Are Congenital Cervical Block Vertebrae a Risk Factor for Adjacent Segment Disease? A Retrospective Cross-Sectional CT and MR Imaging Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASDI | Adjacent segment disease |
| CT | Computed tomography |
| IRB | Institutional review board |
| MRI | Magnetic resonance imaging |
| PACS | Picture archiving and communication system |
References
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. The Developing Human: Clinically Oriented Embryology, 8th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2015. [Google Scholar]
- Bethany, M.U.; Mette, N.C. A Sequential developmental field defect of the vertebrae, ribs and sternum, in a young woman of the 12th Cenury AD. Am. J. Phys. Anthropol. 2000, 3, 355–367. [Google Scholar]
- Witzmann, F.; Rothschild, B.M.; Hampe, O.; Sobral, G.; Gubin, Y.M.; Asbach, P. Congenital malformations of the vertebral column in ancient amphibians. Anat. Histol. Embryol. 2014, 43, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Brückl, R.; Brückl, N. Der kongenitale HWS-blockwirbel. Arch. Orthop. Trauma Surg. 1979, 95, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Erdil, H.; Yildiz, N.; Cimen, M. Congenital fusion of cervical vertebrae and its clinical significance. J. Anat. Soc. India 2003, 52, 125–127. [Google Scholar]
- Kumar, R.; Guinto, F.C.; Madewell, J.E.; Swischuk, L.E.; David, R. The vertebral body: Radiographic configurations in various congenital and acquired disorders. Radiographics 1988, 8, 455–485. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.W.; Romaine, C.B.; Skandalikis, J.E. Collective review: Congenital fusion of the cervical vertebrae. Surg. Ob. Gyn. 1964, 118, 373–384. [Google Scholar]
- Lee, C.K.; Weiss, A.B. Isolated congenital cervical block vertebrae below the axis with neurological symptoms. Spine 1981, 6, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Sasso, R.C.; Smucker, J.D.; Hacker, R.J.; Heller, J.G. Artificial disk versus fusion: A prospective, randomized study with 2-year follow-up on 99 patients. Spine 2007, 32, 2933–2940. [Google Scholar] [CrossRef] [PubMed]
- Shriver, M.F.; Lewis, D.J.; Kshettry, V.R.; Rosenbaum, B.P.; Benzel, E.C.; Mroz, T.E. Pseudoarthrosis rates in anterior cervical diskectomy and fusion: A meta-analysis. Spine J. 2015, 15, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, M.D.; Bevevino, A.J.; Hilibrand, A.S. Update on the evidence for adjacent segment degeneration and disease. Spine J. 2013, 13, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.S.; Kim, S.S.; Lee, B.J.; Moon, J.L.; Lin, J.F.; Moon, Y.W. Radiographic assessment of congenital C2-3 synostosis. J. Orthop. Surg. 2010, 18, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Leivseth, G.; Frobin, W.; Brinckmann, P. Congenital cervical block vertebrae are associated with caudally adjacent disks. Clin. Biomech. 2005, 20, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.S.; Kim, S.S.; Yoon, M.G.; Seo, Y.H.; Lee, B.J.; Moon, H.; Kim, S.S. Radiographic assessment of effect of congenital monosegment synostosis of lower cervical spine between C2–C6 on adjacent mobile segments. Asian Spine J. 2014, 8, 615–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soni, P.; Sharma, V.; Sengupta, J. Cervical vertebrae anomalies-incidental findings on lateral cephalograms. Angle Orthod. 2008, 78, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Grifka, P.D.; Krämer, J. Wirbelsäule. In Orthopädie Unfallchirurgie; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Dunsker, S.B.; Brown, O.; Thomson, N. Craniovertebral anomalies. Clin. Neurosurg. 1980, 27, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Guille, J.T.; Miller, A.; Bowen, J.R.; Forlin, E.; Caro, P.A. The natural history of Klippel-Feil syndrome: Clinical, roentgenographic, and magnetic resonance imaging findings at adulthood. J. Pediatric Orthop. 1995, 15, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Hensinger, R.N. Congenital anomalies of the cervical spine. Clin. Orthop. Relat. Res. 1991, 264, 16–38. [Google Scholar] [CrossRef]
- Eck, J.C.; Humphreys, S.C.; Lim, T.H.; Jeong, S.T.; Kim, J.G.; Hodges, S.D.; An, H.S. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiskal pressure and segmental motion. Spine 2002, 27, 2431–2434. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Roughley, P.J. What is intervertebral disk degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Schmatz, C.; Schultz, A.B. Lumbar disk degeneration: Correlation with age, sex, and spine level in 600 autopsy specimens. Spine 1988, 13, 173–178. [Google Scholar] [CrossRef] [PubMed]
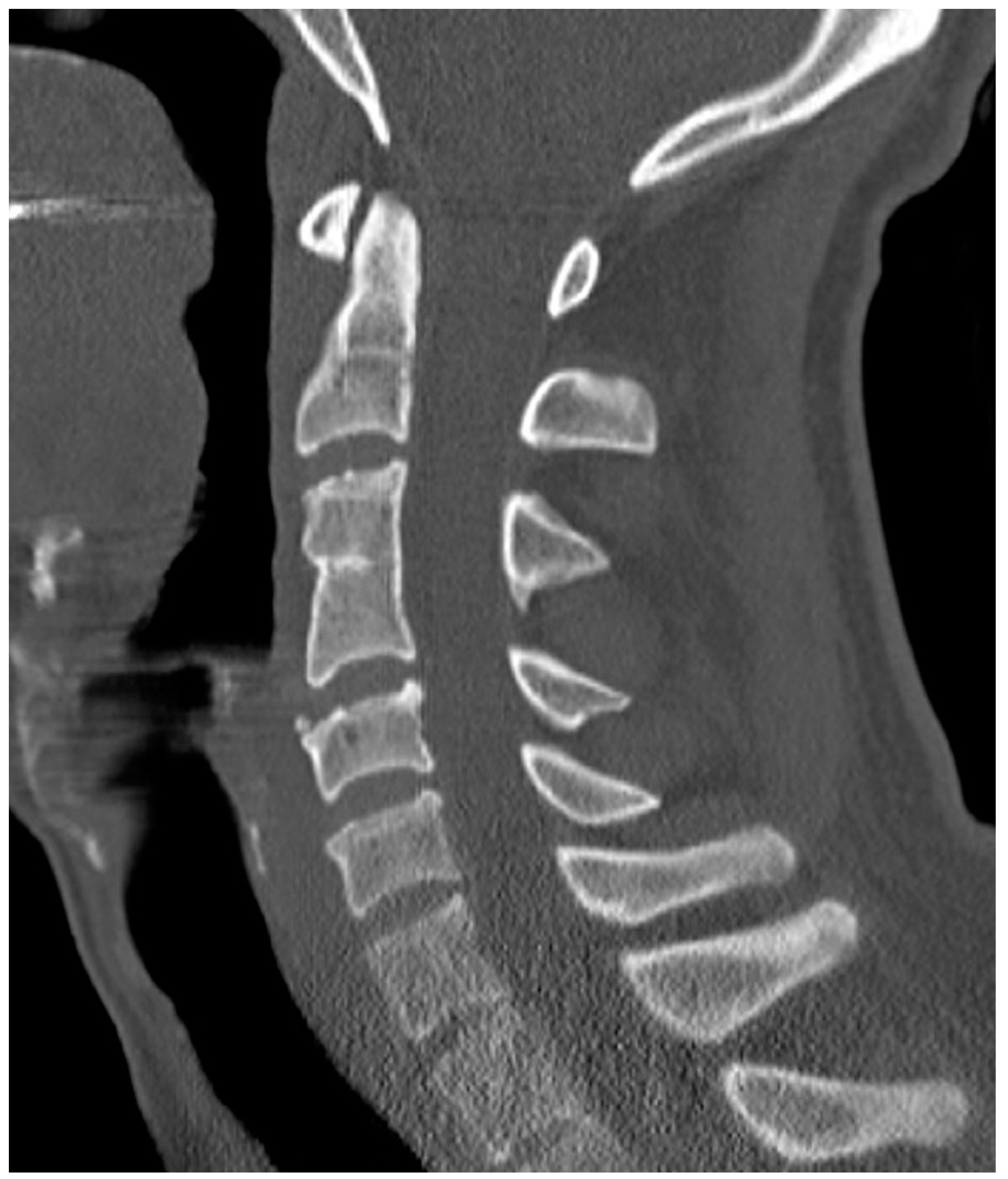
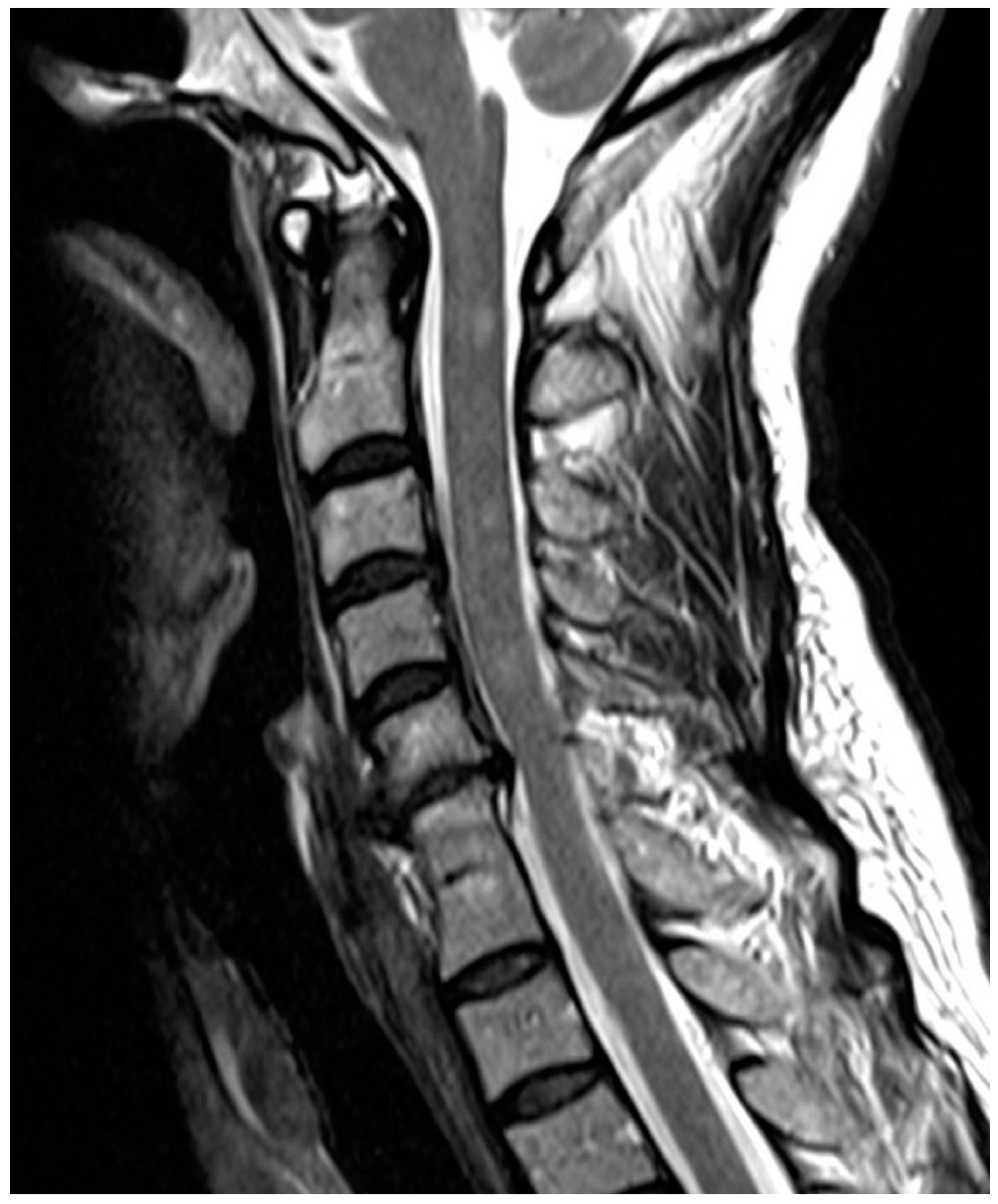
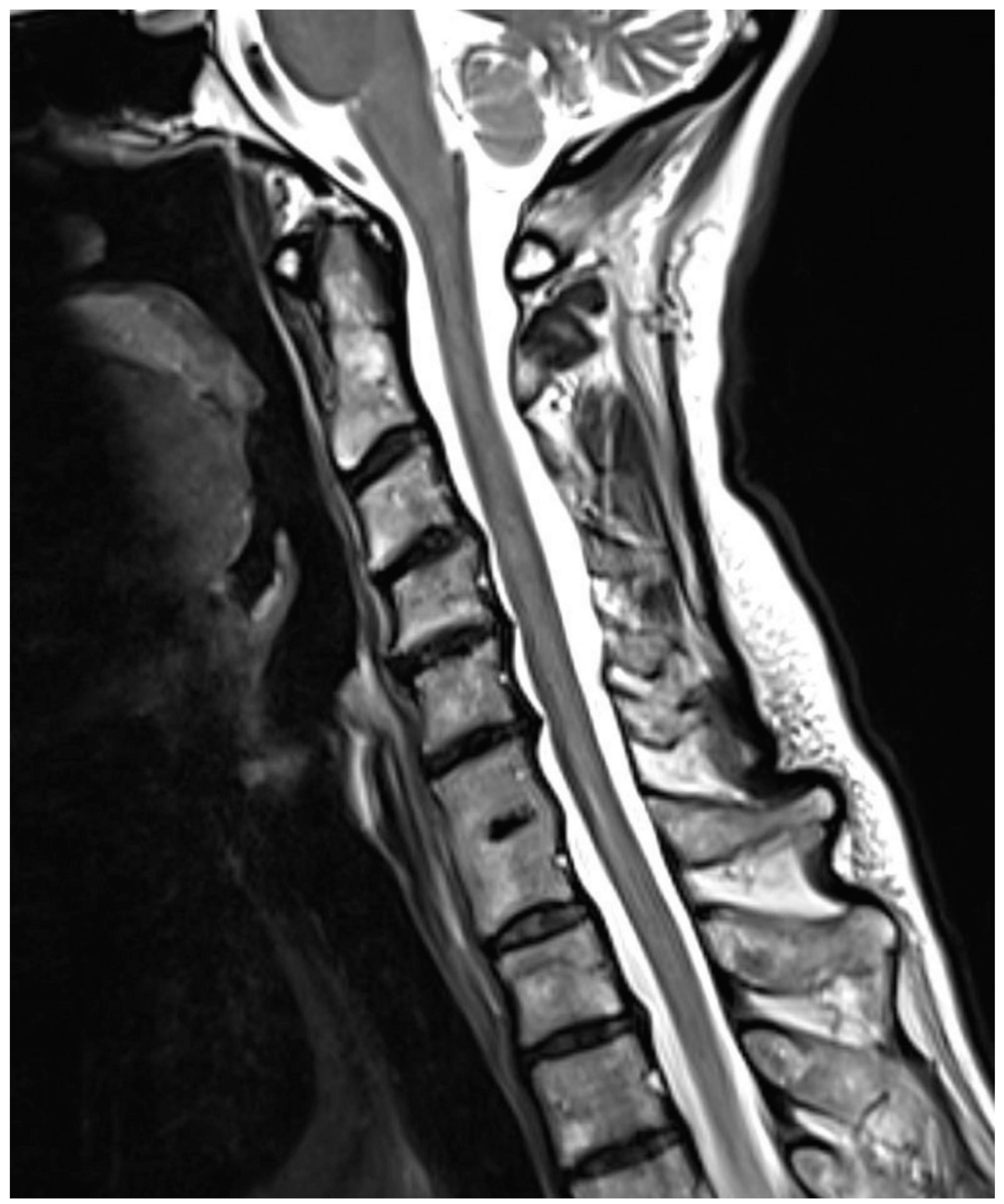
| Fusion of intervertebral disk | Normal Disk | 1 point |
| hypoplastic disk, without bone contact of corresponding endplates | 2 points | |
| hypoplastic disk, with bone contact of corresponding endplates | 3 points | |
| complete fusion of vertebral bodies (with or without residual disk) | 4 points | |
| Fusion of the arches | both arches not fused | 1 point |
| unilaterally fused arches | 2 points | |
| bilaterally fused arches | 3 points | |
| Fusion of spinous process | processes not fused | 1 point |
| processes fused | 2 points |
| Intervertebral Disk Degeneration | Loss of Height of the Intervertebral Disk, Disk Bulging over the Dorsal Level, Evidence of Retrospondylophytes | 1 Point |
| Segment C1/2: Atlantodental degeneration | joint space narrowing, cystic changes of the odontoid process, ligamentous hypertrophy | 1 point |
| Facet joint degeneration | joint space narrowing and subchondral sclerosis of the facet joint, hypertrophic yellow ligaments, joint effusion, evidence of osteophytes | 1 point |
| Segment C1/2: Atlantoaxial degeneration | joint space narrowing, joint effusion, evidence of osteophytes | 1 point |
| Neuroforaminal stenosis | narrowing through diskal, osseous, or ligamental component | 1 point |
| Mean | SD | Range | Mean (SD) Age Group 12–43 Years | Mean (SD) Age Group 45–92 Years | |
|---|---|---|---|---|---|
| adjacent segment ratio | 0.307 | ±0.303 | 0–1 | 0.192 (±0.274) | 0.427 (±0.289) |
| degeneration ratio | 0.188 | ±0.232 | 0–1 | 0.139 (±0.182) | 0.240 (±0.268) |
| instability ratio | 0.119 | ±0.325 | 0–1 | 0.054 (±0.284) | 0.187 (±0.355) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, C.; Asbach, P.; Niehues, S.M. Are Congenital Cervical Block Vertebrae a Risk Factor for Adjacent Segment Disease? A Retrospective Cross-Sectional CT and MR Imaging Study. Diagnostics 2022, 12, 90. https://doi.org/10.3390/diagnostics12010090
Jung C, Asbach P, Niehues SM. Are Congenital Cervical Block Vertebrae a Risk Factor for Adjacent Segment Disease? A Retrospective Cross-Sectional CT and MR Imaging Study. Diagnostics. 2022; 12(1):90. https://doi.org/10.3390/diagnostics12010090
Chicago/Turabian StyleJung, Cornelius, Patrick Asbach, and Stefan M. Niehues. 2022. "Are Congenital Cervical Block Vertebrae a Risk Factor for Adjacent Segment Disease? A Retrospective Cross-Sectional CT and MR Imaging Study" Diagnostics 12, no. 1: 90. https://doi.org/10.3390/diagnostics12010090
APA StyleJung, C., Asbach, P., & Niehues, S. M. (2022). Are Congenital Cervical Block Vertebrae a Risk Factor for Adjacent Segment Disease? A Retrospective Cross-Sectional CT and MR Imaging Study. Diagnostics, 12(1), 90. https://doi.org/10.3390/diagnostics12010090






