Electric Source Imaging in Presurgical Evaluation of Epilepsy: An Inter-Analyser Agreement Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and EEG Recordings
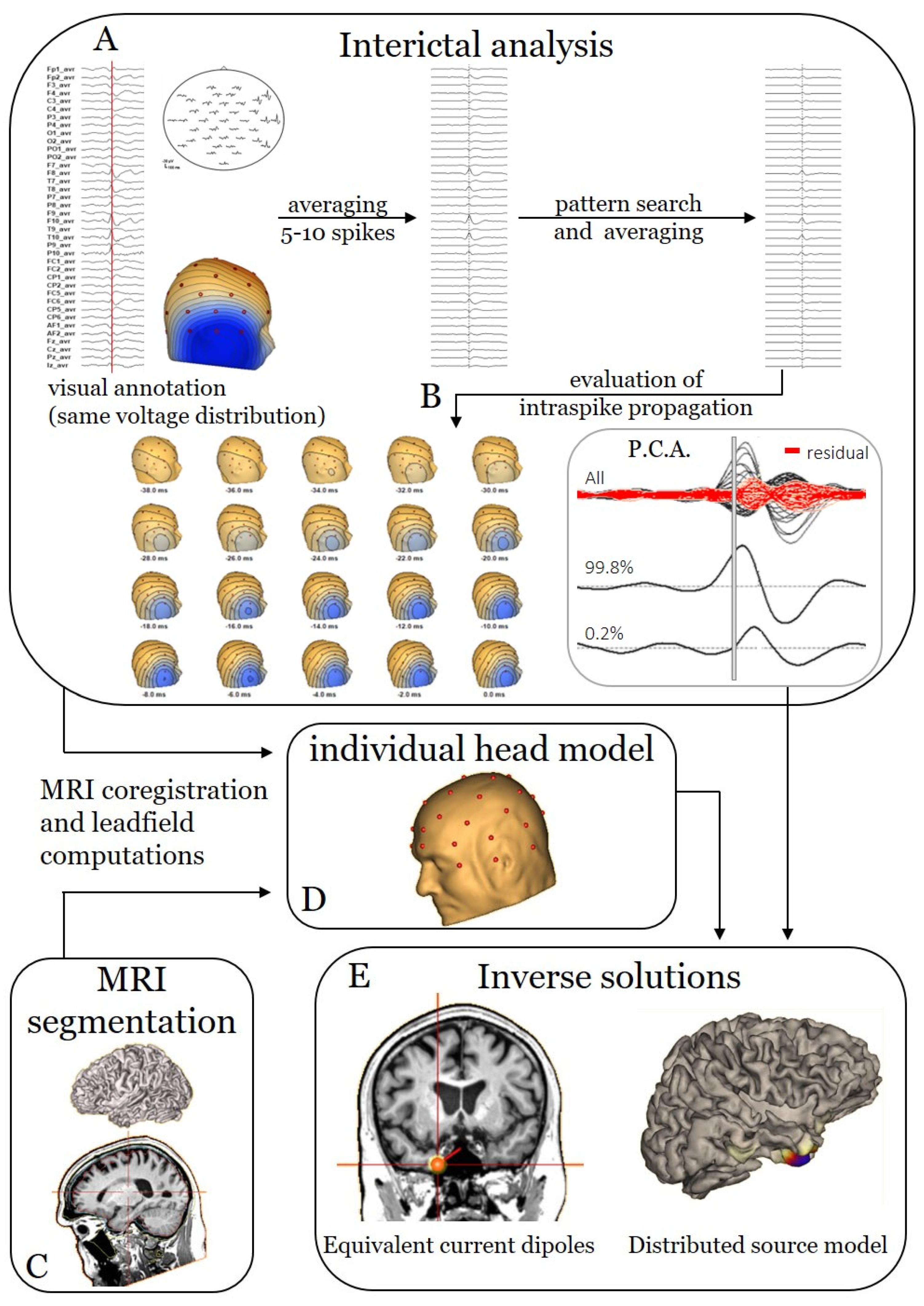
2.2. Electric Source Imaging Pipeline
2.2.1. FEM Leadfield Generation
2.2.2. Interictal ESI
2.2.3. Ictal ESI
2.3. Sublobar Localisation
2.4. Statistical Analysis
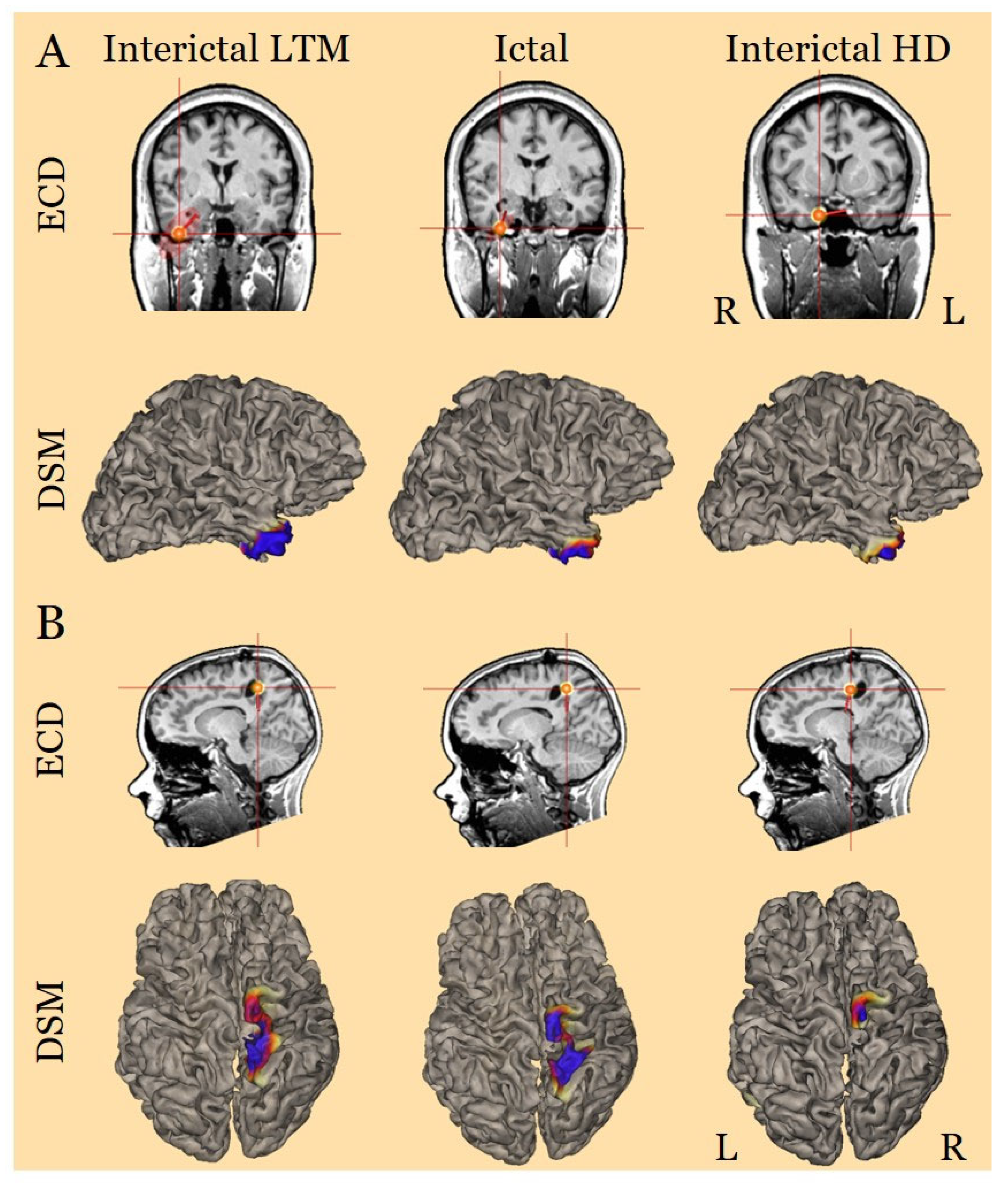
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- DeGiorgio, C.M.; Markovic, D.; Mazumder, R.; Moseley, B.D. Ranking the Leading Risk Factors for Sudden Unexpected Death in Epilepsy. Front. Neurol. 2017, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Ryvlin, P.; Cross, H.; Rheims, S. Epilepsy surgery in children and adults. Lancet Neurol. 2014, 13, 1114–1126. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Early Identification of Refractory Epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, F.; Lüders, H. Presurgical evaluation of epilepsy. Brain 2001, 124, 1683–1700. [Google Scholar] [CrossRef]
- Mégevand, P.; Seeck, M. Electric source imaging for presurgical epilepsy evaluation: Current status and future prospects. Expert Rev. Med. Devices 2020, 17, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Brunet, D. EEG Source Imaging: A Practical Review of the Analysis Steps. Front. Neurol. 2019, 10, 325. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Murray, M.M.; Lantz, G.; Gonzalez, S.; Spinelli, L.; de Peralta, R.G. EEG source imaging. Clin. Neurophysiol. 2004, 115, 2195–2222. [Google Scholar] [CrossRef] [PubMed]
- Duez, L.; Tankisi, H.; Hansen, P.O.; Sidenius, P.; Sabers, A.; Pinborg, L.H.; Fabricius, M.; Rásonyi, G.; Rubboli, G.; Pedersen, B.; et al. Electromagnetic source imaging in presurgical workup of patients with epilepsy: A Prospective Study. Neurology 2019, 92, e576–e586. [Google Scholar] [CrossRef] [PubMed]
- Tamilia, E.; AlHilani, M.; Tanaka, N.; Tsuboyama, M.; Peters, J.M.; Grant, P.E.; Madsen, J.R.; Stufflebeam, S.M.; Pearl, P.L.; Papadelis, C. Assessing the localization accuracy and clinical utility of electric and magnetic source imaging in children with epilepsy. Clin. Neurophysiol. 2019, 130, 491–504. [Google Scholar] [CrossRef]
- Brodbeck, V.; Spinelli, L.; Lascano, A.M.; Wissmeier, M.; Vargas, M.I.; Vulliemoz, S.; Pollo, C.; Schaller, K.; Michel, C.M.; Seeck, M. Electroencephalographic source imaging: A prospective study of 152 operated epileptic patients. Brain 2011, 134, 2887–2897. [Google Scholar] [CrossRef]
- Sharma, P.; Scherg, M.; Pinborg, L.H.; Fabricius, M.; Rubboli, G.; Pedersen, B.; Leffers, A.; Uldall, P.; Jespersen, B.; Brennum, J.; et al. Ictal and interictal electric source imaging in pre-surgical evaluation: A prospective study. Eur. J. Neurol. 2018, 25, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Rosenzweig, I.; Scherg, M.; Jordanov, T.; Lanfer, B.; Lantz, G.; Larsson, P.G. Ictal EEG source imaging in presurgical evaluation: High agreement between analysis methods. Seizure 2016, 43, 1–5. [Google Scholar] [CrossRef][Green Version]
- Sharma, P.; Seeck, M.; Beniczky, S. Accuracy of Interictal and Ictal Electric and Magnetic Source Imaging: A Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Vorderwülbecke, B.J.; Baroumand, A.G.; Spinelli, L.; Seeck, M.; van Mierlo, P.; Vulliémoz, S. Automated interictal source localisation based on high-density EEG. Seizure 2021, 92, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Davey, C.; Poulsen, C.; Luu, P.; Turovets, S.; Anderson, E.; Li, K.; Tucker, D. EEG source localization: Sensor density and head surface coverage. J. Neurosci. Methods 2015, 256, 9–21. [Google Scholar] [CrossRef]
- Lantz, G.; de Peralta, R.G.; Spinelli, L.; Seeck, M.; Michel, C. Epileptic source localization with high density EEG: How many electrodes are needed? Clin. Neurophysiol. 2003, 114, 63–69. [Google Scholar] [CrossRef]
- Baroumand, A.G.; van Mierlo, P.; Strobbe, G.; Pinborg, L.H.; Fabricius, M.; Rubboli, G.; Leffers, A.-M.; Uldall, P.; Jespersen, B.; Brennum, J.; et al. Automated EEG source imaging: A retrospective, blinded clinical validation study. Clin. Neurophysiol. 2018, 129, 2403–2410. [Google Scholar] [CrossRef]
- Seeck, M.; Koessler, L.; Bast, T.; Leijten, F.; Michel, C.; Baumgartner, C.; He, B.; Beniczky, S. The standardized EEG electrode array of the IFCN. Clin. Neurophysiol. 2017, 128, 2070–2077. [Google Scholar] [CrossRef]
- Bernasconi, A.; Cendes, F.; Theodore, W.H.; Gill, R.S.; Koepp, M.J.; Hogan, R.E.; Jackson, G.D.; Federico, P.; Labate, A.; Vaudano, A.E.; et al. Recommendations for the use of structural magnetic resonance imaging in the care of patients with epilepsy: A consensus report from the International League Against Epilepsy Neuroimaging Task Force. Epilepsia 2019, 60, 1054–1068. [Google Scholar] [CrossRef]
- Györfi, O.; Ip, C.-T.; Justesen, A.B.; Gam-Jensen, M.L.; Rømer, C.; Fabricius, M.; Pinborg, L.H.; Beniczky, S. Accuracy of high-density EEG electrode position measurement using an optical scanner compared with the photogrammetry method. Clin. Neurophysiol. Pract. 2022, 7, 135–138. [Google Scholar] [CrossRef]
- Beniczky, S.; Aurlien, H.; Brøgger, J.C.; Hirsch, L.J.; Schomer, D.L.; Trinka, E.; Pressler, R.M.; Wennberg, R.; Visser, G.H.; Eisermann, M.; et al. Standardized computer-based organized reporting of EEG: SCORE—Second version. Clin. Neurophysiol. 2017, 128, 2334–2346. [Google Scholar] [CrossRef]
- Gwet, K.L. Handbook of Inter-Rater Reliability: How to Estimate the Level of Agreement between Two or Multiple Raters; STATAXIS Publishing Company: Gaithersburg, MD, USA, 2001. [Google Scholar]
- Feinstein, A.R.; Cicchetti, D.V. High agreement but low Kappa: I. the problems of two paradoxes. J. Clin. Epidemiol. 1990, 43, 543–549. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Justesen, A.B.; Foged, M.T.; Fabricius, M.; Skaarup, C.; Hamrouni, N.; Martens, T.; Paulson, O.B.; Pinborg, L.H.; Beniczky, S. Diagnostic yield of high-density versus low-density EEG: The effect of spatial sampling, timing and duration of recording. Clin. Neurophysiol. 2019, 130, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.C.; Abdel-Baki, S.G.; Weedon, J.; Arnedo, V.; Chari, G.; Koziorynska, E.; Lushbough, C.; Maus, D.; McSween, T.; Mortati, K.A.; et al. EEG interpretation reliability and interpreter confidence: A large single-center study. Epilepsy Behav. 2014, 32, 102–107. [Google Scholar] [CrossRef]
- van Donselaar, C.A.; Schimsheimer, R.-J.; Geerts, A.T.; Declerck, A.C. Value of the Electroencephalogram in Adult Patients With Untreated Idiopathic First Seizures. Arch. Neurol. 1992, 49, 231–237. [Google Scholar] [CrossRef]
- Hirsch, L.J.; Fong, M.W.; Leitinger, M.; LaRoche, S.M.; Beniczky, S.; Abend, N.S.; Lee, J.W.; Wusthoff, C.J.; Hahn, C.D.; Westover, M.B.; et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J. Clin. Neurophysiol. 2021, 38, 1–29. [Google Scholar] [CrossRef]
- Kural, M.A.; Qerama, E.; Johnsen, B.; Fuchs, S.; Beniczky, S. The influence of the abundance and morphology of epileptiform discharges on diagnostic accuracy: How many spikes you need to spot in an EEG. Clin. Neurophysiol. 2021, 132, 1543–1549. [Google Scholar] [CrossRef]
- Kural, M.A.; Tankisi, H.; Duez, L.; Hansen, V.S.; Udupi, A.; Wennberg, R.; Rampp, S.; Larsson, P.G.; Schulz, R.; Beniczky, S. Optimized set of criteria for defining interictal epileptiform EEG discharges. Clin. Neurophysiol. 2020, 131, 2250–2254. [Google Scholar] [CrossRef] [PubMed]

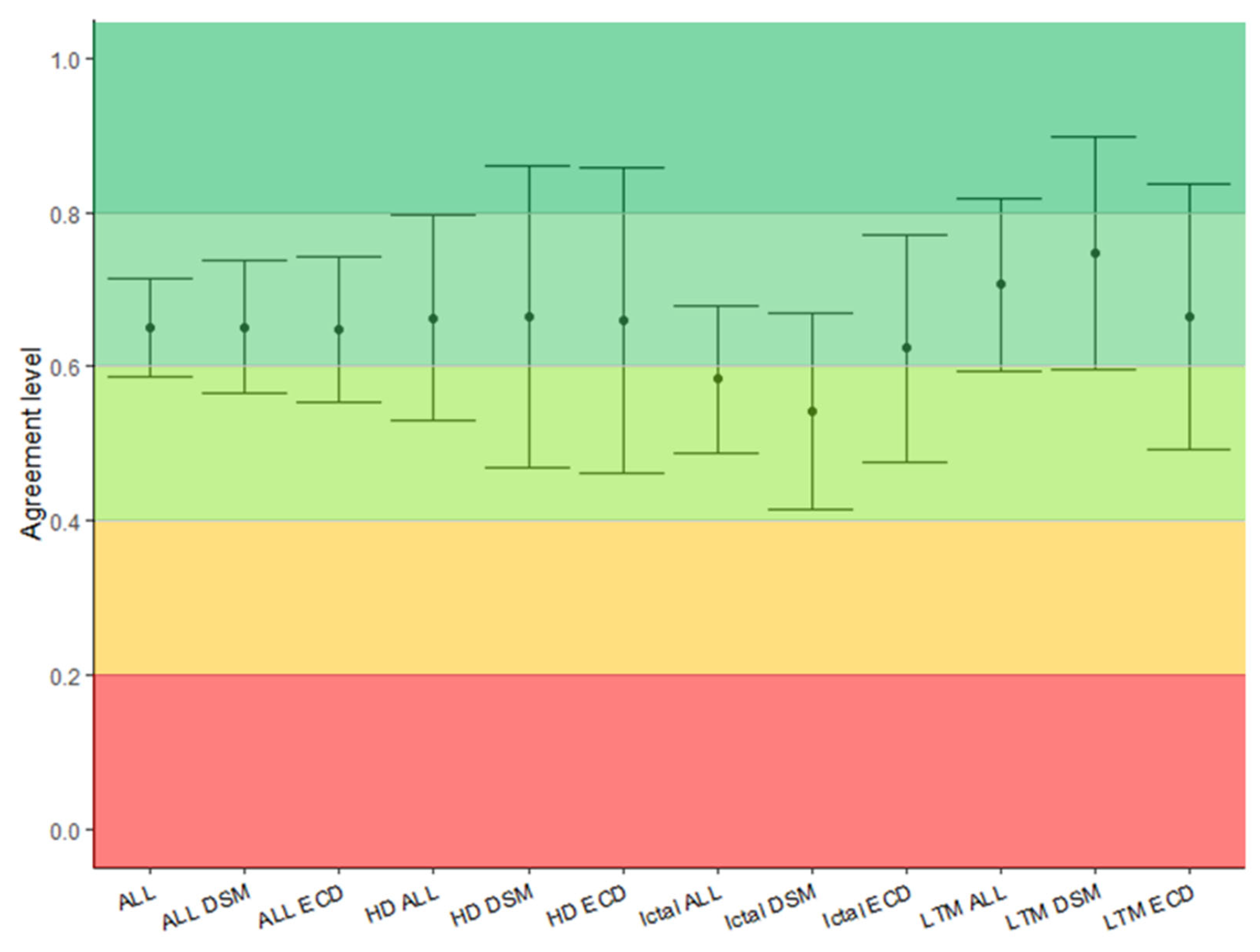
| Overall | ECD | DSM | |
|---|---|---|---|
| Overall | 0.65 [0.59–0.71] | 0.65 [0.56–0.74] | 0.65 [0.57–0.74] |
| Interictal HD | 0.66 [0.53–0.80] | 0.66 [0.46–0.86] | 0.66 [0.47–0.86] |
| Interictal LTM | 0.71 [0.60–0.82] | 0.67 [0.49–0.89] | 0.75 [0.60–0.90] |
| Ictal | 0.58 [0.50–0.68] | 0.62 [0.48–0.77] | 0.54 [0.42–0.67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattioli, P.; Cleeren, E.; Hadady, L.; Cossu, A.; Cloppenborg, T.; Arnaldi, D.; Beniczky, S. Electric Source Imaging in Presurgical Evaluation of Epilepsy: An Inter-Analyser Agreement Study. Diagnostics 2022, 12, 2303. https://doi.org/10.3390/diagnostics12102303
Mattioli P, Cleeren E, Hadady L, Cossu A, Cloppenborg T, Arnaldi D, Beniczky S. Electric Source Imaging in Presurgical Evaluation of Epilepsy: An Inter-Analyser Agreement Study. Diagnostics. 2022; 12(10):2303. https://doi.org/10.3390/diagnostics12102303
Chicago/Turabian StyleMattioli, Pietro, Evy Cleeren, Levente Hadady, Alberto Cossu, Thomas Cloppenborg, Dario Arnaldi, and Sándor Beniczky. 2022. "Electric Source Imaging in Presurgical Evaluation of Epilepsy: An Inter-Analyser Agreement Study" Diagnostics 12, no. 10: 2303. https://doi.org/10.3390/diagnostics12102303
APA StyleMattioli, P., Cleeren, E., Hadady, L., Cossu, A., Cloppenborg, T., Arnaldi, D., & Beniczky, S. (2022). Electric Source Imaging in Presurgical Evaluation of Epilepsy: An Inter-Analyser Agreement Study. Diagnostics, 12(10), 2303. https://doi.org/10.3390/diagnostics12102303








