Diagnostic Performance of Contrast-Enhanced Ultrasound in the Evaluation of Small Renal Masses: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Evidence Acquisition
2.2. Literature Search
- Population—patients with US diagnosis of SMRs
- Intervention—CEUS
- Comparator— final histopathological examination
- Outcome—diagnostic accuracy of CEUS (sensitivity, specificity, accuracy, positive predictive value, and negative predictive value)
- Study design—prospective and retrospective cohort studies
2.3. Risk of Bias Assessment
2.4. Data Collection
2.5. Statistical Analysis
3. Results
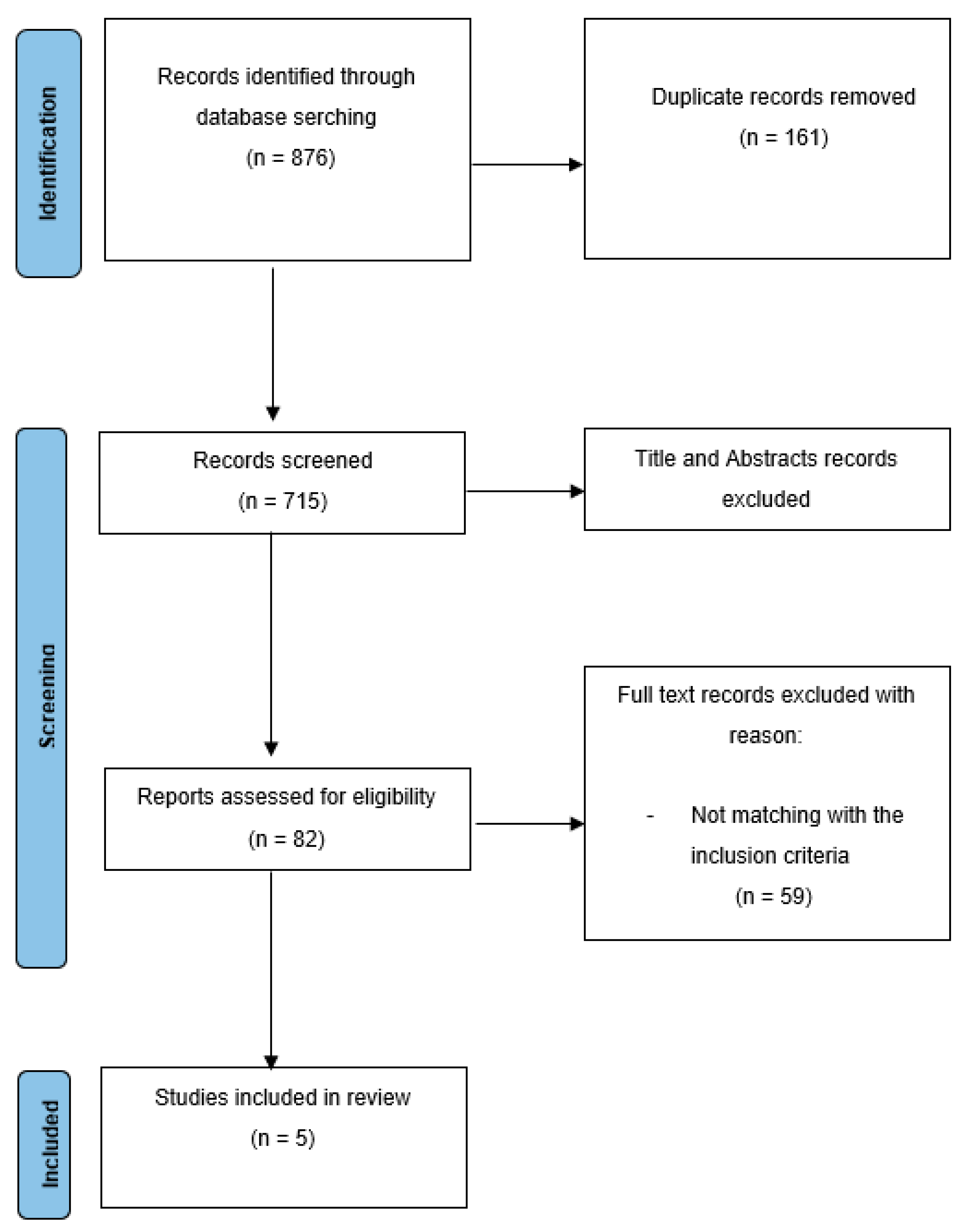
3.1. Characteristics of Included Studies
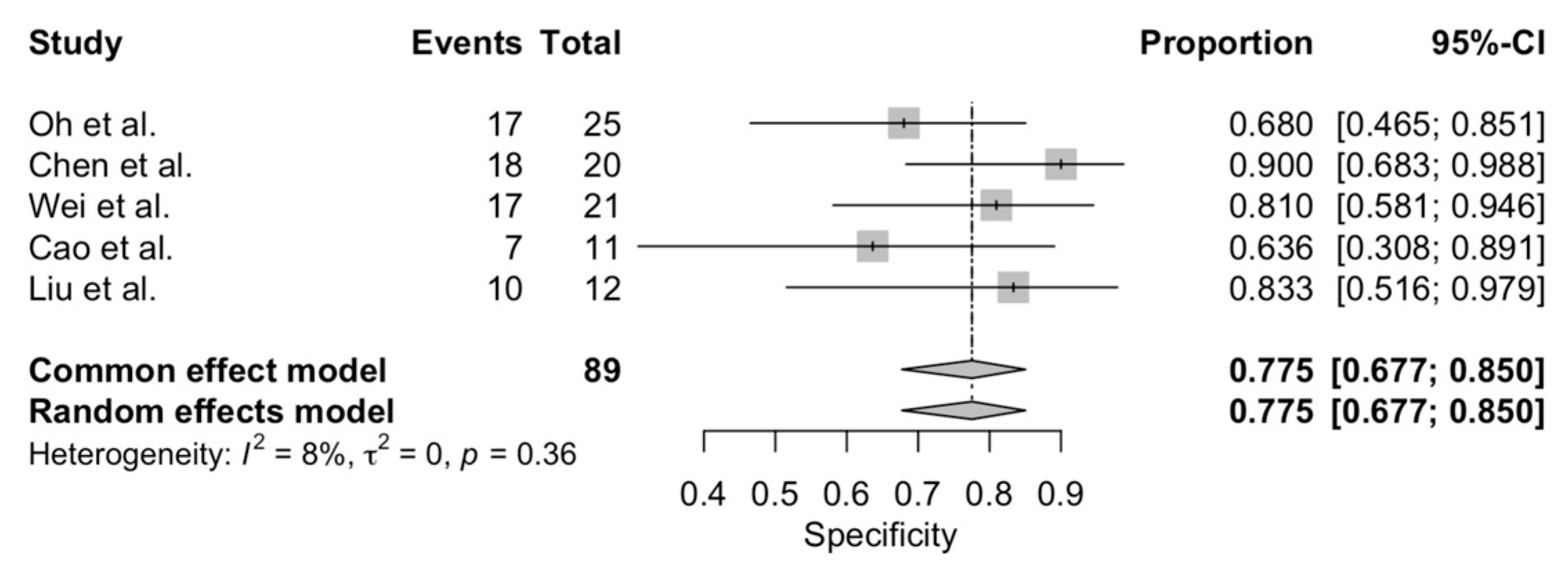
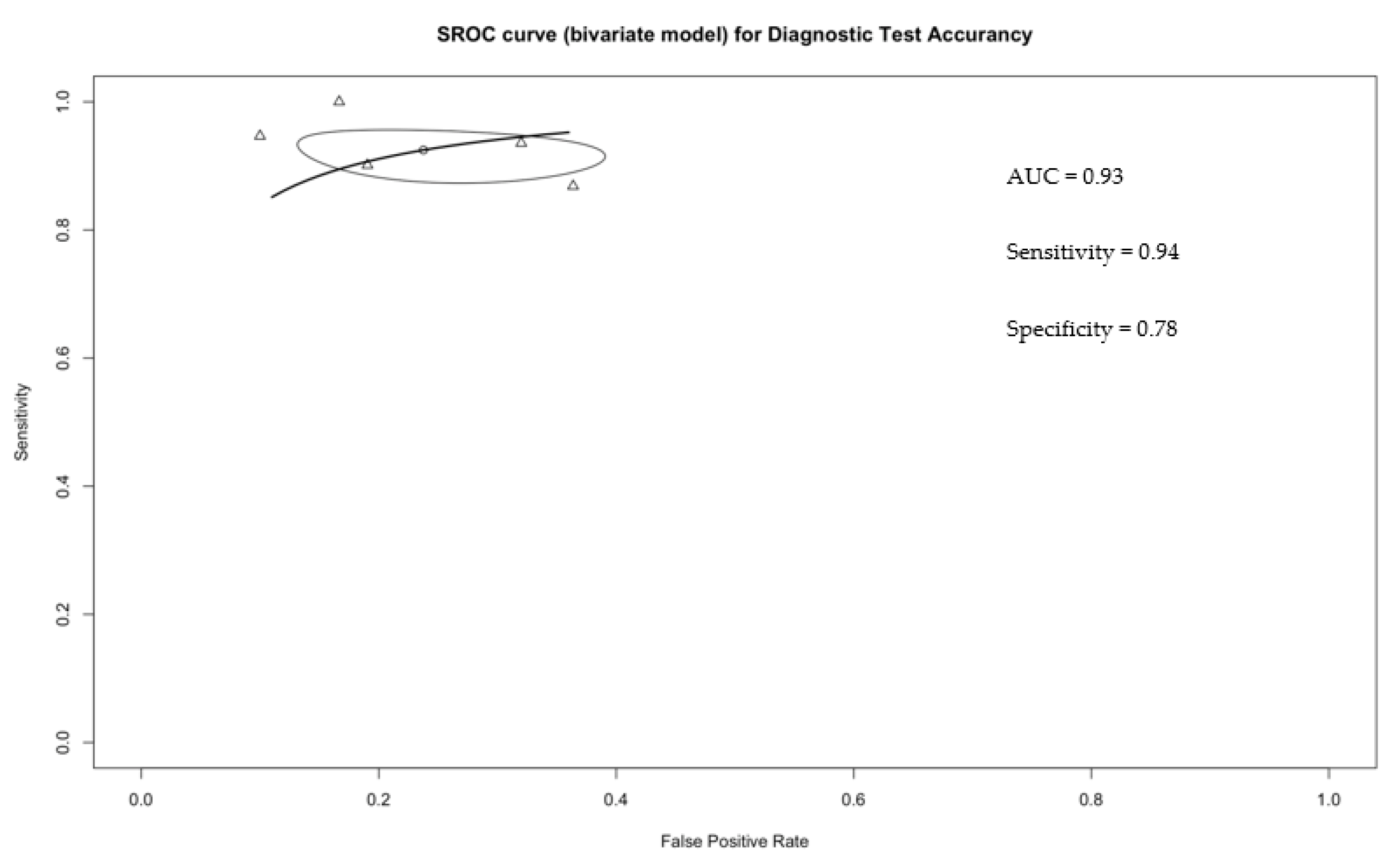
3.2. Accurancy of CEUS in Distinguishing Malignant from Benign Small Renal Masses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Frank, I.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Weaver, A.L.; Zincke, H. Solid Renal Tumors: An Analysis of Pathological Features Related to Tumor Size. J. Urol. 2003, 170, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Kurta, J.M.; Kaag, M.; Tickoo, S.K.; Kundu, S.; Katz, D.; Nogueira, L.; Reuter, V.E.; Russo, P. Tumor Size is Associated with Malignant Potential in Renal Cell Carcinoma Cases. J. Urol. 2009, 181, 2033–2036. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef]
- Correas, J.-M.; Claudon, M.; Tranquart, F.; Hélénon, A.O. The kidney: Imaging with microbubble contrast agents. Ultrasound Q. 2006, 22, 53–66. [Google Scholar]
- Marschner, C.A.; Rübenthaler, J.; Froelich, M.F.; Schwarze, V.; Clevert, D.-A. Benefits of contrast-enhanced ultrasonography for interventional procedures. Ultrasonography 2021, 40, 207–216. [Google Scholar] [CrossRef]
- Zhou, L.; Tang, L.; Yang, T.; Chen, W. Comparison of contrast-enhanced ultrasound with MRI in the diagnosis of complex cystic renal masses: A meta-analysis. Acta Radiol. 2018, 59, 1254–1263. [Google Scholar] [CrossRef]
- Vogel, D.W.T.; Kiss, B.; Heverhagen, J.T.; Benackova, K.; Burkhard, F.; Müller, M.; Uehlinger, D.; Arampatzis, S. Prospective Comparison of Contrast-Enhanced Ultrasound and Magnetic Resonance Imaging to Computer Tomography for the Evaluation of Complex Cystic Renal Lesions. Urology 2021, 154, 320–325. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.-A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, e2–e44. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.; Kirby, J.; Roderick, P. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Heal. Technol. Assess. 2005, 9, 1–113. [Google Scholar] [CrossRef]
- Radzina, M.; Ratniece, M.; Putrins, D.S.; Saule, L.; Cantisani, V. Performance of Contrast-Enhanced Ultrasound in Thyroid Nodules: Review of Current State and Future Perspectives. Cancers 2021, 13, 5469. [Google Scholar] [CrossRef] [PubMed]
- Tufano, A.; Flammia, R.S.; Antonelli, L.; Minelli, R.; Franco, G.; Leonardo, C.; Cantisani, V. The Value of Contrast-Enhanced Ultrasound (CEUS) in Differentiating Testicular Masses: A Systematic Review and Meta-Analysis. Appl. Sci. 2021, 11, 8990. [Google Scholar] [CrossRef]
- Tufano, A.; Drudi, F.M.; Angelini, F.; Polito, E.; Martino, M.; Granata, A.; Di Pierro, G.B.; Kutrolli, E.; Sampalmieri, M.; Canale, V.; et al. Contrast-Enhanced Ultrasound (CEUS) in the Evaluation of Renal Masses with Histopathological Validation—Results from a Prospective Single-Center Study. Diagnostics 2022, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, H.; Chen, L.; Fang, L.; Liu, Y.; Zhan, J.; Diao, X.; Chen, Y. The quantitative evaluation of contrast-enhanced ultrasound in the differentiation of small renal cell carcinoma subtypes and angiomyolipoma. Quant. Imaging Med. Surg. 2022, 12, 106–118. [Google Scholar] [CrossRef]
- Oh, T.H.; Lee, Y.H.; Seo, I.Y. Diagnostic Efficacy of Contrast-Enhanced Ultrasound for Small Renal Masses. Korean J. Urol. 2014, 55, 587–592. [Google Scholar] [CrossRef][Green Version]
- Cao, H.; Fang, L.; Chen, L.; Zhan, J.; Diao, X.; Liu, Y.; Lu, C.; Zhang, Z.; Chen, Y. The independent indicators for differentiating renal cell carcinoma from renal angiomyolipoma by contrast-enhanced ultrasound. BMC Med. Imaging 2020, 20, 32. [Google Scholar] [CrossRef]
- Tsili, A.C.; Argyropoulou, M.I.; Gousia, A.; Kalef-Ezra, J.; Sofikitis, N.; Malamou-Mitsi, V.; Tsampoulas, K. Renal Cell Carcinoma: Value of Multiphase MDCT With Multiplanar Reformations in the Detection of Pseudocapsule. Am. J. Roentgenol. 2012, 199, 379–386. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.; Zhou, Y.; Zhang, H. Clear cell renal cell carcinoma: Contrast-enhanced ultrasound features relation to tumor size. Eur. J. Radiol. 2010, 73, 162–167. [Google Scholar] [CrossRef]
- Xu, Z.-F.; Xu, H.-X.; Xie, X.-Y.; Liu, G.-J.; Zheng, Y.-L.; Lu, M.-D. Renal cell carcinoma and renal angiomyolipoma: Differential diagnosis with real-time contrast-enhanced ultrasonography. J. Ultrasound Med. 2010, 29, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Van Oostenbrugge, T.J.; Runneboom, W.; Bekers, E.; Heidkamp, J.; Langenhuijsen, J.F.; Veltien, A.; Maat, A.; Mulders, P.F.A.; de Kaa, C.A.H.-V.; Fütterer, J.J. MRI as a tool to assess surgical margins and pseudocapsule features directly following partial nephrectomy for small renal masses. Eur. Radiol. 2019, 29, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ascenti, G.; Gaeta, M.; Magno, C.; Mazziotti, S.; Blandino, A.; Melloni, D.; Zimbaro, G. Contrast-Enhanced Second-Harmonic Sonography in the Detection of Pseudocapsule in Renal Cell Carcinoma. Am. J. Roentgenol. 2004, 182, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Diao, X.; Qian, W.; Fang, L.; Pang, Y.; Zhan, J.; Chen, Y. The diagnostic value of contrast-enhanced ultrasound in differentiating small renal carcinoma and angiomyolipoma. Biosci. Trends. 2015, 9, 252–258. [Google Scholar] [CrossRef]
- Li, C.-X.; Lu, Q.; Huang, B.-J.; Xue, L.-Y.; Yan, L.-X.; Zheng, F.-Y.; Wen, J.-X.; Wang, W.-P. Quantitative evaluation of contrast-enhanced ultrasound for differentiation of renal cell carcinoma subtypes and angiomyolipoma. Eur. J. Radiol. 2016, 85, 795–802. [Google Scholar] [CrossRef]
- Cao, H.; Fang, L.; Chen, L.; Zhan, J.; Diao, X.; Liu, Y.; Lu, C.; Zhang, Z.; Chen, Y. The Value of Contrast-Enhanced Ultrasound in Diagnosing Small Renal Cell Carcinoma Subtypes and Angiomyolipoma. J. Ultrasound Med. 2022, 41, 1415–1423. [Google Scholar] [CrossRef]
- Liu, Y.; Kan, Y.; Zhang, J.; Li, N.; Wang, Y. Characteristics of contrast-enhanced ultrasound for diagnosis of solid clear cell renal cell carcinomas ≤4 cm: A meta-analysis. Cancer Med. 2021, 10, 8288–8299. [Google Scholar] [CrossRef]
- Lou, L.; Teng, J.; Lin, X.; Zhang, H. Ultrasonographic features of renal oncocytoma with histopathologic correlation. J. Clin. Ultrasound 2014, 42, 129–133. [Google Scholar] [CrossRef]
- Wei, S.-P.; Xu, C.-L.; Zhang, Q.; Zhang, Q.-R.; Zhao, Y.-E.; Huang, P.-F.; Xie, Y.-D.; Zhou, C.-S.; Tian, F.-L.; Yang, B. Contrast-enhanced ultrasound for differentiating benign from malignant solid small renal masses: Comparison with contrast-enhanced CT. Abdom. Radiol. 2017, 42, 2135–2145. [Google Scholar] [CrossRef]
- Warren, H.; Neves, J.B.; Tran, M.G.B. Oncocytoma: Risk of promoting unnecessary surgery. World J. Urol. 2020, 39, 4003–4004. [Google Scholar] [CrossRef]

| Risk of Bias | Applicability Concerns | ||||||
|---|---|---|---|---|---|---|---|
| Study | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard |
| Chen et al. | L | L | L | U | L | L | L |
| Cao et al. | U | L | L | L | U | L | L |
| Liu et al. | L | L | L | L | L | L | L |
| Oh et al. | H | L | L | U | H | L | L |
| Wei et al. | L | L | L | U | L | L | L |
| Study | Year | Mono/ Multi | Study Design | Patient, n | Lesion, n | Age, yrs (SD) | Tum Size, cm Mean (SD) | Contrast Agent, mL | Reader, n | Inter-obServer Agreement, K Value (95% CI) | Hystopatological Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oh et al. | 2014 | mono | retrospective | 49 | 49 | 61 | RCCs: 2.89 (±0.81); AMLs: 2.85 (±0.85) | SonoVue | 1 | N.R. | 38 RCCs; 11 AMLs |
| Chen et al. | 2015 | mono | retrospective | 99 | 102 | RCCs: 56.6 (±16.5); AMLs: 56.6 (±16.5) | RCCs: 1.81 (±0.59); AMLs: 1.77 (±0.52) | SonoVue, 1.2 ml | 2 | N.R. | 81 RCCs (68 clear cell carcinoma, 8 papillary carcinoma, 4 chromophobe carcinoma, 1 collecting duct carcinoma); 21 AML |
| Wei et al. | 2017 | mono | retrospective | 118 | 118 | Benign: 52.6 (±10.6); Malignant: 52.8 (±11.4) | Benign: 2.76 (±0.76); Malignant: 2.74(±0.73) | SonoVue, 1.6–2.4 mL | 2 | 0.89 (0.79–0.98) | 25 benign (25/118, 21.2%): 20 AMLs, 3 oncocytomas, and 2 metanephric adenomas; 93 malignant (93/118,78.8%): 75 clear cell RCCs, 13 papillary RCCs, 2 chromophobe RCCs, 1 unclassified RCC, 1 Xp11.2 translocation/TFE3 gene fusion RCC, and 1 mucinous tubular and spindle cell carcinoma. |
| Cao et al. | 2021 | mono | retrospective | 151 | 151 | RCCs: 59.8 (±12.0); AMLs: 57.4 (±14.8) | RCCs: 2.7 (±0.8); AMLs: 2.2 (±1.0) | SonoVue, 1.2–2.0 mL | 2 | 0.74 | 131 RCCs (110 ccRCCs, 12 pRCCs, and 9 chRCCs); 20 AMLs |
| Liu et al. | 2022 | mono | retrospective | 97 | 97 | RCCs: 60.5 (±12.1); AMLs: 54.8 (±8.6) | RCCs: 2.9 (±0.7); AMLs: 2.3 (±1.0) | SonoVue, 1.6–2.4 mL | 1 | N.R. | RCCs (71 ccRCCs, 7 pRCCs, 7 chRCCs); 12 AMLs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tufano, A.; Antonelli, L.; Di Pierro, G.B.; Flammia, R.S.; Minelli, R.; Anceschi, U.; Leonardo, C.; Franco, G.; Drudi, F.M.; Cantisani, V. Diagnostic Performance of Contrast-Enhanced Ultrasound in the Evaluation of Small Renal Masses: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2310. https://doi.org/10.3390/diagnostics12102310
Tufano A, Antonelli L, Di Pierro GB, Flammia RS, Minelli R, Anceschi U, Leonardo C, Franco G, Drudi FM, Cantisani V. Diagnostic Performance of Contrast-Enhanced Ultrasound in the Evaluation of Small Renal Masses: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(10):2310. https://doi.org/10.3390/diagnostics12102310
Chicago/Turabian StyleTufano, Antonio, Luca Antonelli, Giovanni Battista Di Pierro, Rocco Simone Flammia, Rocco Minelli, Umberto Anceschi, Costantino Leonardo, Giorgio Franco, Francesco Maria Drudi, and Vito Cantisani. 2022. "Diagnostic Performance of Contrast-Enhanced Ultrasound in the Evaluation of Small Renal Masses: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 10: 2310. https://doi.org/10.3390/diagnostics12102310
APA StyleTufano, A., Antonelli, L., Di Pierro, G. B., Flammia, R. S., Minelli, R., Anceschi, U., Leonardo, C., Franco, G., Drudi, F. M., & Cantisani, V. (2022). Diagnostic Performance of Contrast-Enhanced Ultrasound in the Evaluation of Small Renal Masses: A Systematic Review and Meta-Analysis. Diagnostics, 12(10), 2310. https://doi.org/10.3390/diagnostics12102310








