Significance of Indoleamine 2,3-Dioxygenase Expression in the Immunological Response of Kidney Graft Recipients
Abstract
1. Introduction
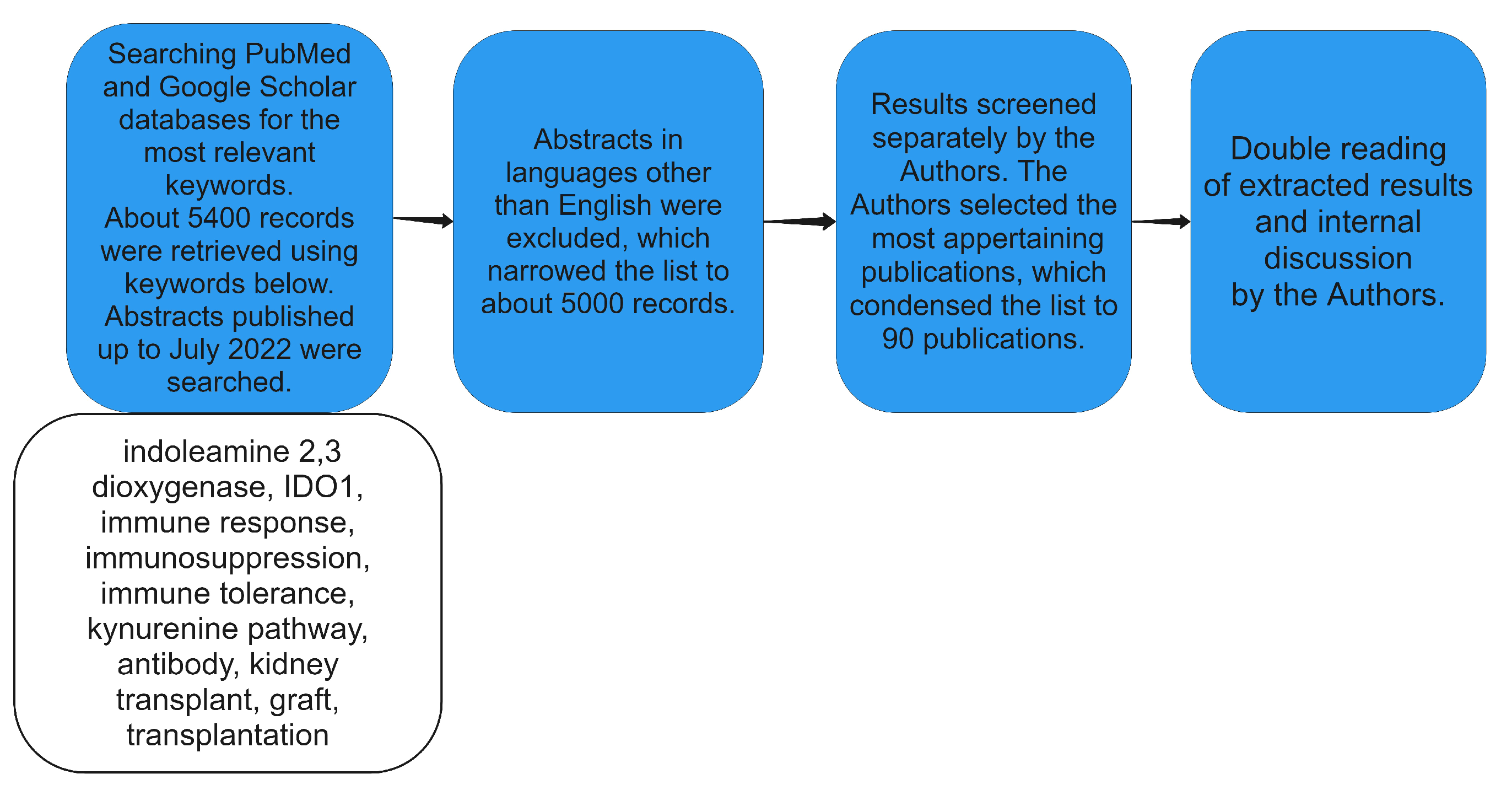
2. Materials and Methods
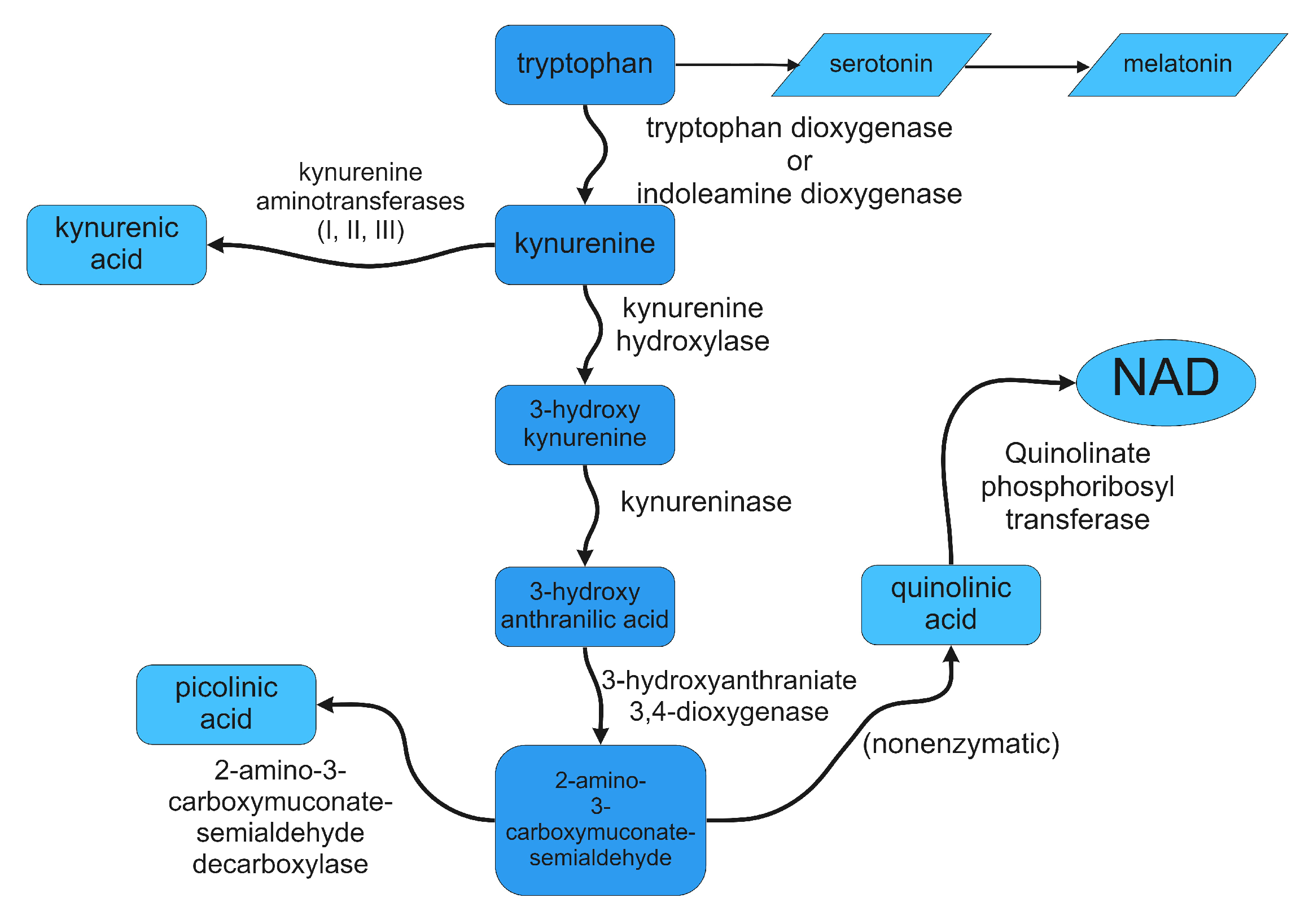
3. Control of Tryptophan Metabolism
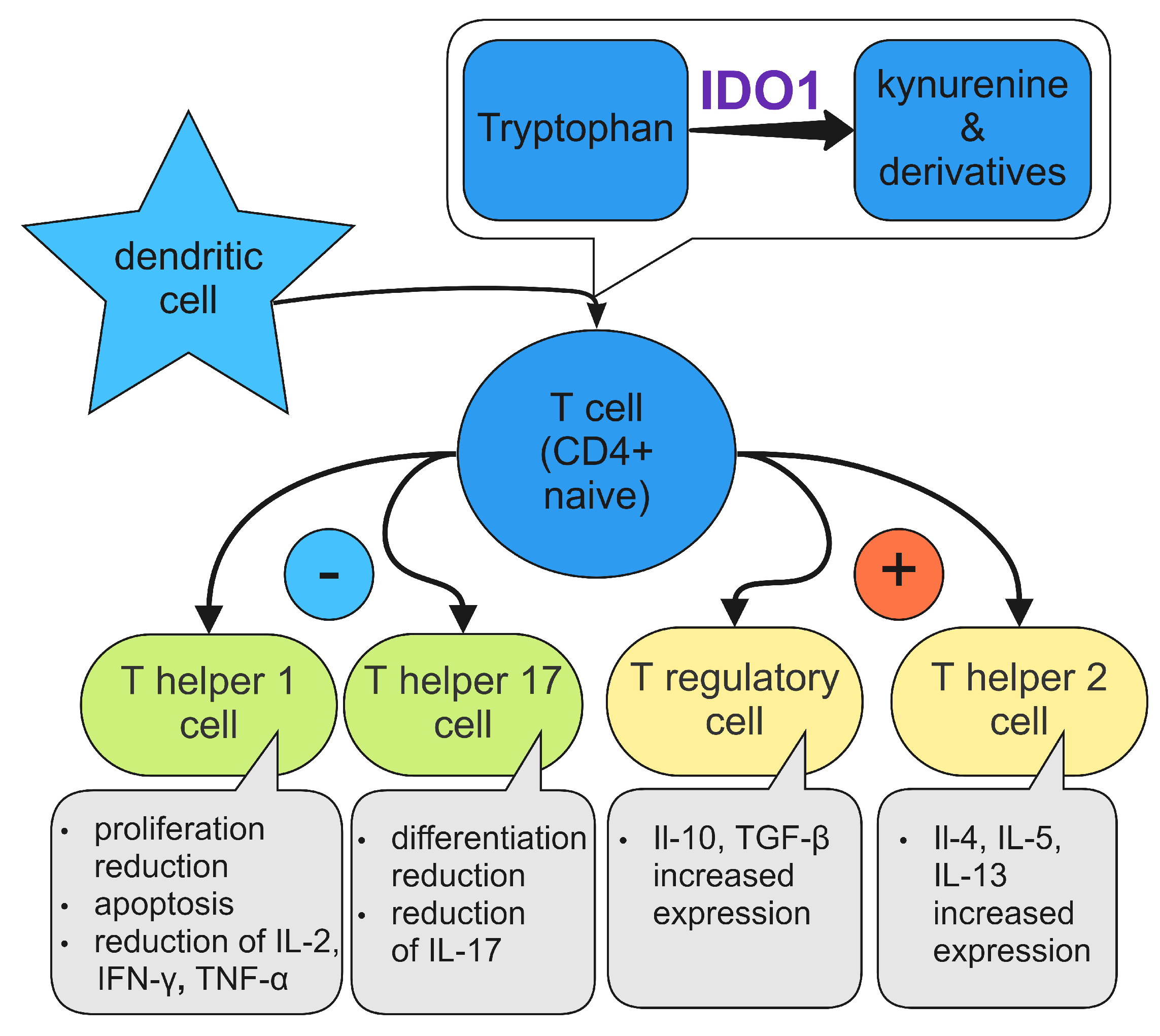
4. Immunosuppressive Mechanisms of IDO1
4.1. IDO1 and Dendritic Cells
4.2. Macrophages
4.3. Natural Killers
4.4. Eosinophils
4.5. Neutrophils
4.6. T Cells
4.7. B Cells
5. The Role of IDO1 in Kidneys
6. The Role of IDO1 in Kidney Allografts
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IDO | indoleamine 2,3-dioxygenase |
| ETAR | endothelin A type receptor |
| HLA | human leukocyte antigen |
| DC | dendritic cell |
References
- Kostro, J.Z.; Hellmann, A.; Kobiela, J.; Skóra, I.; Lichodziejewska-Niemierko, M.; Dębska-Ślizień, A.; Śledziński, Z. Quality of Life After Kidney Transplantation: A Prospective Study. Transpl. Proc. 2016, 48, 50–54. [Google Scholar] [CrossRef]
- Brennan, D.C.; Malone, A.; Vella, J. Kidney transplantation in adults: Treatment of acute T cell-mediated (cellular) rejection of the renal allograft. UpToDate. 2020. Available online: https://www.uptodate.com/contents/kidney-transplantation-in-adults-treatment-of-acute-t-cell-mediated-cellular-rejection (accessed on 1 July 2022).
- Cooper, J.E. Evaluation and Treatment of Acute Rejection in Kidney Allografts. Clin. J. Am. Soc. Nephrol. 2020, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Shabir, S.; Chand, S.; Cockwell, P.; Ball, S.; Borrows, R. Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation. J. Am. Soc. Nephrol. 2011, 22, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Mulley, W.R.; Nikolic-paterson, D.J. Indoleamine 2,3-dioxygenase in transplantation. Nephrology 2008, 13, 204–211. [Google Scholar] [CrossRef]
- Lee, G.K.; Park, H.J.; Macleod, M.; Chandler, P.; Munn, D.H.; Mellor, A.L. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 2002, 107, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Shafizadeh, E.; Attwood, J.T.; Bondarev, I.; Pashine, A.; Mellor, A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 1999, 189, 1363–1372. [Google Scholar] [CrossRef]
- Suarez-Fuentetaja, N.; Domenech-Garcia, N.; Paniagua-Martin, M.J.; Marzoa-Rivas, R.; Barge-Caballero, E.; Grille-Cancela, Z.; Pombo-Otero, J.; Muñiz-García, J.; Castro-Beiras, A.; Crespo-Leiro, M.G. Indoleamine, 2-3 dioxygenase activity could be an early marker of graft rejection in heart transplantation. Transpl. Proc. 2012, 44, 2645–2648. [Google Scholar] [CrossRef]
- Bauer, T.M.; Jiga, L.P.; Chuang, J.J.; Randazzo, M.; Opelz, G.; Terness, P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: Tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl. Int. 2005, 18, 95–100. [Google Scholar] [CrossRef]
- Quan, J.; Tan, P.H.; MacDonald, A.; Friend, P.J. Manipulation of indoleamine 2,3-dioxygenase (IDO) for clinical transplantation: Promises and challenges. Expert Opin. Biol. Ther. 2008, 8, 1705–1719. [Google Scholar] [CrossRef]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef]
- Grohmann, U.; Volpi, C.; Fallarino, F.; Bozza, S.; Bianchi, R.; Vacca, C.; Orabona, C.; Belladonna, M.L.; Ayroldi, E.; Nocentini, G.; et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat. Med. 2007, 13, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Sharma, M.D.; Lee, J.R.; Jhaver, K.G.; Johnson, T.S.; Keskin, D.B.; Marshall, B.; Chandler, P.; Antonia, S.J.; Burgess, R.; et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2002, 297, 1867–1870. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meinhardt, A.; Roehrich, M.E.; Golshayan, D.; Dudler, J.; Pagnotta, M.; Trucco, M.; Vassalli, G. Indoleamine 2,3-dioxygenase gene transfer prolongs cardiac allograft survival. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3415–H3423. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem. Biophys. Res. Commun. 2005, 338, 12–19. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, G. Nutritionally Essential Amino Acids. Adv. Nutr. 2018, 9, 849–851. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Krupa, A.; Kowalska, I. The Kynurenine Pathway-New Linkage between Innate and Adaptive Immunity in Autoimmune Endocrinopathies. Int. J. Mol. Sci. 2021, 22, 9879. [Google Scholar] [CrossRef]
- Routy, J.P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77. [Google Scholar] [CrossRef]
- Knox, W.E. [32] Tryptophan oxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1955; Volume 2, pp. 242–253. [Google Scholar]
- Wang, X.F.; Wang, H.S.; Wang, H.; Zhang, F.; Wang, K.F.; Guo, Q.; Zhang, G.; Cai, S.H.; Du, J. The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: Focus on macrophage polarization of THP-1 cells. Cell. Immunol. 2014, 289, 42–48. [Google Scholar] [CrossRef]
- Kai, S.; Goto, S.; Tahara, K.; Sasaki, A.; Kawano, K.; Kitano, S. Inhibition of indoleamine 2,3-dioxygenase suppresses NK cell activity and accelerates tumor growth. J. Exp. Ther. Oncol. 2003, 3, 336–345. [Google Scholar] [CrossRef]
- Shortman, K.; Liu, Y.J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002, 2, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Young, L.J.; Wilson, N.S.; Schnorrer, P.; Proietto, A.; ten Broeke, T.; Matsuki, Y.; Mount, A.M.; Belz, G.T.; O’Keeffe, M.; Ohmura-Hoshino, M.; et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat. Immunol. 2008, 9, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Espey, M.G.; Namboodiri, M.A. Selective metabolism of kynurenine in the spleen in the absence of indoleamine 2,3-dioxygenase induction. Immunol. Lett. 2000, 71, 67–72. [Google Scholar] [CrossRef]
- Moreau, M.; Lestage, J.; Verrier, D.; Mormede, C.; Kelley, K.W.; Dantzer, R.; Castanon, N. Bacille Calmette-Guérin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J. Infect. Dis. 2005, 192, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Widner, B.; Sepp, N.; Kowald, E.; Ortner, U.; Wirleitner, B.; Fritsch, P.; Baier-Bitterlich, G.; Fuchs, D. Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology 2000, 201, 621–630. [Google Scholar] [CrossRef]
- Forrest, C.M.; Kennedy, A.; Stone, T.W.; Stoy, N.; Darlington, L.G. Kynurenine and neopterin levels in patients with rheumatoid arthritis and osteoporosis during drug treatment. Adv. Exp. Med. Biol. 2003, 527, 287–295. [Google Scholar]
- Hassanain, H.H.; Chon, S.Y.; Gupta, S.L. Differential regulation of human indoleamine 2,3-dioxygenase gene expression by interferons-gamma and -alpha. Analysis of the regulatory region of the gene and identification of an interferon-gamma-inducible DNA-binding factor. J. Biol. Chem. 1993, 268, 5077–5084. [Google Scholar] [CrossRef]
- Belladonna, M.L.; Orabona, C.; Grohmann, U.; Puccetti, P. TGF-β and kynurenines as the key to infectious tolerance. Trends Mol. Med. 2009, 15, 41–49. [Google Scholar] [CrossRef]
- Yuasa, H.J.; Takubo, M.; Takahashi, A.; Hasegawa, T.; Noma, H.; Suzuki, T. Evolution of Vertebrate Indoleamine 2,3-Dioxygenases. J. Mol. Evol. 2007, 6, 705–714. [Google Scholar] [CrossRef]
- Terness, P.; Bauer, T.M.; Röse, L.; Dufter, C.; Watzlik, A.; Simon, H.; Opelz, G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: Mediation of suppression by tryptophan metabolites. J. Exp. Med. 2002, 196, 447–457. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Al Khasawneh, E.; Gupta, S.; Tuli, S.Y.; Shahlaee, A.H.; Garrett, T.J.; Schechtman, K.B.; Dharnidharka, V.R. Stable pediatric kidney transplant recipients run higher urine indoleamine 2, 3 dioxygenase (IDO) levels than healthy children. Pediatr. Transpl. 2014, 18, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Mediavilla-Varela, M.; Antonia, S. Indoleamine 2,3-Dioxygenase: Is it an immune suppressor? Cancer J. 2010, 16, 354–359. [Google Scholar] [CrossRef]
- Kadowaki, N.; Antonenko, S.; Lau, J.Y.N.; Liu, Y.J. Natural Interferon α/β–Producing Cells Link Innate and Adaptive Immunity. J. Exp. Med. 2000, 192, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Dong, C. TH17 cells in development: An updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008, 8, 337–348. [Google Scholar] [CrossRef]
- Blum, A.; Chaperot, L.; Molens, J.P.; Foissaud, V.; Plantaz, D.; Plumas, J. Mechanisms of TRAIL-induced apoptosis in leukemic plasmacytoid dendritic cells. Exp. Hematol. 2006, 34, 1655–1662. [Google Scholar] [CrossRef]
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity 2016, 44, 683–697. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T Cell Apoptosis by Kynurenines. In Developments in Tryptophan and Serotonin Metabolism; Springer: Boston, MA, USA, 2003. [Google Scholar]
- Hwu, P.; Du, M.X.; Lapointe, R.; Do, M.; Taylor, M.W.; Young, H.A. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 2000, 164, 3596–3599. [Google Scholar] [CrossRef]
- Harden, J.L.; Egilmez, N.K. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol. Invest. 2012, 41, 738–764. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Kimura, A.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 19961–19966. [Google Scholar] [CrossRef]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.T.; Gao, F.; Gu, K.; Chen, D.K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019, 10, 1140. [Google Scholar] [CrossRef]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol. Oncol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kai, S.; Goto, S.; Tahara, K.; Sasaki, A.; Tone, S.; Kitano, S. Indoleamine 2,3-Dioxygenase is Necessary for Cytolytic Activity of Natural Killer Cells. Scandinavian J. Immunol. 2004, 59, 177–182. [Google Scholar] [CrossRef]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived Catabolites Are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol. Spectr. 2016, 4, 4–5. [Google Scholar] [CrossRef]
- Jung, Y.J.; Woo, S.Y.; Jang, M.H.; Miyasaka, M.; Ryu, K.H.; Park, H.K.; Seoh, J.Y. Human Eosinophils Show Chemotaxis to Lymphoid Chemokines and Exhibit Antigen-Presenting-Cell-Like Properties upon Stimulation with IFN-γ, IL-3 and GM-CSF. Int. Arch. Allergy Immunol. 2008, 146, 227–234. [Google Scholar] [CrossRef]
- Odemuyiwa, S.O.; Ghahary, A.; Li, Y.; Puttagunta, L.; Lee, J.E.; Musat-Marcu, S.; Ghahary, A.; Moqbel, R. Cutting Edge: Human Eosinophils Regulate T Cell Subset Selection through Indoleamine 2,3-Dioxygenase. J. Immunol. 2004, 173, 5909–5913. [Google Scholar] [CrossRef]
- Astigiano, S.; Morandi, B.; Costa, R.; Mastracci, L.; D’Agostino, A.; Ratto, G.B.; Melioli, G.; Frumento, G. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia 2005, 7, 390–396. [Google Scholar] [CrossRef]
- Spencer, L.A.; Szela, C.T.; Perez, S.A.C.; Kirchhoffer, C.L.; Neves, J.S.; Radke, A.L.; Weller, P.F. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 2009, 85, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Tulic, M.K.; Sly, P.D.; Andrews, D.; Crook, M.; Davoine, F.; Odemuyiwa, S.O.; Charles, A.; Hodder, M.L.; Prescott, S.L.; Holt, P.G.; et al. Thymic indoleamine 2,3-dioxygenase-positive eosinophils in young children: Potential role in maturation of the naive immune system. Am. J. Pathol. 2009, 175, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.; Saffarzadeh, M.; Weber, A.N.R.; Rieber, N.; Radsak, M.; von Bernuth, H.; Benarafa, C.; Roos, D.; Skokowa, J.; Hartl, D. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015, 11, e1004651. [Google Scholar] [CrossRef] [PubMed]
- Novitskiy, S.V.; Pickup, M.W.; Gorska, A.E.; Owens, P.; Chytil, A.; Aakre, M.; Wu, H.; Shyr, Y.; Moses, H.L. TGF-β Receptor II Loss Promotes Mammary Carcinoma Progression by Th17-Dependent Mechanisms. Cancer Discov. 2011, 1, 411–430. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef]
- Lazarevic, V.; Glimcher, L.H.; Lord, G.M. T-bet: A bridge between innate and adaptive immunity. Nat. Rev. Immunol. 2013, 13, 777–789. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Chen, Y.; Wang, Q.; Tian, Z.; Pan, S.; Zeng, X.; Ye, S. Anti-endothelin receptor type A autoantibodies in systemic lupus erythematosus-associated pulmonary arterial hypertension. Arthritis Rheumatol. 2015, 67, 2394–2402. [Google Scholar] [CrossRef]
- Wong, C.P.; Nguyen, L.P.; Noh, S.K.; Bray, T.M.; Bruno, R.S.; Ho, E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol. Lett. 2011, 139, 7–13. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Munn, D.H.; Sharma, M.D.; Mellor, A.L. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 2004, 172, 4100–4110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; McGrath, B.C.; Reinert, J.; Olsen, D.S.; Lei, L.; Gill, S.; Wek, S.A.; Vattem, K.M.; Wek, R.C.; Kimball, S.R.; et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 2002, 22, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Walsh, M.C.; Hoehn, K.L.; James, D.E.; Wherry, E.J.; Choi, Y. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J. Immunol. 2014, 192, 3190–3199. [Google Scholar] [CrossRef]
- Ge, W.; Jiang, J.; Arp, J.; Liu, W.; Garcia, B.; Wang, H. Regulatory T-Cell Generation and Kidney Allograft Tolerance Induced by Mesenchymal Stem Cells Associated With Indoleamine 2,3-Dioxygenase Expression. Transplantation 2010, 2, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, J.D.; Le Buanec, H.; Saussine, A.; Bensussan, A.; Bagot, M. IL-10 producing regulatory B cells in mice and humans: State of the art. Curr. Mol. Med. 2012, 12, 519–527. [Google Scholar] [CrossRef]
- Lemoine, S.; Morva, A.; Youinou, P.; Jamin, C. Regulatory B cells in autoimmune diseases: How do they work? Ann. N. Y. Acad. Sci. 2009, 1173, 260–267. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Chesneau, M.; Michel, L.; Degauque, N.; Brouard, S. Regulatory B cells and tolerance in transplantation: From animal models to human. Front. Immunol. 2013, 4, 497. [Google Scholar] [CrossRef]
- Scott, G.N.; DuHadaway, J.; Pigott, E.; Ridge, N.; Prendergast, G.C.; Muller, A.J.; Mandik-Nayak, L. The immunoregulatory enzyme IDO paradoxically drives B cell-mediated autoimmunity. J. Immunol. 2009, 182, 7509–7517. [Google Scholar] [CrossRef]
- Vinay, D.S.; Kim, C.H.; Chang, K.H.; Kwon, B.S. PDCA expression by B lymphocytes reveals important functional attributes. J. Immunol. 2010, 184, 807–815. [Google Scholar] [CrossRef]
- Zheng, S.G.; Wang, J.H.; Stohl, W.; Kim, K.S.; Gray, J.D.; Horwitz, D.A. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J. Immunol. 2006, 176, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Nouël, A.; Pochard, P.; Simon, Q.; Ségalen, I.; Le Meur, Y.; Pers, J.O.; Hillion, S. B-Cells induce regulatory T cells through TGF-β/IDO production in A CTLA-4 dependent manner. J. Autoimmun. 2015, 59, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.G.; Jensen, M.S.; Tingskov, S.J.; Olinga, P.; Nørregaard, R.; Mutsaers, H.A.M. Local Inhibition of Indoleamine 2,3-Dioxygenase Mitigates Renal Fibrosis. Biomedicines 2021, 9, 856. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Jankovic, M.G.; Djokovic, B.; Jovicic, N.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N.; Lukic, M.L. Galectin 3 protects from cisplatin-induced acute kidney injury by promoting TLR-2-dependent activation of IDO1/Kynurenine pathway in renal DCs. Theranostics 2019, 9, 5976–6001. [Google Scholar] [CrossRef]
- Cook, C.H.; Bickerstaff, A.A.; Wang, J.J.; Nadasdy, T.; Della Pelle, P.; Colvin, R.B.; Orosz, C.G. Spontaneous Renal Allograft Acceptance Associated with “Regulatory” Dendritic Cells and IDO. J. Immunol. 2008, 180, 3103–3112. [Google Scholar] [CrossRef]
- Na, N.; Luo, Y.; Zhao, D.; Yang, S.; Hong, L.; Li, H.; Miao, B.; Qiu, J. Prolongation of kidney allograft survival regulated by indoleamine 2, 3-dioxygenase in immature dendritic cells generated from recipient type bone marrow progenitors. Mol. Immunol. 2016, 79, 22–31. [Google Scholar] [CrossRef]
- Brandacher, G.; Cakar, F.; Winkler, C.; Schneeberger, S.; Obrist, P.; Bösmüller, C.; Werner-Felmayer, G.; Werner, E.R.; Bonatti, H.; Margreiter, R.; et al. Non-invasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney Int. 2007, 71, 60–67. [Google Scholar]
- Banasik, M.; Kuriata-Kordek, M.; Donizy, P.; Nowańska, K.; Wiśnicki, K.; Letachowicz, K.; Zmonarski, S.; Kamińska, D.; Mazanowska, O.; Dawiskiba, T.; et al. The Summarized Assessment of Endothelin A Receptor Expression in Renal Transplant Compartments Associated with Antibody-Mediated Rejection. Diagnostics 2021, 11, 2366. [Google Scholar] [CrossRef]
- Terasaki, P.I. Humoral theory of transplantation. Am. J. Transpl. 2003, 3, 665–673. [Google Scholar] [CrossRef]
- Sellarés, J.; de Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transpl. 2012, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C. Immune response to non-HLA antigens and renal allograft loss. Lancet 2019, 393, 854–856. [Google Scholar] [CrossRef]
- Dragun, D.; Hegner, B. Non-HLA antibodies post-transplantation: Clinical relevance and treatment in solid organ transplantation. Contrib. Nephrol. 2009, 162, 129–139. [Google Scholar]
- Lefaucheur, C.; Viglietti, D.; Bouatou, Y.; Philippe, A.; Pievani, D.; Aubert, O.; Duong Van Huyen, J.P.; Taupin, J.L.; Glotz, D.; Legendre, C.; et al. Non-HLA agonistic anti-angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody-mediated rejection in kidney transplant recipients. Kidney Int. 2019, 96, 189–201. [Google Scholar] [CrossRef]
- Banasik, M.; Boratyńska, M.; Nowakowska, B.; Haloń, A.; Kościelska-Kasprzak, K.; Drulis-Fajdasz, D.; Patrzałek, D.; Weyde, W.; Klinger, M. Variability in donor-specific alloantibody production after transplantation. Transpl. Proc. 2007, 39, 2715–2717. [Google Scholar] [CrossRef] [PubMed]
- Nowańska, K.; Wiśnicki, K.; Kuriata-Kordek, M.; Krajewska, M.; Banasik, M. The role of endothelin II type A receptor (ETAR) in transplant injury. Transpl. Immunol. 2022, 70, 101505. [Google Scholar] [CrossRef]
- Shinde, R.; Shimoda, M.; Chaudhary, K.; Liu, H.; Mohamed, E.; Bradley, J.; Kandala, S.; Li, X.; Liu, K.; McGaha, T.L. B Cell–Intrinsic IDO1 Regulates Humoral Immunity to T Cell–Independent Antigens. J. Immunol. 2015, 195, 2374–2382. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśnicki, K.; Donizy, P.; Remiorz, A.; Janczak, D.; Krajewska, M.; Banasik, M. Significance of Indoleamine 2,3-Dioxygenase Expression in the Immunological Response of Kidney Graft Recipients. Diagnostics 2022, 12, 2353. https://doi.org/10.3390/diagnostics12102353
Wiśnicki K, Donizy P, Remiorz A, Janczak D, Krajewska M, Banasik M. Significance of Indoleamine 2,3-Dioxygenase Expression in the Immunological Response of Kidney Graft Recipients. Diagnostics. 2022; 12(10):2353. https://doi.org/10.3390/diagnostics12102353
Chicago/Turabian StyleWiśnicki, Krzysztof, Piotr Donizy, Agata Remiorz, Dariusz Janczak, Magdalena Krajewska, and Mirosław Banasik. 2022. "Significance of Indoleamine 2,3-Dioxygenase Expression in the Immunological Response of Kidney Graft Recipients" Diagnostics 12, no. 10: 2353. https://doi.org/10.3390/diagnostics12102353
APA StyleWiśnicki, K., Donizy, P., Remiorz, A., Janczak, D., Krajewska, M., & Banasik, M. (2022). Significance of Indoleamine 2,3-Dioxygenase Expression in the Immunological Response of Kidney Graft Recipients. Diagnostics, 12(10), 2353. https://doi.org/10.3390/diagnostics12102353






