Beckmann, Grit Mehlhorn, Carla E. Schulmeyer, and et al. 2022. "Concordance Rate of Colposcopy in Detecting Cervical Intraepithelial Lesions" Diagnostics 12, no. 10: 2436. https://doi.org/10.3390/diagnostics12102436
APA StyleStuebs, F. A., Dietl, A. K., Behrens, A., Adler, W., Geppert, C., Hartmann, A., Knöll, A., Beckmann, M. W., Mehlhorn, G., Schulmeyer, C. E., Gass, P., & Koch, M. C. (2022). Concordance Rate of Colposcopy in Detecting Cervical Intraepithelial Lesions. Diagnostics, 12(10), 2436. https://doi.org/10.3390/diagnostics12102436








