Network Analysis Reveals That Headache-Related, Psychological and Psycho–Physical Outcomes Represent Different Aspects in Women with Migraine
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Migraine-Related Variables
2.3. Disability-Related Variables
2.4. Emotional/Psychological Variables
2.5. Psycho–Physical Variables
2.6. Statistical Analysis
2.6.1. Software and Packages
2.6.2. Exploratory Analysis
2.6.3. Network Estimation
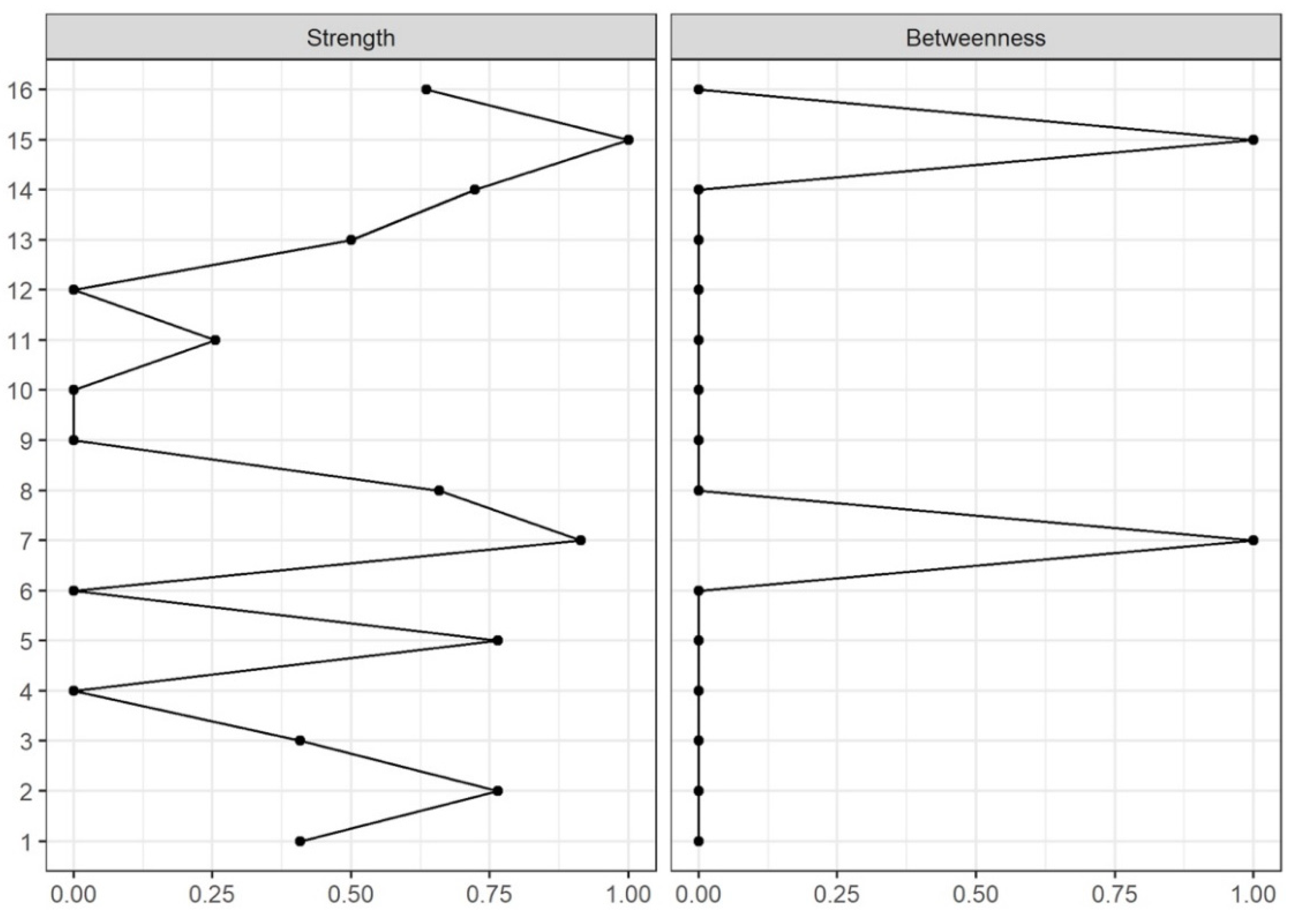
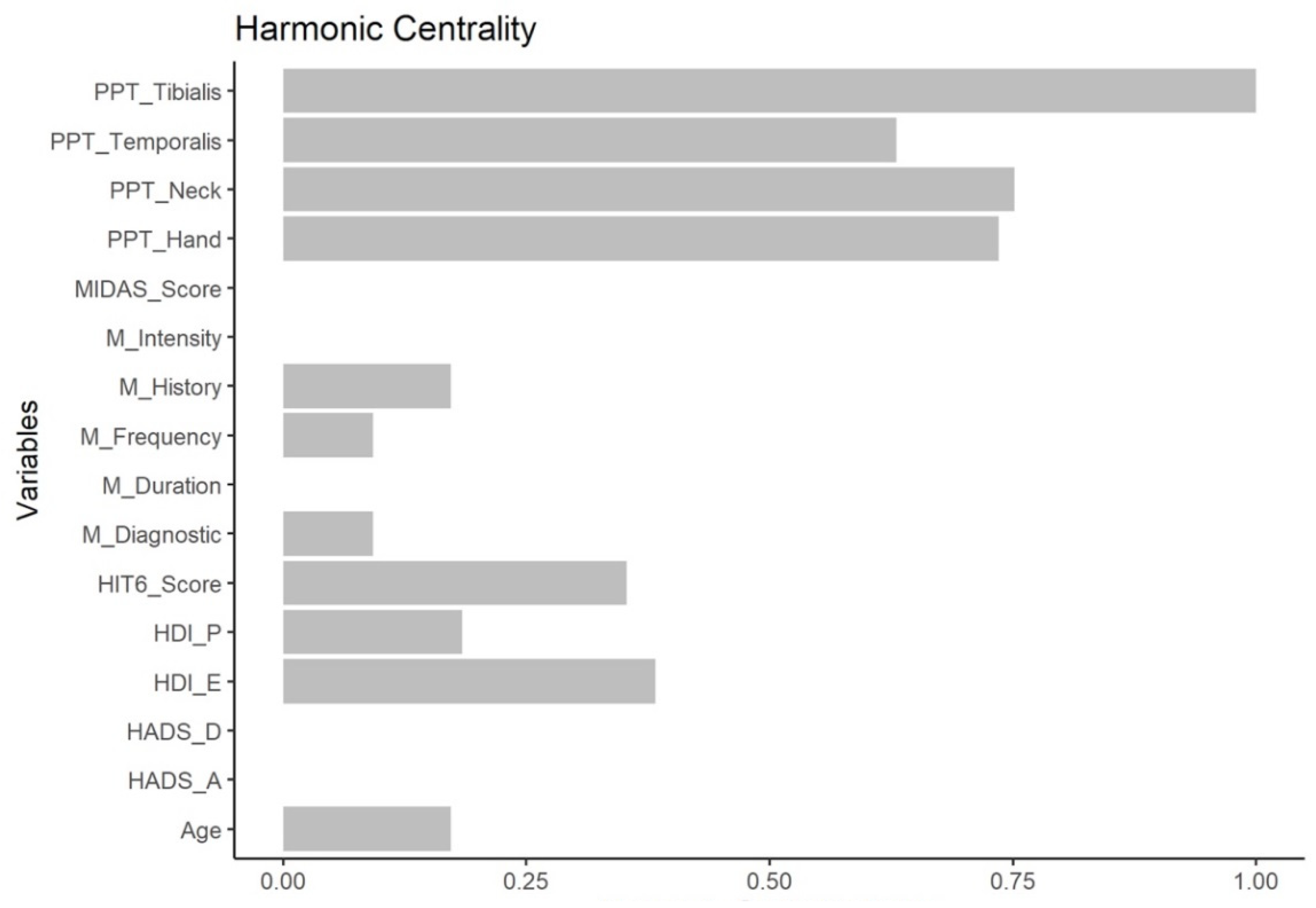
2.6.4. Node Centrality
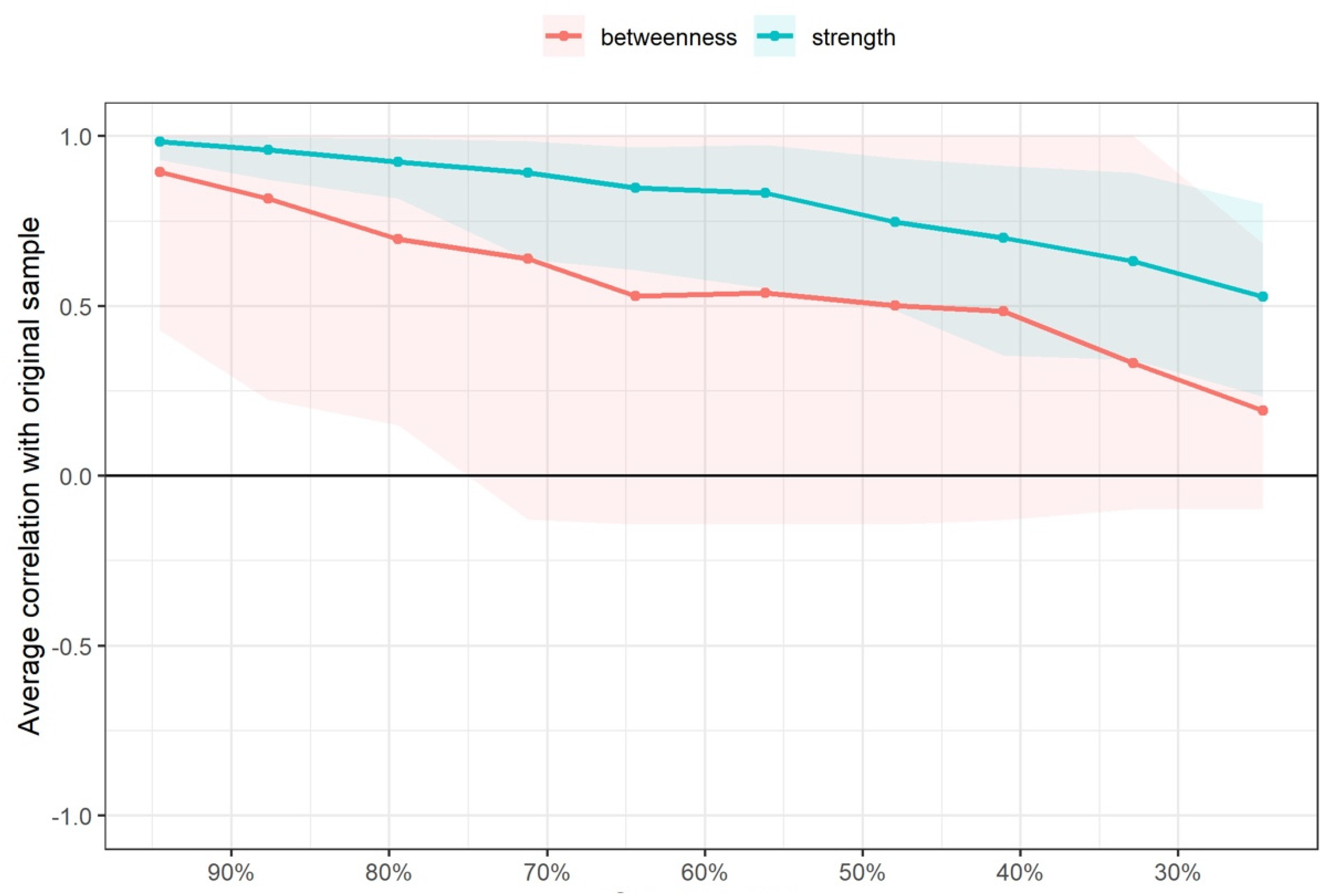
2.6.5. Network Edge and Node Centrality Variability
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Steiner, T.J.; Stovner, L.J.; Vos, T. GBD 2015: Migraine is the third cause of disability in under 50s. J. Headache Pain 2016, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Woldeamanuel, Y.W.; Cowan, R.P. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J. Neurol. Sci. 2017, 372, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Shefi, T.; Schneider, V.J., 2nd; Li, D.; Ding, L.; King, C.D.; Coghill, R.C. Quantitative sensory testing in patients with migraine: A systemic review and meta-analysis. Pain 2018, 159, 1202–1223. [Google Scholar] [CrossRef]
- Garramone, F.; Baiano, C.; Russo, A.; D’Iorio, A.; Tedeschi, G.; Trojano, L.; Santangelo, G. Personality profile and depression in migraine: A meta-analysis. Neurol. Sci. 2020, 41, 543–554. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Fernández-Muñoz, J.J.; Palacios-Ceña, M.; Parás-Bravo, P.; Cigarán-Méndez, M.; Navarro-Pardo, E. Sleep disturbances in tension-type headache and migraine. Ther. Adv. Neurol. Dis. 2018, 11, 1756285617745444. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, R.; Xiao, T.; Liu, X. Genetic variants in migraine: A field synopsis and systematic re-analysis of meta-analyses. J. Headache Pain 2020, 21, 13. [Google Scholar] [CrossRef]
- Epskamp, S.; Fried, E.I. A tutorial on regularized partial correlation networks. Psychol. Methods 2018, 23, 617–634. [Google Scholar] [CrossRef]
- Schmittmann, V.D.; Cramer, A.O.J.; Waldorp, L.J.; Epskamp, S.; Kievit, R.A.; Borsboom, D. Deconstructing the construct: A network perspective on psychological phenomena. New Ideas Psychol. 2013, 31, 43–53. [Google Scholar] [CrossRef]
- Valente, T.W. Network Interventions. Science 2012, 337, 49. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Palacios-Ceña, M.; Valera-Calero, J.A.; Cuadrado, M.L.; Guerrero-Peral, A.; Pareja, J.A.; Arendt-Nielsen, L.; Varol, U. Understanding the interaction between clinical, emotional and psychophysical outcomes underlying tension-type headache: A network analysis approach. J. Neurol. 2022, 269, 4525–4534. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Arendt-Nielsen, L.; Cigarán-Méndez, M.; Fernández-de-las-Peñas, C.; Varol, U. Network analysis for better understanding the complex psycho-biological mechanisms behind fibromyalgia syndrome. Diagnostics 2022, 12, 1845. [Google Scholar] [CrossRef]
- Liew, B.X.W.; de-la-Llave-Rincón, A.I.; Arias-Buría, J.L.; Ortega-Santiago, R.; Fernández-de-las-Peñas, C. Understanding the psychophysiological mechanisms related to widespread pressure pain hyperalgesia underpinning carpal tunnel syndrome: A network analysis approach. Pain Med. 2021, 22, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Herrero-Montes, M.; Cancela-Cilleruelo, I.; Rodríguez-Jiménez, J.; Parás-Bravo, P.; Varol, U.; Del-Valle-Loarte, P.; Flox-Benítez, G.; Arendt-Nielsen, L.; Valera-Calero, J.A. Understanding sensitization, cognitive and neuropathic associated mechanisms behind post-COVID Pain: A network analysis. Diagnostics 2022, 12, 1538. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Phillip, D.; Lyngberg, A.; Jensen, R. Assessment of headache diagnosis. A comparative population study of a clinical interview with a diagnostic headache diary. Cephalalgia 2007, 27, 1–8. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Ramadan, N.M.; Aggarwal, S.K.; Newman, C.W. The Henry Ford Hospital Headache Disability Inventory (HDI). Neurology 1994, 44, 837–842. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Ramadan, N.M.; Norris, L.; Newman, C.W. Headache disability inventory (HDI): Short-term test-retest reliability and spouse perceptions. Headache 1995, 35, 534–539. [Google Scholar] [CrossRef]
- Kosinski, M.; Bayliss, M.S.; Bjorner, J.B.; Ware, J.E.; Garber, W.H.; Batenhorst, A.; Cady, R.; Dahlöf, C.G.H.; Dowson, A.; Tepper, S. A six-item short-form survey for measuring headache impact: The HIT-6TM. Qual. Life Res. 2003, 12, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Lipton, R.B.; Kolodner, K.; Sawyer, J.; Lee, C.; Liberman, J.N. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000, 88, 41–52. [Google Scholar] [CrossRef]
- Rodríguez-Almagro, D.; Achalandabaso, A.; Rus, A.; Obrero-Gaitán, E.; Zagalaz-Anula, N.; Lomas-Vega, R. Validation of the Spanish version of the migraine disability assessment questionnaire (MIDAS) in university students with migraine. BMC Neurol. 2020, 20, 67. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Juang, K.D.; Wang, S.J.; Lin, C.H.; Fuh, J.L. Use of the hospital anxiety and depression scale as a screening tool for patients with headache. Zhonghua Yi Xue Za Zhi 1999, 62, 749–755. [Google Scholar] [PubMed]
- Palacios-Ceña, M.; Lima Florencio, L.; Natália Ferracini, G.; Barón, J.; Guerrero, Á.L.; Ordás-Bandera, C.; Arendt-Nielsen, L.; Fernández-de-las-Peñas, C. Women with chronic and episodic migraine exhibit similar widespread pressure pain sensitivity. Pain Med. 2016, 17, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Glasso: Graphical Lasso Estimation of Gaussian Graphical Models. R Package Version 20141. Available online: http://www-stat.stanford.edu/~tibs/glasso (accessed on 1 March 2022).
- Stekhoven, D.J.; Bühlmann, P. MissForest: Non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- Lauritzen, S.L.; Wermuth, N. Graphical models for associations between variables, some of which are qualitative and some quantitative. Ann. Stat. 1989, 17, 31–57. [Google Scholar] [CrossRef]
- Haslbeck, J.M.B.; Waldorp, L.J. How well do network models predict observations? On the importance of predictability in network models. Behav. Res. Methods 2018, 50, 853–861. [Google Scholar] [CrossRef]
- Bloch, F.; Jackson, M.O.; Tebaldi, P. Centrality Measures in Networks. 1 June 2019. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2749124 (accessed on 1 March 2022).
- Freeman, L.C. Centrality in social networks conceptual clarification. Soc. Netw. 1978, 1, 215–239. [Google Scholar] [CrossRef]
- Newman, M.E.J. Analysis of weighted networks. Phys. Rev. E 2004, 70, 056131. [Google Scholar] [CrossRef] [PubMed]
- Opsahl, T.; Agneessens, F.; Skvoretz, J. Node centrality in weighted networks: Generalizing degree and shortest paths. Soc. Netw. 2010, 32, 245–251. [Google Scholar] [CrossRef]
- Rochat, Y. Closeness centrality extended to unconnected graphs: The harmonic centrality index. In Proceedings of the ASNA 2009, Zurich, Switzerland, 27–28 August 2009. Application of Social Network Analysis Conference. [Google Scholar]
- Kindelan-Calvo, P.; Gil-Martínez, A.; Paris-Alemany, A.; Pardo-Montero, J.; Muñoz-García, D.; Angulo-Díaz-Parreño, S.; La Touche, R. Effectiveness of therapeutic patient education for adults with migraine. A systematic review and meta-analysis of randomized controlled trials. Pain Med. 2014, 15, 1619–1636. [Google Scholar] [CrossRef] [PubMed]
- La Touche, R.; Fernández Pérez, J.J.; Proy Acosta, A.; González Campodónico, L.; Martínez García, S.; Adraos Juárez, D.; Serrano García, B.; Angulo-Díaz-Parreño, S.; Cuenca-Martínez, F.; Suso-Martí, L.; et al. Is aerobic exercise helpful in patients with migraine? A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2020, 30, 965–982. [Google Scholar] [CrossRef]
- Hübscher, M.; Moloney, N.; Leaver, A.; Rebbeck, T.; McAuley, J.H.; Refshauge, K.M. Relationship between quantitative sensory testing and pain or disability in people with spinal pain-a systematic review and meta-analysis. Pain 2013, 154, 1497–1504. [Google Scholar] [CrossRef]
- Georgopoulos, V.; Akin-Akinyosoye, K.; Zhang, W.; McWilliams, D.F.; Hendrick, P.; Walsh, D.A. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: A systematic review and meta-analysis. Pain 2019, 160, 1920–1932. [Google Scholar] [CrossRef]
- Caponnetto, V.; Deodato, M.; Robotti, M.; Koutsokera, M.; Pozzilli, V.; Galati, C.; Nocera, G.; De Matteis, E.; De Vanna, G.; Fellini, E.; et al. Comorbidities of primary headache disorders: A literature review with meta-analysis. J. Headache Pain 2021, 22, 71. [Google Scholar] [CrossRef]
- Probyn, K.; Bowers, H.; Mistry, D.; Caldwell, F.; Underwood, M.; Patel, S.; Sandhu, H.K.; Matharu, M.; Pincus, T.; CHESS Team. Non-pharmacological self-management for people living with migraine or tension-type headache: A systematic review including analysis of intervention components. BMJ Open 2017, 7, e016670. [Google Scholar] [CrossRef]
- Perlini, C.; Donisi, V.; Del Piccolo, L. From research to clinical practice: A systematic review of the implementation of psychological interventions for chronic headache in adults. BMC Health Serv. Res. 2020, 20, 459. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Cho, E.Y.; Kim, S.M.; Yoon, S. Efficacy of psychological treatment for headache disorder: A systematic review and meta-analysis. J. Headache Pain 2019, 20, 17. [Google Scholar] [CrossRef]
- Falsiroli Maistrello, L.; Rafanelli, M.; Turolla, A. Manual therapy and quality of life in people with headache: Systematic review and meta-analysis of randomized controlled trials. Curr. Pain Headache Rep. 2019, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Rist, P.M.; Hernandez, A.; Bernstein, C.; Kowalski, M.; Osypiuk, K.; Vining, R.; Long, C.R.; Goertz, C.; Song, R.; Wayne, P.M. The impact of spinal manipulation on migraine pain and disability: A systematic review and meta-analysis. Headache 2019, 59, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Florencio, L.L.; Plaza-Manzano, G.; Arias-Buría, J.L. Clinical reasoning behind non-pharmacological interventions for the management of headaches: A narrative literature review. Int. J. Environ. Res. Public Health 2020, 17, 4126. [Google Scholar] [CrossRef] [PubMed]

| Variable | Baseline Scores | Missing Values (n; %) |
|---|---|---|
| Age (years) | 42.3 ± 12.1 | 0; 0 |
| Migraine Type (n; %) | ||
| Episodic (1) | 56; 75.6 | 0; 0 |
| Chronic (2) | 18; 24.4 | 0; 0 |
| Years with Migraine (years) | 19.6 ± 13.9 | 0; 0 |
| Migraine Intensity (0–10) | 8.1 ± 2.0 | 0; 0 |
| Migraine Frequency (n/month) | 9.9 ± 8.1 | 0; 0 |
| Migraine Duration (hours/episode) | 24.2 ± 20.6 | 0; 0 |
| HDI-E (0–52) | 27.0 ± 13.4 | 0; 0 |
| HDI-P (0–48) | 34.7 ± 11.5 | 0; 0 |
| HADS-A (0–21) | 12.3 ± 2.5 | 0; 0 |
| HADS-D (0–21) | 10.5 ± 3.0 | 0; 0 |
| HIT6 (36–78) | 63.0 ± 7.3 | 0; 0 |
| MIDAS (days missed work) | 46.3 ± 69.3 | 1; 1.35 |
| PPT Neck (kPa) | 135.4 ± 46.5 | 0; 0 |
| PPT Temporalis (kPa) | 156.7 ± 61.6 | 0; 0 |
| PPT Hand (kPa) | 194.5 ± 64.1 | 0; 0 |
| PPT Tibialis Anterior (kPa) | 327.0 ± 114.2 | 0; 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-de-las-Peñas, C.; Florencio, L.L.; Varol, U.; Pareja, J.A.; Ordás-Bandera, C.; Valera-Calero, J.A. Network Analysis Reveals That Headache-Related, Psychological and Psycho–Physical Outcomes Represent Different Aspects in Women with Migraine. Diagnostics 2022, 12, 2318. https://doi.org/10.3390/diagnostics12102318
Fernández-de-las-Peñas C, Florencio LL, Varol U, Pareja JA, Ordás-Bandera C, Valera-Calero JA. Network Analysis Reveals That Headache-Related, Psychological and Psycho–Physical Outcomes Represent Different Aspects in Women with Migraine. Diagnostics. 2022; 12(10):2318. https://doi.org/10.3390/diagnostics12102318
Chicago/Turabian StyleFernández-de-las-Peñas, César, Lidiane L. Florencio, Umut Varol, Juan A. Pareja, Carlos Ordás-Bandera, and Juan A. Valera-Calero. 2022. "Network Analysis Reveals That Headache-Related, Psychological and Psycho–Physical Outcomes Represent Different Aspects in Women with Migraine" Diagnostics 12, no. 10: 2318. https://doi.org/10.3390/diagnostics12102318
APA StyleFernández-de-las-Peñas, C., Florencio, L. L., Varol, U., Pareja, J. A., Ordás-Bandera, C., & Valera-Calero, J. A. (2022). Network Analysis Reveals That Headache-Related, Psychological and Psycho–Physical Outcomes Represent Different Aspects in Women with Migraine. Diagnostics, 12(10), 2318. https://doi.org/10.3390/diagnostics12102318









