The Efficacy of Trabecular Titanium Cages to Induce Reparative Bone Activity after Lumbar Arthrodesis Studied through the 18f-Naf PET/CT Scan: Observational Clinical In-Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
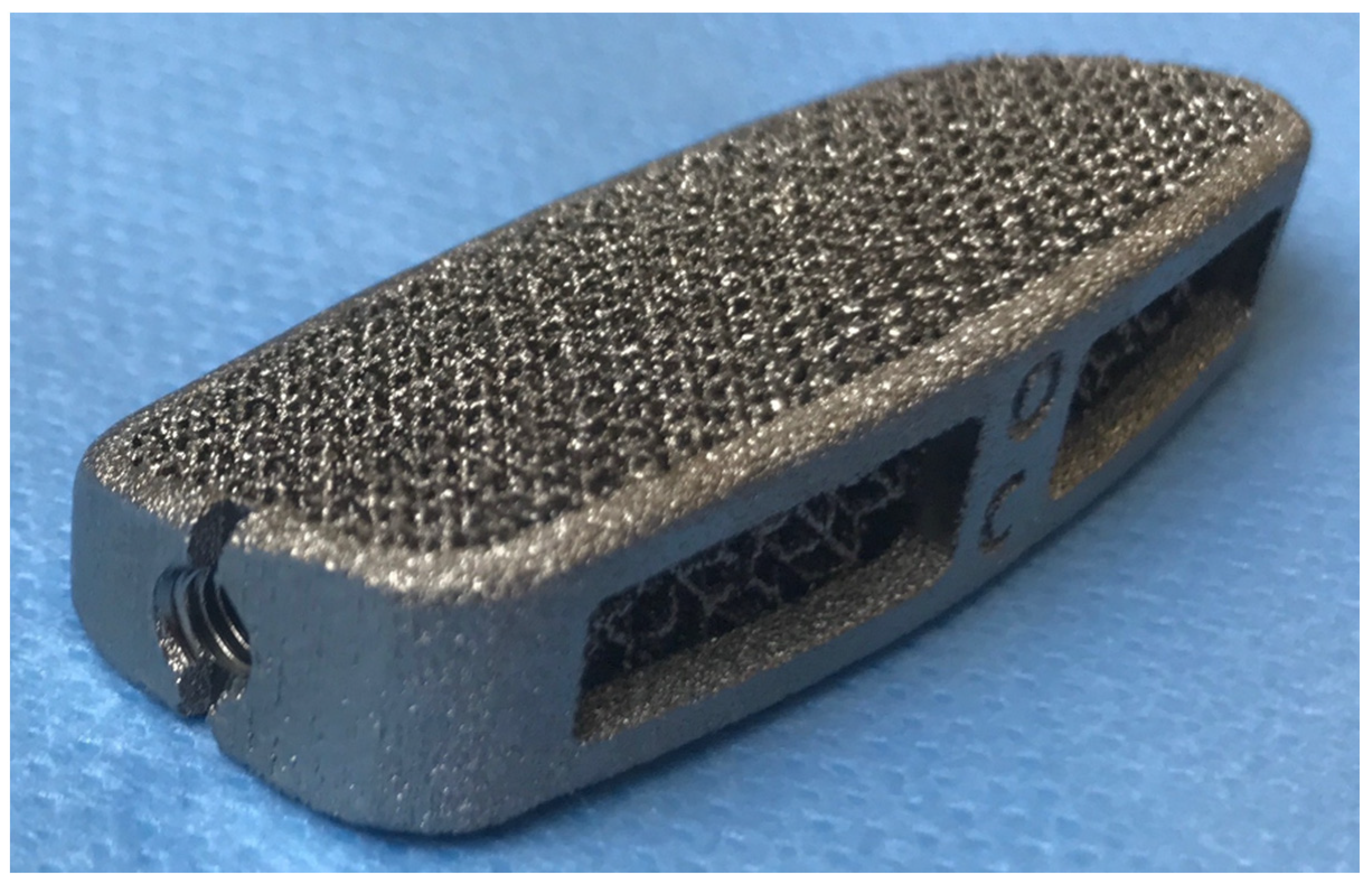
2.2. Interbody Implants and Surgery
2.3. 18F-NaF PET/CT Imaging Protocol
2.4. Metabolic Parameters
2.5. Size, Statistics, and a Potential Source of Bias
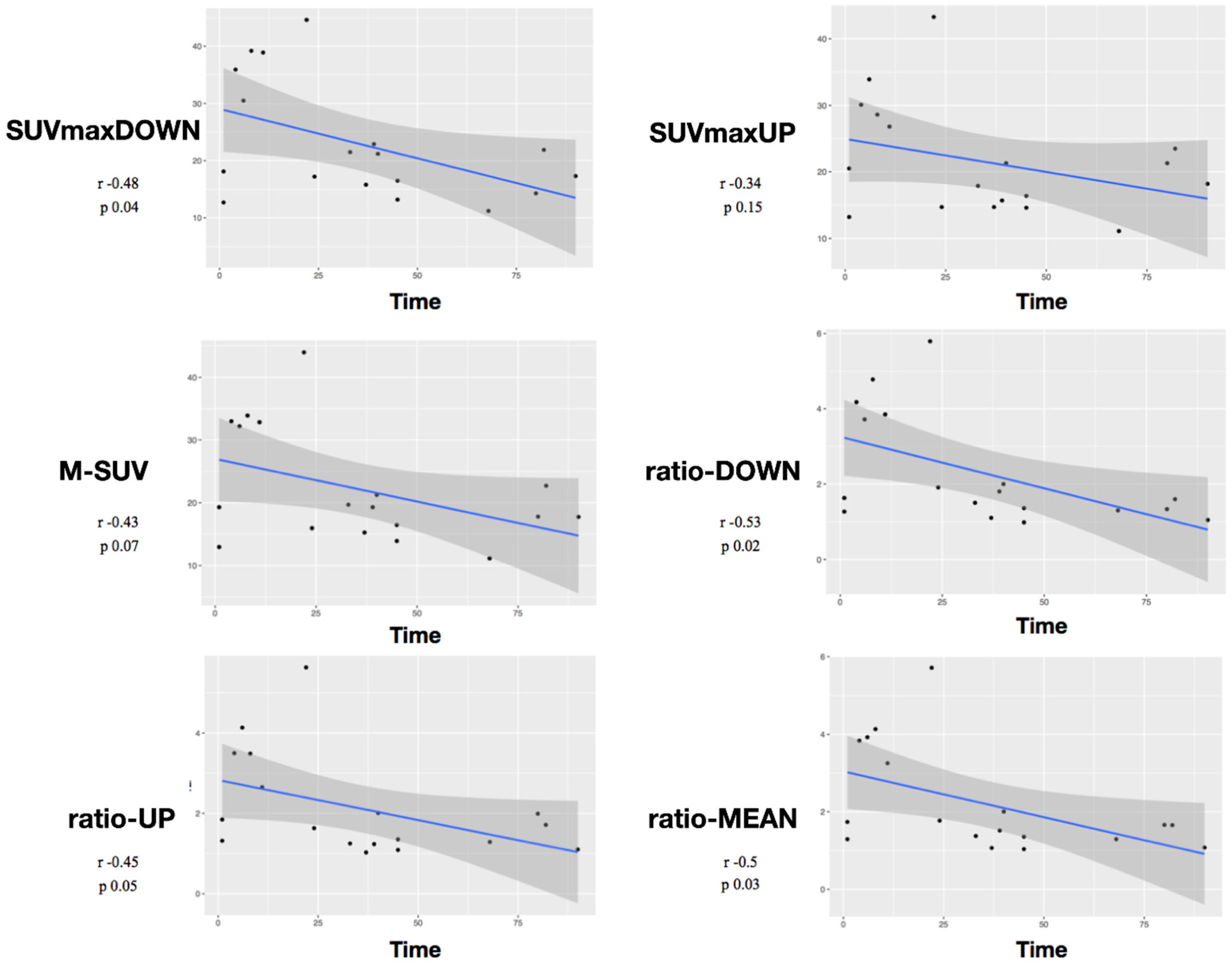
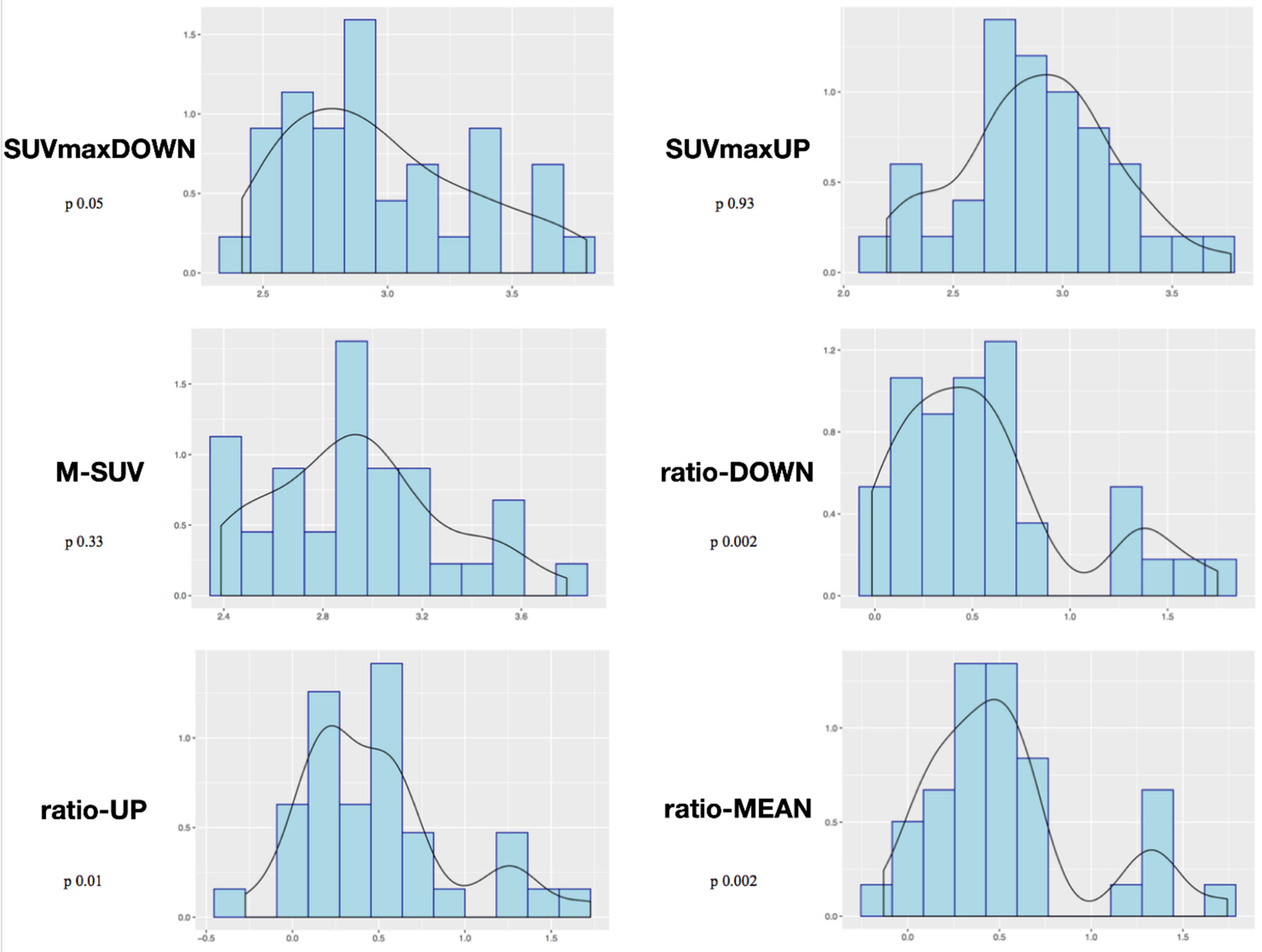
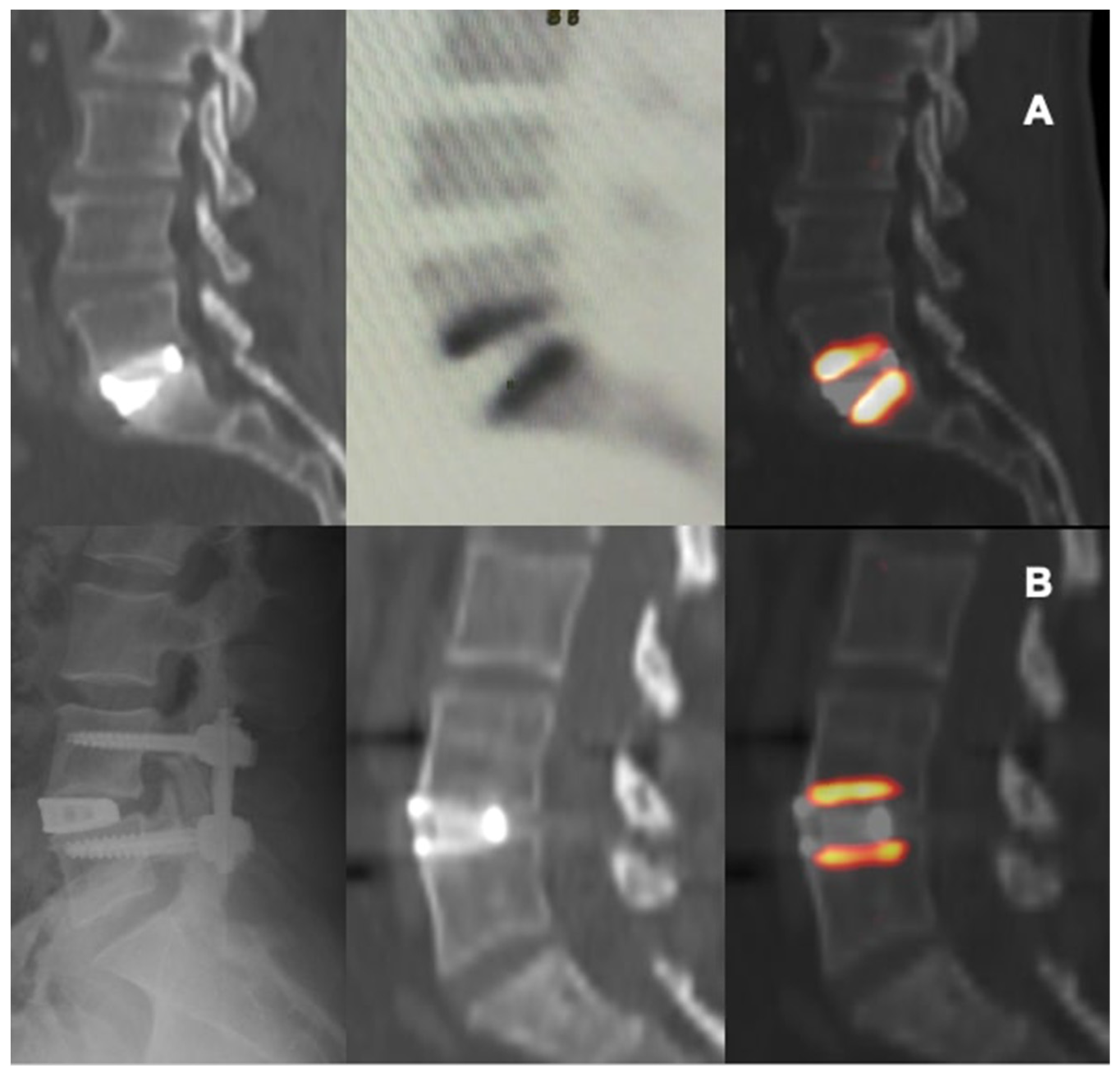
3. Results
Study of Metabolic Results
4. Discussion
Limitations and Further Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Mehren, C.; Mayer, H.M.; Siepe, C.; Grochulla, F.; Korge, A. The minimally invasive anterolateral approach to L2-5. Oper. Orthop. Traumatol. 2010, 22, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Chun, D.S.; Baker, K.C.; Hsu, W.K. Lumbar pseudarthrosis: A review of current diagnosis and treatment. Neurosurg. Focus 2015, 39, E10. [Google Scholar] [CrossRef]
- Rothenfluh, D.A.; Mueller, D.A.; Rothenfluh, E.; Min, K. Pelvic incidencelumbar lordosis mismatch predisposes to adjacent segment disease after lumbar spinal fusion. Eur. Spine J. 2015, 24, 1251–1258. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okuda, S.; Maeno, T.; Yamashita, T.; Yamasaki, R.; Sugiura, T.; Iwasaki, M. Spinopelvic sagittal imbalance as a risk factor for adjacent-segment disease after single-segment posterior lumbar interbody fusion. J. Neurosurg. Spine 2017, 26, 435–440. [Google Scholar] [CrossRef]
- Makino, T.; Kaito, T.; Fujiwara, H.; Honda, H.; Sakai, Y.; Takenaka, S.; Yoshikawa, H.; Yonenobu, K. Risk factors for poor patientreported quality of life outcomes after posterior lumbar interbody fusion: An analysis of two-year follow-up. Spine 2017, 42, 1502–1510. [Google Scholar] [CrossRef]
- Uribe, J.S.; Smith, D.A.; Dakwar, E.; Baaj, A.A.; Mundis, G.M.; Turner, A.W.L.; Cornwall, G.B.; Akbarnia, B.A. Lordosis restoration after anterior longitudinal ligament release and placement of lateral hyperlordotic interbody cages during the minimally invasive lateral transpsoas approach: A radiographic study in cadavers. J. Neurosurg. Spine 2012, 17, 476–485. [Google Scholar] [CrossRef]
- Beutler, W.J.; Peppelman, W.C., Jr. Anterior lumbar fusion with paired BAK standard and paired BAK Proximity cages: Subsidence incidence, subsidence factors, and clinical outcome. Spine J. 2003, 3, 289–293. [Google Scholar] [CrossRef]
- Choi, J.Y.; Sung, K.H. Subsidence after anterior lumbar interbody fusion using paired stand-alone rectangular cages. Eur. Spine J. 2006, 15, 16–22. [Google Scholar] [CrossRef]
- Le, T.V.; Baaj, A.A.; Dakwar, E.; Burkett, C.J.; Murray, G.; Smith, D.A.; Uribe, J.S. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012, 37, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Marchi, L.; Abdala, N.; Oliveira, L.; Amaral, R.; Coutinho, E.; Pimenta, L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J. Neurosurg. Spine 2013, 19, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Armocida, D.; Pesce, A.; Cimatti, M.; Proietti, L.; Santoro, A.; Frati, A. Minimally invasive transforaminal lumbar interbody fusion using expandable cages: Increased risk of late postoperative subsidence without a real improvement of perioperative outcomes: A clinical monocentric study. World Neurosurg. 2021, 156, e57–e63. [Google Scholar] [CrossRef] [PubMed]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2019, 4, 101–110. [Google Scholar] [CrossRef]
- Bari, E.; Tartara, F.; Cofano, F.; Di Perna, G.; Garbossa, D.; Perteghella, S.; Sorlini, M.; Mandracchia, D.; Giovannelli, L.; Gaetani, P.; et al. Freeze-dried secretome (lyosecretome) from mesenchymal stem/stromal cells promotes the osteoinductive and osteoconductive properties of titanium cages. Int. J. Mol. Sci. 2021, 22, 8445. [Google Scholar] [CrossRef]
- Ragni, E.; Orfei, C.P.; Bidossi, A.; De Vecchi, E.; Francaviglia, N.; Romano, A.; Maestretti, G.; Tartara, F.; de Girolamo, L. Superior osteo-inductive and osteo-conductive properties of trabecular titanium vs. PEEK scaffolds on human mesenchymal stem cells: A proof of concept for the use of fusion cages. Int. J. Mol. Sci. 2021, 22, 2379. [Google Scholar] [CrossRef]
- Caliogna, L.; Bina, V.; Botta, L.; Benazzo, F.M.; Medetti, M.; Maestretti, G.; Mosconi, M.; Cofano, F.; Tartara, F.; Gastaldi, G. Osteogenic potential of human adipose derived stem cells (hASCs) seeded on titanium trabecular spinal cages. Sci. Rep. 2020, 10, 18284. [Google Scholar] [CrossRef]
- McGilvray, K.C.; Easley, J.; Seim, H.B.; Regan, D.; Berven, S.H.; Hsu, W.K.; Mroz, T.E.; Puttlitz, C.M. Bony ingrowth potential of 3D-printed porous titanium alloy: A direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J. 2018, 18, 1250–1260. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Gittens, R.A.; Schneider, J.M.; Hyzy, S.L.; Haithcock, D.A.; Ullrich, P.F.; Schwartz, Z.; Boyan, B.D. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012, 12, 265–272. [Google Scholar] [CrossRef]
- Aaltonen, L.; Koivuviita, N.; Seppänen, M.; Burton, I.S.; Kröger, H.; Löyttyniemi, E.; Metsärinne, K. Bone histomorphometry and 18F-sodium fluoride positron emission tomography imaging: Comparison between only bone turnover-based and unified TMV-based classification of renal osteodystrophy. Calcif. Tissue Int. 2021, 109, 605–614. [Google Scholar] [CrossRef]
- Sarikaya, I.; Elgazzar, A.H.; Sarikaya, A.; Alfeeli, M. Normal bone and soft tissue distribution of fluorine-18-sodium fluoride and artifacts on 18F-NaF PET/CT bone scan: A pictorial review. Nucl. Med. Commun. 2017, 38, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Lamartina, C.; Berjano, P. Classification of sagittal imbalance based on spinal alignment and compensatory mechanisms. Eur. Spine J. 2014, 23, 1177–1189. [Google Scholar] [CrossRef]
- Schwab, F.J.; Diebo, B.G.; Smith, J.S.; Hostin, R.A.; Shaffrey, C.I.; Cunningham, M.E.; Mundis, G.M.; Ames, C.P.; Burton, D.C.; Bess, S.; et al. Fine-tuned surgical planning in adult spinal deformity: Determining the lumbar lordosis necessary by accounting for both thoracic kyphosis and pelvic incidence. Spine J. 2014, 14, S73. [Google Scholar] [CrossRef]
- Cofano, F.; Zenga, F.; Mammi, M.; Altieri, R.; Marengo, N.; Ajello, M.; Pacca, P.; Melcarne, A.; Junemann, C.; Ducati, A.; et al. Intraoperative neurophysiological monitoring during spinal surgery: Technical review in open and minimally invasive approaches. Neurosurg. Rev. 2019, 42, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Mottaghy, F.M.; Paycha, F.; Behrendt, F.F.F.; Van den Wyngaert, T.; Fogelman, I.; Strobel, K.; Celli, M.; Fanti, S.; Giammarile, F.; et al. (18)F-NaF PET/CT: EANM procedure guidelines for bone imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Segall, G.; Delbeke, D.; Stabin, M.G.; Even-Sapir, E.; Fair, J.; Sajdak, R.; Smith, G.T. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J. Nucl. Med. 2010, 51, 1813–1820. [Google Scholar] [CrossRef]
- Kinahan, P.E.; Fletcher, J.W. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin. Ultrasound CT MR 2010, 31, 496–505. [Google Scholar] [CrossRef]
- Boellaard, R. Standards for PET image acquisition and quantitative data analysis. J. Nucl. Med. 2009, 50 (Suppl. 1), 11S–20S. [Google Scholar] [CrossRef]
- Rao, P.J.; Pelletier, M.H.; Walsh, W.R.; Mobbs, R.J. Spine interbody implants: Material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop. Surg. 2014, 6, 81–89. [Google Scholar] [CrossRef]
- Ramakrishna, S.M.J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Stadelmann, V.A.; Terrier, A.; Pioletti, D.P. Microstimulation at the bone-implant interface upregulates osteoclast activation pathways. Bone 2008, 42, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Lee, J.K.; Moon, S.J.; Seo, S.K.; Lee, J.H.; Kim, S.H. The efficacy of the synthetic interbody cage and Grafton for anterior cervical fusion. Spine (Phila Pa 1976) 2009, 34, E591–E595. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Takemoto, M.; Neo, M.; Matsushita, T.; Kokubo, T.; Doi, K.; Ito, T.; Shimizu, A.; Nakamura, T. A novel synthetic material for spinal fusion: A prospective clinical trial of porous bioactive titanium metal for lumbar interbody fusion. Eur. Spine J. 2011, 20, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; So, K.; Akiyama, N.; Matsushita, T.; Kokubo, T.; Nakamura, T. A porous bioactive titanium implant for spinal interbody fusion: An experimental study using a canine model. J. Neurosurg. Spine 2007, 7, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, H.; Fogel, G.R.; Liao, Z.; Li, Y.; Liu, W. Biomechanical analysis of porous additive manufactured cages for lateral lumbar interbody fusion: A finite element analysis. World Neurosurg. 2018, 111, e581–e591. [Google Scholar] [CrossRef]
- Tartara, F.; Bongetta, D.; Pilloni, G.; Colombo, E.V.; Giombelli, E. Custom-made trabecular titanium implants for the treatment of lumbar degenerative discopathy via ALIF/XLIF techniques: Rationale for use and preliminary results. Eur. Spine J. 2020, 29, 314–320. [Google Scholar] [CrossRef]
- Fischer, D.R.; Zweifel, K.; Treyer, V.; Hesselmann, R.; Johayem, A.; Stumpe, K.D.M.; Von Schulthess, G.K.; Hany, T.F.; Strobel, K. Assessment of successful incorporation of cages after cervical or lumbar intercorporal fusion with [(18)F]fluoride positron-emission tomography/computed tomography. Eur. Spine J. 2011, 20, 640–648. [Google Scholar] [CrossRef]
- Ayubcha, C.; Zirakchian Zadeh, M.; Stochkendahl, M.J.; Al-Zaghal, A.; Hartvigsen, J.; Rajapakse, C.S.; Borja, A.J.; Arani, L.S.; Gerke, O.; Werner, T.J.; et al. Quantitative evaluation of normal spinal osseous metabolism with 18F-NaF PET/CT. Nucl. Med. Commun. 2018, 39, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Brans, B.; Weijers, R.; Halders, S.; Wierts, R.; Peters, M.; Punt, I.; Willems, P. Assessment of bone graft incorporation by 18F-fluoride positron-emission tomography/computed tomography in patients with persisting symptoms after posterior lumbar interbody fusion. EJNMMI Res. 2012, 2, 42. [Google Scholar] [CrossRef]
- Seifen, T.; Rodrigues, M.; Rettenbacher, L.; Piotrowski, W.; Holzmannhofer, J.; Mc Coy, M.; Pirich, C. The value of (18)F-fluoride PET/CT in the assessment of screw loosening in patients after intervertebral fusion stabilization. EJNMMI 2015, 42, 272–277. [Google Scholar]
- Peters, M.; Willems, P.; Weijers, R.; Wierts, R.; Jutten, L.; Urbach, C.; Arts, J.; Van Rhijn, L.; Brans, B. Pseudarthrosis after lumbar spinal fusion: The role of 18F-fluoride PET/CT. EJNMMI 2015, 42, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Pouldar, D.; Bakshian, S.; Matthews, R.; Rao, V.; Manzano, M.; Dardashti, S. Utility of 18F sodium fluoride PET/CT imaging in the evaluation of postoperative pain following surgical spine fusion. Muscoloskelet Surg. 2017, 101, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Quon, A.; Dodd, R.; Iagaru, A.; de Abreu, M.R.; Hennemann, S.; Neto, J.M.A.; Sprinz, C. Initial investigation of 18F-NaF PET/CT for identification of vertebral sites amenable to surgical revision after spinal fusion surgery. EJNMMI 2012, 39, 1737–1744. [Google Scholar] [CrossRef]

| Patients | 15 (7 M, 8 F) |
|---|---|
| Age (mean/min/max) | 61/41/77 |
| Levels | 4 L1-L2 (11.5%) 6 L2-L3 (17.1%) 7 L3-L4 (20%) 12 L4-L5 (34.3%) 6 L5-S1 (17.1%) |
| Type of fusion | XLIF (26), ALIF (9) |
| Number of levels of surgery | 4 Single level (26.7%) 6 Two levels (40%) 2 Three levels (13.3%) 2 Four levels (13.3%) 1 Five levels (6.7%) |
| Follow-up (mean/min/max) | 22.2/13/32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofano, F.; Armocida, D.; Ruffini, L.; Scarlattei, M.; Baldari, G.; Di Perna, G.; Pilloni, G.; Zenga, F.; Ballante, E.; Garbossa, D.; et al. The Efficacy of Trabecular Titanium Cages to Induce Reparative Bone Activity after Lumbar Arthrodesis Studied through the 18f-Naf PET/CT Scan: Observational Clinical In-Vivo Study. Diagnostics 2022, 12, 2296. https://doi.org/10.3390/diagnostics12102296
Cofano F, Armocida D, Ruffini L, Scarlattei M, Baldari G, Di Perna G, Pilloni G, Zenga F, Ballante E, Garbossa D, et al. The Efficacy of Trabecular Titanium Cages to Induce Reparative Bone Activity after Lumbar Arthrodesis Studied through the 18f-Naf PET/CT Scan: Observational Clinical In-Vivo Study. Diagnostics. 2022; 12(10):2296. https://doi.org/10.3390/diagnostics12102296
Chicago/Turabian StyleCofano, Fabio, Daniele Armocida, Livia Ruffini, Maura Scarlattei, Giorgio Baldari, Giuseppe Di Perna, Giulia Pilloni, Francesco Zenga, Elena Ballante, Diego Garbossa, and et al. 2022. "The Efficacy of Trabecular Titanium Cages to Induce Reparative Bone Activity after Lumbar Arthrodesis Studied through the 18f-Naf PET/CT Scan: Observational Clinical In-Vivo Study" Diagnostics 12, no. 10: 2296. https://doi.org/10.3390/diagnostics12102296
APA StyleCofano, F., Armocida, D., Ruffini, L., Scarlattei, M., Baldari, G., Di Perna, G., Pilloni, G., Zenga, F., Ballante, E., Garbossa, D., & Tartara, F. (2022). The Efficacy of Trabecular Titanium Cages to Induce Reparative Bone Activity after Lumbar Arthrodesis Studied through the 18f-Naf PET/CT Scan: Observational Clinical In-Vivo Study. Diagnostics, 12(10), 2296. https://doi.org/10.3390/diagnostics12102296













