Acute Distal Vertebral Artery Occlusion in Patients with Asymmetrical Vertebral Artery Geometry: Role of Black-Blood-Enhanced MR Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Procedure
2.3. Clinical Data Analysis
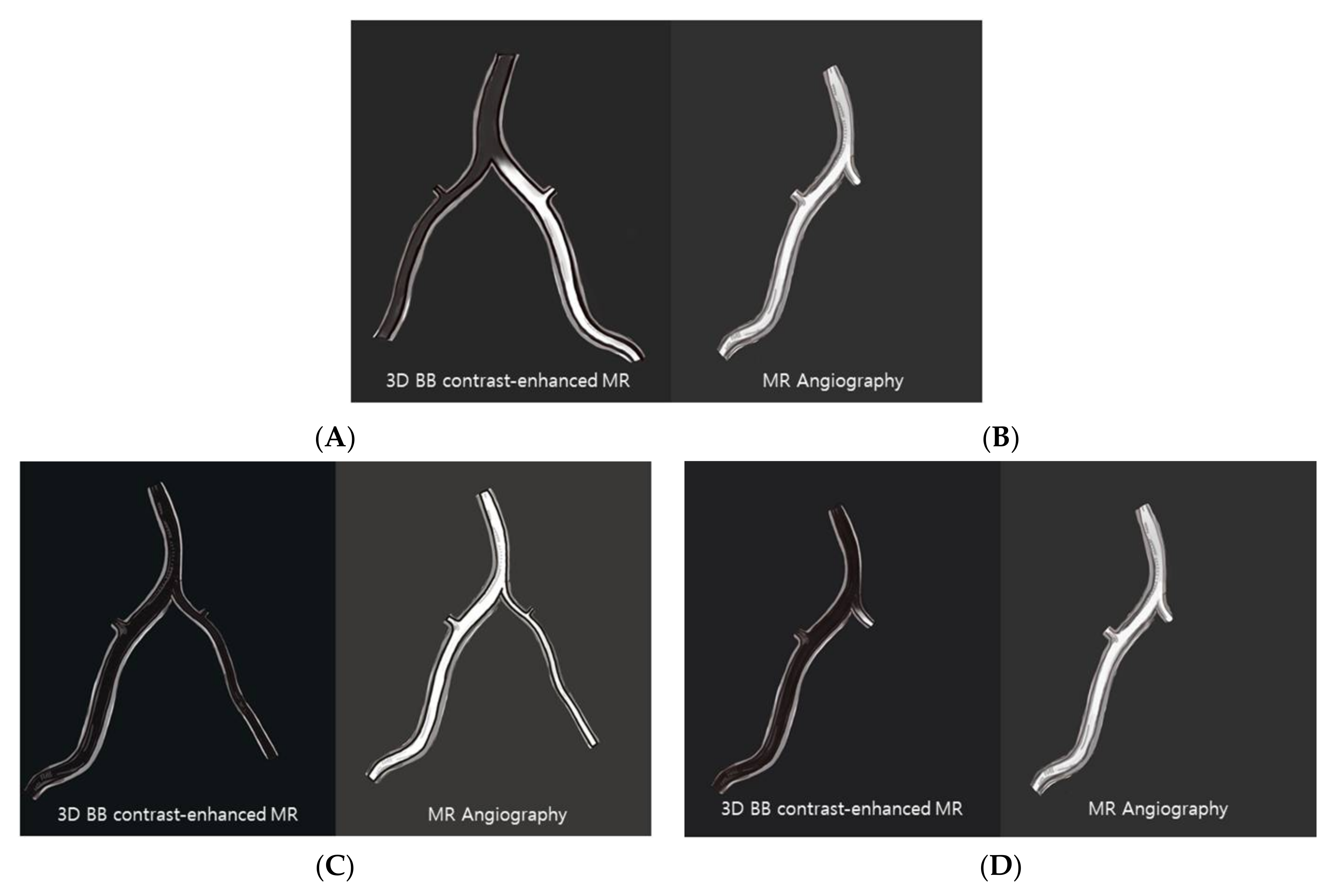
2.4. MR Imaging Analysis
2.5. Statistical Analyses
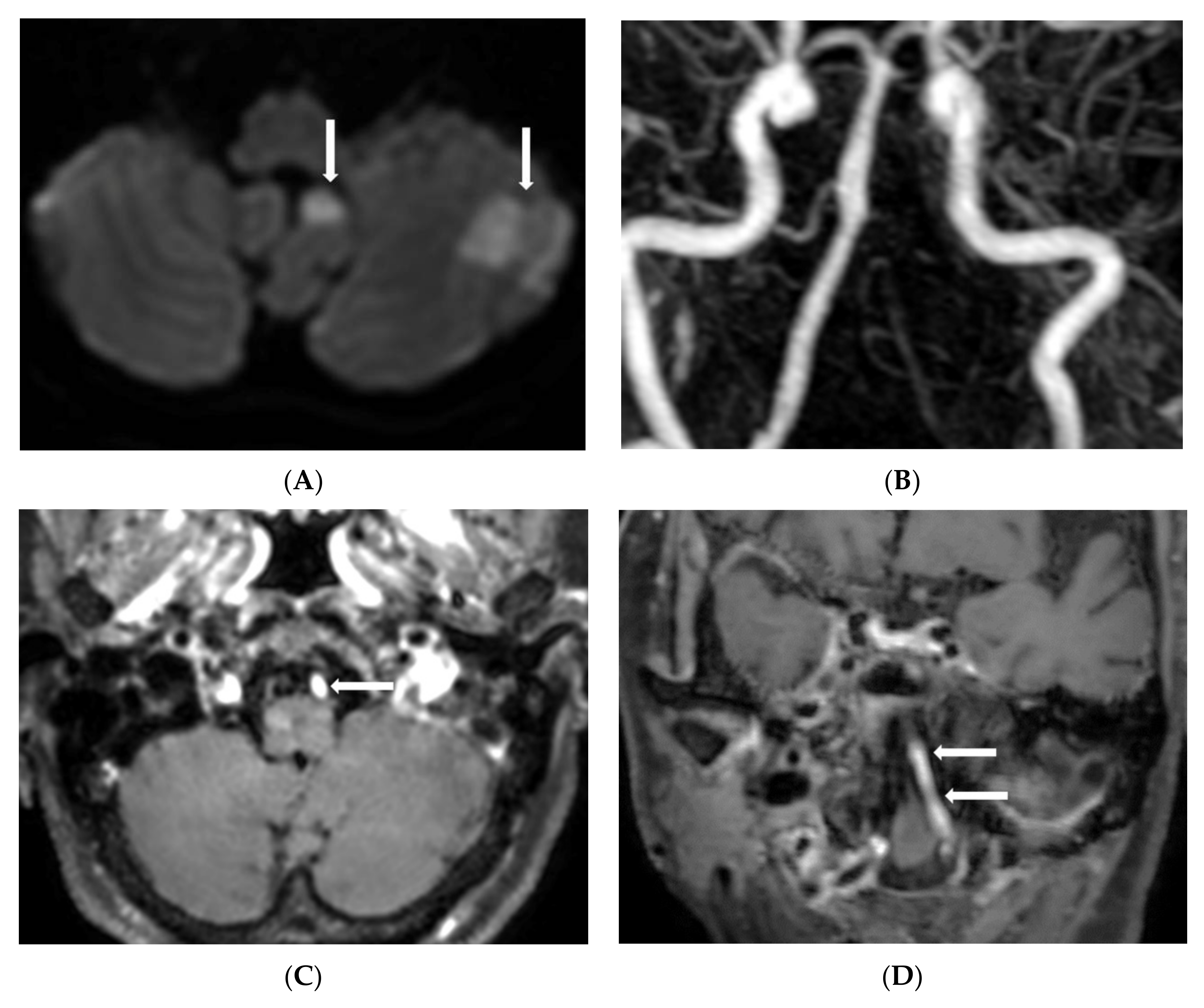
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caplan, L.R.; Wityk, R.; Pazdera, L.; Chang, H.M.; Pessin, M.; Dewitt, L.D. New England Medical Center Posterior Circulation Stroke Registry II. Vascular Lesions. J. Clin. Neurol. 2005, 1, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Qin, H.; Zhang, H. Evaluation of recanalisation treatment on posterior circulation ischemic stroke by Solitaire device-A multicenter retrospective study. Neurol. Neurochir. Pol. 2017, 51, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Tariq, N.; Maud, A.; Shah, Q.A.; Suri, M.F.; Qureshi, A.I. Clinical outcome of patients with acute posterior circulation stroke and bilateral vertebral artery occlusion. J. Vasc. Interv. Neurol. 2011, 4, 9. [Google Scholar] [PubMed]
- Derex, L.; Nighoghossian, N.; Hermier, M.; Adeleine, P.; Froment, J.C.; Trouillas, P. Early detection of cerebral arterial occlusion on magnetic resonance angiography: Predictive value of the baseline NIHSS score and impact on neurological outcome. Cerebrovasc. Dis. 2002, 13, 225–229. [Google Scholar] [CrossRef]
- Legrand, L.; Naggara, O.; Turc, G.; Mellerio, C.; Roca, P.; Calvet, D.; Labeyrie, M.A.; Baron, J.C.; Mas, J.L.; Meder, J.F.; et al. Clot burden score on admission T2*-MRI predicts recanalization in acute stroke. Stroke 2013, 44, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Park, M.G.; Oh, S.J.; Baik, S.K.; Jung, D.S.; Park, K.P. Susceptibility-Weighted Imaging for Detection of Thrombus in Acute Cardioembolic Stroke. J. Stroke 2016, 18, 73–79. [Google Scholar] [CrossRef]
- Wang, N.; Hu, Q.; Wang, Q.; Zhao, J.; Dedhia, N.; Caplan, L.R. Intracranial Posterior Circulation Large Artery Thrombi Visualized Using Susceptibility-Weighted MRI. J. Neuroimaging 2017, 27, 505–510. [Google Scholar] [CrossRef]
- Jang, W.; Kwak, H.S.; Chung, G.H.; Hwang, S.B. Three-dimensional black-blood contrast-enhanced MRI improves detection of intraluminal thrombi in patients with acute ischaemic stroke. Eur. Radiol. 2018, 28, 3840–3847. [Google Scholar] [CrossRef]
- Lee, K.Y.; Suh, S.H.; Ahn, S.J. Significance of hyperintense arteries on Gd-enhanced 3D T1W black-blood imaging in acute stroke. Eur. Radiol. 2019, 29, 1329–1337. [Google Scholar] [CrossRef]
- Duron, L.; Savatovsky, J.; Obadia, M.; Metten, M.A.; Roux, P.; Blanc, R.; Sadik, J.C.; Dhundass, S.; Lecler, A. Magnetic resonance post-contrast vascular hyperintensities at 3 T: A new highly sensitive sign of vascular occlusion in acute ischaemic stroke. Eur. Radiol. 2018, 28, 2903–2913. [Google Scholar] [CrossRef]
- Chung, G.H.; Hwang, S.B.; Kwak, H.S. Use of 3-Dimensional, Black-Blood, Contrast-Enhanced, T1-Weighted Magnetic Resonance Imaging to Identify Vascular Occlusion in the Posterior Circulation After Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 104373. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kwak, H.S.; Hwang, S.B.; Chung, G.H. Three-dimensional black blood contrast enhanced magnetic resonance imaging in patients with acute ischemic stroke and negative susceptibility vessel sign. Eur. J. Radiol. 2018, 102, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gerretsen, S.C.; Maki, J.H.; Jaarsma, C.; Kooi, M.E.; Herzka, D.; Chu, B.; Yarnykh, V.L.; Yuan, C.; Leiner, T. Time-efficient black blood RCA wall imaging at 3T using improved motion sensitized driven equilibrium (iMSDE): Feasibility and reproducibility. PLoS ONE 2011, 6, e26567. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yarnykh, V.L.; Yuan, C. Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. J. Magn. Reson. Imaging 2010, 31, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Head, T.; Daunert, S.; Goldschmidt-Clermont, P.J. The Aging Risk and Atherosclerosis: A Fresh Look at Arterial Homeostasis. Front. Genet. 2017, 8, 216. [Google Scholar] [CrossRef]
- Al-Ali, F.; Barrow, T.; Duan, L.; Jefferson, A.; Louis, S.; Luke, K.; Major, K.; Smoker, S.; Walker, S.; Yacobozzi, M. Vertebral artery ostium atherosclerotic plaque as a potential source of posterior circulation ischemic stroke: Result from borgess medical center vertebral artery ostium stenting registry. Stroke 2011, 42, 2544–2549. [Google Scholar] [CrossRef]
- Gulli, G.; Khan, S.; Markus, H.S. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke 2009, 40, 2732–2737. [Google Scholar] [CrossRef]
- Floßmann, E.; Rothwell, P.M. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain J. Neurol. 2003, 126, 1940–1954. [Google Scholar] [CrossRef]
- Bogousslavsky, J.; Van Melle, G.; Regli, F. The Lausanne Stroke Registry: Analysis of 1,000 consecutive patients with first stroke. Stroke 1988, 19, 1083–1092. [Google Scholar] [CrossRef]
- Caplan, L.R.; Wityk, R.J.; Glass, T.A.; Tapia, J.; Pazdera, L.; Chang, H.M.; Teal, P.; Dashe, J.F.; Chaves, C.J.; Breen, J.C.; et al. New England Medical Center Posterior Circulation registry. Ann. Neurol. 2004, 56, 389–398. [Google Scholar] [CrossRef]
- Madonis, S.M.; Jenkins, J.S. Vertebral artery stenosis. Prog. Cardiovasc. Dis. 2021, 65, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Sparaco, M.; Ciolli, L.; Zini, A. Posterior circulation ischaemic stroke-a review part I: Anatomy, aetiology and clinical presentations. Neurol. Sci. 2019, 40, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Morris, L. Non-union of the vertebral arteries: Case report. Br. J. Radiol. 1962, 35, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Li, C.S.; Fu, J.H.; Zhang, K.; Xu, R.; Xu, W.J. Evaluation of Cranial and Cervical Arteries and Brain Tissue in Transient Ischemic Attack Patients with Magnetic Resonance Angiography and Diffusion-Weighted Imaging. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1726. [Google Scholar] [CrossRef]
- Morimoto, K.; Nagahata, M.; Ono, S.; Miura, H.; Tsushima, F.; Seino, H.; Kakehata, S.; Basaki, K.; Uno, S.; Abe, Y. Incidence of unilateral distal vertebral artery aplasia: Evaluation by combining basiparallel anatomic scanning-magnetic resonance imaging (BPAS-MRI) and magnetic resonance angiography. Jpn. J. Radiol. 2009, 27, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Al-Smadi, A.S.; Abdalla, R.N.; Elmokadem, A.H.; Shaibani, A.; Hurley, M.C.; Potts, M.B.; Jahromi, B.S.; Carroll, T.J.; Ansari, S.A. Diagnostic Accuracy of High-Resolution Black-Blood MRI in the Evaluation of Intracranial Large-Vessel Arterial Occlusions. AJNR Am. J. Neuroradiol. 2019, 40, 954–959. [Google Scholar] [CrossRef]
- Gang, M.; Lee, H.; Kwak, H.S.; Hwang, S.B.; Chung, G.H. Strong enhancement on three-dimensional black-blood enhanced magnetic resonance imaging: Comparison intracranial stenosis and complete occlusion. Eur. J. Radiol. 2021, 137, 109580. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Meng, Y.; Zhang, Y.; Zhang, J.; Song, Y.; Sun, L.; Zheng, M.; Wang, W.; Yin, H.; et al. Symptomatic Atherosclerotic Non-Acute Intracranial Vertebral Artery Total Occlusion: Clinical Features, Imaging Characteristics, Endovascular Recanalization, and Follow-up Outcomes. Front. Neurol. 2020, 11, 598795. [Google Scholar] [CrossRef]
- Mercier, P.H.; Brassier, G.; Fournier, H.D.; Picquet, J.; Papon, X.; Lasjaunias, P. Vascular microanatomy of the pontomedullary junction, posterior inferior cerebellar arteries, and the lateral spinal arteries. Interv. Neuroradiol. 2008, 14, 49–58. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.H.; Choi, C.G. Patterns of lateral medullary infarction: Vascular lesion-magnetic resonance imaging correlation of 34 cases. Stroke 1998, 29, 645–652. [Google Scholar] [CrossRef]
- Gaigalaite, V.; Vilimas, A.; Ozeraitiene, V.; Dementaviciene, J.; Janilionis, R.; Kalibatiene, D.; Rocka, S. Association between vertebral artery hypoplasia and posterior circulation stroke. BMC Neurol. 2016, 16, 118. [Google Scholar] [CrossRef] [PubMed]
| N = 288 | |
|---|---|
| Age, mean and SD | 71.6 ± 1.2 |
| Male, n (%) | 167 (58.0) |
| Hypertension, n (%) | 219 (76.0) |
| Diabetes, n (%) | 110 (38.2) |
| Smoking | 78 (27.1) |
| Hyperlipidemia, n (%) | 93 (32.3) |
| Previous stroke history, n (%) | 97 (33.7) |
| Previous heart problem, n (%) | 78 (27.1) |
| Pattern of contrast-enhanced VA, n (%) | |
| Type 1 | 65 (22.6) |
| Type 2 | 17 (5.9) |
| Type 3 | 129 (44.8) |
| Type 4 | 77 (26.7) |
| Contrast-enhanced VA (type 1 + 2), n (%) | 82 (28.5) |
| Positive DWI, n (%) | 51 (17.7) |
| Medulla | 22 |
| Pons | 9 |
| PICA | 20 |
| BB Enhanced Group (n =82) | BB Non-Enhanced Group (n = 206) | p | |
|---|---|---|---|
| Mean age ± SD | 73.1 ± 1.2 | 71.0 ± 0.9 | 0.216 |
| Male, n (%) | 56 (68.3) | 111 (53.9) | 0.025 |
| Rt, n (%) | 43 (52.4) | 127 (62.0) | 0.201 |
| Hypertension, n (%) | 71 (91.5) | 144 (69.9) | <0.001 |
| Diabetes, n (%) | 35 (42.7) | 75 (36.4) | 0.323 |
| Smoking, n (%) | 25 (30.5) | 53 (25.7) | 0.412 |
| Hyperlipidemia, n (%) | 30 (36.6) | 63 (30.6) | 0.326 |
| Previous stroke history, n (%) | 28 (34.1) | 69 (33.5) | 0.916 |
| Previous heart problem, n (%) | 19 (23.2) | 59 (28.6) | 0.346 |
| Positive DWI, n (%) | 41 (50.0) | 10 (4.9) | <0.001 |
| Medulla | 19 | 3 | |
| Pons | 7 | 2 | |
| PICA | 15 | 5 |
| Positive DWI (n = 51) | Negative DWI (n = 237) | p | |
|---|---|---|---|
| Mean age ± SD | 71.7 ± 1.6 | 71.5 ± 0.8 | 0.825 |
| Male, n (%) | 35 (68.6) | 132 (55.7) | 0.09 |
| Rt, n (%) | 43 (52.4) | 127 (62.0) | 0.247 |
| Hypertension, n (%) | 47 (92.2) | 172 (72.6) | 0.003 |
| Diabetes, n (%) | 28 (54.9) | 82 (34.6) | 0.007 |
| Smoking, n (%) | 21 (41.2) | 57 (24.1) | 0.013 |
| Hyperlipidemia, n (%) | 21 (41.2) | 72 (30.4) | 0.135 |
| Previous stroke history, n (%) | 16 (34.1) | 81 (34.2) | 0.701 |
| Previous heart problem, n (%) | 17 (33.3) | 61 (25.7) | 0.268 |
| VA geometry pattern | <0.001 | ||
| Type 1 | 35 (68.6) | 30 (12.7) | |
| Type 2 | 6 (11.8) | 11 (4.6) | |
| Type 3 | 8 (15.7) | 122 (51.5) | |
| Type 4 | 2 (3.9) | 74 (31.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Park, C.S.; Lee, U.Y.; Hwang, S.B.; Chung, G.H.; Kwak, H.S. Acute Distal Vertebral Artery Occlusion in Patients with Asymmetrical Vertebral Artery Geometry: Role of Black-Blood-Enhanced MR Imaging. Diagnostics 2022, 12, 2391. https://doi.org/10.3390/diagnostics12102391
Jeong Y, Park CS, Lee UY, Hwang SB, Chung GH, Kwak HS. Acute Distal Vertebral Artery Occlusion in Patients with Asymmetrical Vertebral Artery Geometry: Role of Black-Blood-Enhanced MR Imaging. Diagnostics. 2022; 12(10):2391. https://doi.org/10.3390/diagnostics12102391
Chicago/Turabian StyleJeong, Youngsun, Chan Sol Park, Ui Yun Lee, Seung Bae Hwang, Gyung Ho Chung, and Hyo Sung Kwak. 2022. "Acute Distal Vertebral Artery Occlusion in Patients with Asymmetrical Vertebral Artery Geometry: Role of Black-Blood-Enhanced MR Imaging" Diagnostics 12, no. 10: 2391. https://doi.org/10.3390/diagnostics12102391
APA StyleJeong, Y., Park, C. S., Lee, U. Y., Hwang, S. B., Chung, G. H., & Kwak, H. S. (2022). Acute Distal Vertebral Artery Occlusion in Patients with Asymmetrical Vertebral Artery Geometry: Role of Black-Blood-Enhanced MR Imaging. Diagnostics, 12(10), 2391. https://doi.org/10.3390/diagnostics12102391






